Abstract
Background
To explore the effects of the respiratory rate (RR) on the venous-to-arterial CO2 tension difference (gapCO2) in septic shock patients undergoing volume mechanical ventilation.
Methods
Adult patients with septic shock underwent volume mechanical ventilation between October 2015 and October 2016. RR was started at 10 breaths/min, and 2 breaths/min were added every 60 min until 16 breaths/min was reached. At every point, central venous and arterial blood gas measurements were obtained simultaneously.
Results
In this study, gapCO2 induced by hyperventilation significantly increased, while the central venous carbon dioxide pressure (PvCO2) and the partial pressure of CO2 (PaCO2) in arteries decreased. The decreasing trend of the PaCO2 was more obvious than that of the PvCO2. HCO3− and ctCO2 were markedly decreased, when the RR was increased (P < 0.05). Central venous oxygen saturation (ScvO2) had a decreasing trend between 14 (77.1 ± 8.3%) and 16 (75.2 ± 8.7%) breaths/min; however, the difference was not significant.
Conclusions
In septic patients undergoing ventilation, respiratory alkalosis induced by hyperventilation caused an increase in the gapCO2. Clinicians should cautiously interpret the gapCO2 in hemodynamically stable ventilated septic shock patients and its relationship with low cardiac output and inadequate perfusion.
Similar content being viewed by others
Background
The current consensus and guidelines for the hemodynamic management of severe sepsis and septic shock advocate clinicians to regard the use of lactate clearance and central venous oxygen saturation (ScvO2) normalization as tissue perfusion endpoints [1,2,3]. Abnormally high ScvO2 values have been related to higher mortality in septic shock patients [4], while lactate clearance has proved to be as beneficial as the ScvO2 in the hemodynamic management of sepsis. However, when clinicians face the uncertainty of a high lactate value, they have to identify whether it indicates persistent hypoperfusion or whether it will eventually normalize. Therefore, normalized ScvO2 values and lactate values do not rule out persistent hypoxia and hypoperfusion during initial resuscitation.
A few authors have reported that the central venous-to-arterial CO2 difference (gapCO2) demonstrated prognostic value in different conditions [5,6,7,8] and have recommended that the gapCO2 combined with the ScvO2 and lactate values should be used to rule out patients with persistent global hypoperfusion [3]. Under steady states of both CO2 production and oxygen consumption, the gapCO2 was observed to be increased in parallel with the reduction in cardiac output [9]. However, spontaneous breathing and hyperventilation may reduce the partial pressure of CO2 in arteries (PaCO2) and prevent the CO2 stagnation-induced rise in central venous carbon dioxide pressure (PvCO2). Mallat et al. demonstrated that acute hyperventilation provoked a significant increase in gapCO2 and oxygen consumption (VO2) [10]. To date, little is known about the gapCO2 and respiratory rate (RR) in septic shock patients undergoing volume mechanical ventilation. Therefore, in this study, we investigated the underlying effect of the RR on the gapCO2 in septic shock patients undergoing volume mechanical ventilation.
Materials and methods
Patients
This observational and prospective study was performed at a single, mixed medical and surgical adult intensive care unit (ICU) with 14 beds at 105 Hospital of Chinese People’s Liberation Army from October 2015 to October 2016. The study was approved by the ethics committee of the 105 Hospital of Chinese People’s Liberation Army. Informed consent was obtained from each subject.
The study included 17 patients within 24 h of septic shock onset. The diagnosis of septic shock was determined according to the Second International Consensus of Definitions for Sepsis and Septic Shock [11]. As part of the routine management of septic shock in our ICU, all patients underwent volume mechanical ventilation with a fractional inspired oxygen level (FiO2) no greater than 65%, using an 840 Ventilator System.
Sedation with propofol and analgesia with remifentanil were provided so that patients without spontaneous breathing could be included in the study. The inclusion criteria were oliguria, mottled skin [12], central venous oxygen saturation (ScvO2) > 65% and lactate level < 4 mmol/L after achieving adequate intravascular volume and adequate mean arterial pressure (MAP) > 65 mmHg, as recommended by the Surviving Sepsis Campaign [1]. The exclusion criteria were pregnancy, COPD, age less than 18 years, unstable hemodynamic condition (change of vasoactive drug dosage or fluid administration within 60 min before the protocol), high blood lactate levels (> 4 mmol/L) after adequate resuscitation, and uncontrolled tachyarrhythmia (heart rate > 140 beats/min).
Measurements
Demographic data, septic shock etiology, and acute physiology and chronic Health evaluation (APACHE) II [13] and sequential organ failure assessment (SOFA) scores were obtained on the day of enrollment. Blood gas analysis was performed using a blood gas analyzer (ABL 900 Radiometer, Copenhagen, Denmark). ScvO2 was determined in a sample taken from the central venous catheter with the tip (confirmed by X-ray) in the superior vena cava near or at the right atrium. The gapCO2 was calculated as the difference between the central PvCO2 tension and PaCO2 tension.
Study protocols
Ventilation parameters and vasopressor drugs were kept constant during the 60-min period prior to the measurements being taken. After the baseline measurements were taken, an RR of 10 breaths/min was started, and 2 breaths/min were added every 60 min until 16 breaths/min was attained. For each procedure, all parameters were measured. The study ended when arterial hypotension (MAP < 60 mmHg), tachycardia (heart rate > 150 beats/min), acute atrial fibrillation, or changes in the ST segment of the electrocardiogram occurred.
Statistical analysis
Statistical analysis was performed using SPSS 10.0 software. A p value (2-tailed) of 0.05 or less was considered significant. Continuous variables were presented as mean ± standard deviation (SD) if normally distributed, or as median if otherwise, and compared using Wilcoxon–Mann–Whitney test. Comparisons of hemodynamic indexes and blood gas in accordance with the RR from 10 to 16 breaths/min were performed by the Bonferroni method.
Results
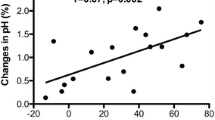
Basic characteristics of the 17 patients are presented in Table 1. All hemodynamic effects following the change in RR are listed in Table 2. Blood pressure, heart rate, lactate level, and BE changes were not statistically significant from 10 to 16 breaths/min. Hyperventilation induced a significant RR-dependent increase in the gapCO2, which was related to the combined trend of decreases in PaCO2 and PvCO2 (Figs. 1 and 2). Changes in HCO3− were paralleled by a significant decrease in ctCO2 (Fig. 3). ScvO2 significantly increased with increasing RR from 10 to 14 breaths/min, but had a decreasing trend from 14 to 16 breaths/min. Hypocapnia provoked by hyperventilation significantly decreased from 10 to 16 breaths/min, while the gapCO2 exhibited an opposite trend (Fig. 4).
Discussion
Septic patients with respiratory failure suffer myocardial systolic and diastolic dysfunction [14]. Mechanical ventilation plays a critical role in these patients. At present, the gapCO2 has been considered an evaluation of tissue hypoperfusion and has been recommended as a guide for resuscitation in septic patients [5, 6]. However, it is unknown whether the clinical significance of the gapCO2 also applies to septic patients with mechanical ventilation. Therefore, we explored the effects of the respiratory rate on the gapCO2 in septic shock patients undergoing volume mechanical ventilation and found a significant difference in the gapCO2 induced by hyperventilation.
When the respiratory rates increased with the tidal volume unchanged, the total minute ventilation and alveolar ventilation, as well as the PaO2, improved. The PaCO2 decreased because excessive ventilation enabled the increased expiration of CO2. Our results showed that the PcvCO2 and PaCO2 decreased with an increasing respiratory rate, and this difference was statistically significant. However, the decreasing trend of the PaCO2 was more obvious than that of the PcvCO2. Consequently, the difference in the gapCO2 values when the RR varied from 10 to 16 breaths/min was significant. We consider that the following physiological factors can explain this phenomenon.
The combination of oxygen and hemoglobin can promote the release of CO2. Furthermore, CO2 more easily combines with hemoglobin at lower oxygen saturation, which is a phenomenon called the Haldane effect [15]. Clearly an increase in alveolar ventilation increases CO2 excretion and alveolar PaO2. Increased alveolar ventilation causes respiratory alkalosis with immediate changes in hydrogen ion concentrations and bicarbonate concentrations. There are transcellular shifts with H ions coming out cells into the plasma and shifts of potassium from the plasma into cells. Renal compensation with bicarbonate excretion takes hours. Nevertheless, respiratory alkalosis can cause peripheral vasoconstriction and decreased flow through the various vascular beds. This is probably not a uniform effect in all vessels and may or may not occur in patients with sepsis. However, if blood flow decreases but metabolism is unchanged, then the CO2 content of venous blood should increase, leading to a higher gapCO2. If patients with sepsis have disordered cellular metabolism, then the production CO2 may decrease, which in turn results in a lower gapCO2. Furthermore, the depression of oxidative adenosine triphosphate (ATP) production also led to pulmonary vasoconstriction [16]. Nitric oxide synthesis defect has shown to be another cause [17]. As a consequence, the reducing trend of the CO2 partial pressure of venous blood is less marked than that of arterial blood. This has been demonstrated by other scholars as well [18]. The increase in alveolar ventilation increases CO2 excretion and should increase the alveolar PaO2 provided other factors stay the same. Increased alveolar ventilation causes respiratory alkalosis with immediate changes in hydrogen ion concentrations and bicarbonate concentrations. There should be transcellular shifts with hydrogen ion coming out cells into the plasma and shifts of potassium from the plasma into cells. Respiratory alkalosis can cause peripheral vasoconstriction and decreased flow through various vascular beds. This is probably not a uniform effect in all vessels and may or may not occur in patients with sepsis. However, if blood flow decreases but metabolism is unchanged, then the CO2 content of venous blood should increase, resulting in a gapCO2. If patients with sepsis have dysfunctional cellular metabolism, then the production CO2 may decrease, which would result in a lower gapCO2. This likely varies in the vascular bed from tissue to tissue. CO2 binding hemoglobin may not have an important effect in these experiments.
When vasoconstriction is caused by hypocapnia, poor oxygen delivery may add tissue oxygen extraction [19]. Hence, when patients are in hypocapnia provoked by hyperventilation, the CO2 partial pressure of the venous blood does not obviously decrease compared with the CO2 partial pressure of the arterial blood. For instance, Khambatta et al. reported that mechanically ventilated dogs undergoing 2 h of hyperventilation had 20% CO2 production and 25% O2 consumption [20]. In this study, the ScvO2 significantly increased with the RR increase from 10 to 14 breaths/min, but there was no significant decreasing trend in ScvO2 between 14 and 16 breaths/min, which was consistent with a previous report [21]. Moreover, it is widely accepted that the gapCO2 depends on measurements from the superior vena cava (SVC) but does not reflect measurements from the inferior vena cava (IVC) and tissues draining into the IVC [22].
Patients with septic shock lack an effective circulating blood volume, which leads to systemic anoxia and metabolic acidosis. Acidosis and excess CO2 can cause mitochondrial dysfunction [23, 24]. Metabolic acidosis and respiratory alkalosis due to excessive hyperventilation further reduce the function of mitochondria oxidative phosphorylation, which may impact CO2 production. In summary, our data suggested that in septic patients with mechanical ventilation, respiratory alkalosis induced by hyperventilation enabled the increase of gapCO2. Clinicians should be cautious when interpreting the gapCO2 as low cardiac output and inadequate perfusion in hemodynamically stable ventilated septic shock patients.
Conclusions
However, there were some limitations in our paper. In this study, we used a central venous sample to measure the gapCO2 instead of using a mixed venous sample, despite the latter having a better correlation. We cannot state the relationship between the gapCO2 and cardiac output, as we did not include the indexes of cardiac function in this study. We included fewer patients than other studies, and the included patients suffered from different etiological factors, so the results from this study should be further confirmed in the future.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
Mesquida J, Borrat X, Lorente JA, Masip J, Baigorri F. Objectives of hemodynamic resuscitation. Med Intens. 2011;35(8):499–508.
Vallet B, Pinsky MR, Cecconi M. Resuscitation of patients with septic shock: “please mind the gap”! Intensive Care Med. 2013;39(9):1653.
van Beest PA, Hofstra JJ, Schultz MJ, Boerma EC, Spronk PE, Kuiper MA. The incidence of low venous oxygen saturation on admission to the intensive care unit: a multi-center observational study in The Netherlands. Crit Care. 2008;12(2):R33.
Vallée F, Vallet B, Mathe O, Parraguette J, Mari A, Silva S, Samii K, Fourcade O, Genestal M. Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock? Intensive Care Med. 2008;34(12):2218.
Futier E, Robin E, Jabaudon M, Guerin R, Petit A, Bazin JE, Constantin JM, Vallet B. Central venous O2 saturation and venous-to-arterial CO2 difference as complementary tools for goal-directed therapy during high-risk surgery. Crit Care. 2010;14(5):R193.
Ospinatascón GA, Bautistarincón DF, Umaña M, Tafur JD, Gutiérrez A, García AF, Bermúdez W, Granados M, Arangodávila C, Hernández G. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care. 2013;17(6):R294.
Beest PAV, Lont MC, Holman ND, Loef B, Kuiper MA, Boerma EC. Central venous-arterial p CO2 difference as a tool in resuscitation of septic patients. Intensive Care Med. 2013;39(6):1034–9.
Rocha M, Costa F, Barreto JEF. Central venous-to-arterial carbon dioxide difference (CO2 GAP) in patients with arterial pulmonary hypertension: a pilot study. Eur Respir J. 2014;40:P3920.
Mallat J, Mohammad U, Lemyze M, Meddour M, Jonard M, Pepy F, Gasan G, Barrailler S, Temime J, Vangrunderbeeck N. Acute hyperventilation increases the central venous-to-arterial PCO2 difference in stable septic shock patients. Ann Intensive Care. 2017;7(1):31.
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brunbuisson C, Beale R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2008;34:17–60.
Ait-Oufella H, Lemoinne S, Boelle PY, Galbois A, Baudel JL, Lemant J, Joffre J, Margetis D, Guidet B, Maury E. Mottling score predicts survival in septic shock. Intensive Care Med. 2011;37(5):801.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1986;13(10):818–29.
Flynn A, Mani BC, Mather PJ. Sepsis-induced cardiomyopathy: a review of pathophysiologic mechanisms. Heart Fail Rev. 2010;15(6):605.
Teboul JL, Scheeren T. Understanding the Haldane effect. Intensive Care Med. 2017;43(1):91–3.
Rounds S, Mcmurtry IF. Inhibitors of oxidative ATP production cause transient vasoconstriction and block subsequent pressor responses in rat lungs. Circ Res. 1981;48(3):393.
Gardiner SM, Kemp PA, Bennett T, Palmer RM, Moncada S. Nitric oxide synthase inhibitors cause sustained, but reversible, hypertension and hindquarters vasoconstriction in Brattleboro rats. Eur J Pharmacol. 1992;213(3):449–51.
Morel J, Gergelé L, Dominé A, Molliex S, Perrot JL, Labeille B, Costes F. The venous-arterial difference in CO2 should be interpreted with caution in case of respiratory alkalosis in healthy volunteers. J Clin Monit Comput. 2017;31(4):701–7.
Mas A, Saura P, Joseph D, Blanch L, Baigorri F, Artigas A, Fernández R. Effect of acute moderate changes in PaCO2 on global hemodynamics and gastric perfusion. Crit Care Med. 2000;28(2):360–5.
Khambatta HJ, Sullivan SF. Effects of respiratory alkalosis on oxygen consumption and oxygenation. Anesthesiology. 1973;38(1):53–8.
Morel J, Gergele L, Verveche D, Costes F, Auboyer C, Molliex S. Do fluctuations of PaCO2 impact on the venous-arterial carbon dioxide gradient? Crit Care. 2011;15(6):456.
Riva JA, Bouchacourt JP, Kohn WE, Hurtado FJ. The changes in the oxygen saturations in the superior vena cava and the pulmonary artery are not the same during cardiac surgery. Rev Esp Anestesiol Reanim. 2015;62(3):140–4.
Jubrias SA, Crowther GJ, Shankland EG, Gronka RK, Conley KE. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol. 2003;553(2):589–99.
Vohwinkel CU, Lecuona E, Sun H, Sommer N, Vadász I, Chandel NS, Sznajder JI. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J Biol Chem. 2011;286(43):37067–76.
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
ZG, YW designed the study; ZG, YW, CX, GH, SG, YL performed the study; ZG, YW, YL analyzed the data; YL drafted the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the 105 Hospital of Chinese People’s Liberation Army.
Consent for publication
Informed consent was obtained from each subject.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, Z., Wang, Y., Xie, C. et al. Effects of respiratory rate on venous-to-arterial CO2 tension difference in septic shock patients undergoing volume mechanical ventilation. Eur J Med Res 25, 6 (2020). https://doi.org/10.1186/s40001-020-00402-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-020-00402-9