Abstract
Background
Acute distress respiratory syndrome (ARDS) patients with veno-venous extra corporeal membrane oxygenation (ECMO) support are particularly exposed to ECMO-associated infection (ECMO-AI). Unfortunately, data regarding AI prophylaxis in this setting are lacking. Selective decontamination regimens decrease AI incidence, including ventilator-associated pneumonia (VAP) and bloodstream infection (BSI) in critically ill patients. We hypothesized that a multiple-site decontamination (MSD) regimen is associated with a reduction in the incidence of AI among VV-ECMO patients.
Methods
We conducted a retrospective observational study in three French ECMO referral centers from January 2010 to December 2021. All adult patients (> 18 years old) who received VV-ECMO support for ARDS were eligible. In addition to standard care (SC), 2 ICUs used MSD, which consists of the administration of topical antibiotics four times daily in the oropharynx and the gastric tube, once daily chlorhexidine body-wash and a 5-day nasal mupirocin course. AIs were compared between the 2 ICUs using MSD (MSD group) and the last ICU using SC.
Results
They were 241 patients available for the study. Sixty-nine were admitted in an ICU that applied MSD while the 172 others received standard care and constituted the SC group.
There were 19 ECMO-AIs (12 VAP, 7 BSI) in the MSD group (1162 ECMO-days) compared to 143 AIs (104 VAP, 39 BSI) in the SC group (2376 ECMO-days), (p < 0.05 for all infection site). In a Poisson regression model, MSD was independently associated with a lower incidence of ECMO-AI (IRR = 0.42, 95% CI [0.23–0.60] p < 0.001). There were 30 multidrug resistant microorganisms (MDRO) acquisition in the SC group as compared with two in the MSD group (IRR = 0.13, 95% CI [0.03–0.56] p = 0.001). Mortality in ICU was similar in both groups (43% in the SC group vs 45% in the MSD group p = 0.90). Results were similar after propensity-score matching.
Conclusion
In this cohort of patients from different hospitals, MSD appeared to be safe in ECMO patients and may be associated with improved outcomes including lower ECMO-AI and MDRO acquisition incidences. Since residual confounders may persist, these promising results deserve confirmation by randomized controlled trials.
Similar content being viewed by others
Background
For the more severe acute distress respiratory syndrome (ARDS) patients, veno-venous extra corporeal membrane oxygenation (VV-ECMO) support is a life saving therapy [1]. Unfortunately, because of associated conditions and ECMO-induced immune dysfunction, up to 55% of patients will subsequently develop an acquired infection during ECMO support (ECMO-AI) [2,3,4,5,6] with implication for mortality [2, 7].
Selective decontamination regimens are infection prevention measures that decreases AI, including ventilator-associated pneumonia (VAP) and bloodstream infection (BSI) and may decrease mortality in specific population [8,9,10,11]. Recently, multiple-site decontamination (MSD) which consists of a combination of topical antibiotic in the oropharyngeal and digestive tract with chlorhexidine body-wash and intra-nasal mupirocin have shown favorable results on VAP, BSI and mortality in ARDS patients [10,11,12]. However, data regarding its effect on the more severe cases with VV-ECMO support are lacking. We hypothesized that MSD was associated with a reduction in the incidence of ECMO-AI among VV-ECMO patients.
Methods
Setting and patients
We conducted a retrospective observational study in three French ECMO referral centers in the medical Intensive Care unit (ICU) of Rennes University Hospital, the medical ICU of La Pitié Salpêtrière in Paris and the polyvalent ICU of Saint-Brieuc. Patients were screened from each hospital from respective ECMO cohorts and all adult patients (> 18 year old) who received VV-ECMO support for ARDS were eligible. In La Pitié Salpétrière, the cohort was constituted from 1st January 2017 until 31st December 2019, whereas in the other ICUs, patients were included from 1st January 2010 to 31st December 2021. Those who received VA-ECMO only and those who were already included in the database during a previous VV-ECMO run were excluded. Patients with liberty deprivation, and patients younger than 18 years were excluded from the study.
Intervention
In addition to standard care (SC), MSD was applied in patients admitted in Rennes and Saint-Brieuc ICU. This strategy is part of the local protocol for the prevention of acquired infections and is systematically applied in intubated patients [12]. MSD is a variant of selective digestive decontamination, and consists of the administration of (i) topical antibiotics including an aminoglycoside (tobramycin, 300 mg per day, in Rennes or gentamicin, 543 mg per day, in Saint-Brieuc), colistin sulfate (400 mg per day) and amphotericin B (2 g per day), four times daily in the oropharynx and the gastric tube, (ii) 4% chlorhexidine body washing daily and (iii) 5-day nasal mupirocin course. MSD do not include a systematic intravenous antimicrobial agent as part of decontamination regimen. In Rennes and Saint-Brieuc ICUs, MSD was systematically applied in all patients who had an expected intubation duration of 24 h or more throughout the duration of intubation. Full details about the MSD regimen have been reported elsewhere [10,11,12]. Patients in La Pitié Salpêtrière medical ICU received standard care alone.
AI prevention measures
In all ICUs, VAP prevention measure consisted of a bundle of care that included semi-recumbent positioning (at a maximum of 30°, and depending on its feasibility and tolerance in patients having femoral cannula), specific oral care with tooth brushing and mouth washing every 6 h and subglottic secretion drainage every 4 h. Topical antibiotic, chlorhexidine body washing and mupirocin were not used in La Pitié-Salpêtrière patients. Mouth washing was performed with 0.2% chlorhexidine in La Pitié-Salpêtrière and Saint-Brieuc ICUs but not in Rennes ICU. Subglottic secretion drainage was performed in La Pitié-Salpêtrière patients every 4 h but not in Rennes and St Brieuc.
In La Pité-Salpêtrière hospital, catheter dressing (central venous and arterial lines) were performed using chlorhexidine-impregnated dressing, except for the first 24–48 h, and changed every 7 days or sooner in case of bleeding. Dressings were performed with dry sterile compresses in Rennes and St Brieuc ICUs and were changed weekly or sooner in case of bleeding. Adherence to these measures was not retrieved in the present report. There were no systematic antibioprophylaxis during cannulation in any centers.
VV-ECMO management
ECMO management in the three ICUs is reported elsewhere [13, 14]. Briefly, VV-ECMO was inserted percutaneously through right femoral (drainage) and right jugular (reinjection) veins. Ultra-sonography guided Seldinger technique was applied for vascular access while chest ultra-sonography and X-ray confirmed intra-thoracic placement. Intravenous unfractioned heparin bolus was administrated unless contra-indications and was followed with continuous administration unless bleeding or treatment-related complications. A nurse-directed sedation and analgesia protocol was routinely used in the three ICUs. Using Richmond Agitation–Sedation Scale (RASS) and Behavioral Pain Scale (BPS), nurses recorded agitation and pain levels hourly and titrated infusion to obtain prescribed targets (RASS between [–1, 0] and BPS < 4).The sedative and analgesic drug choice was at the clinician’s discretion. The weaning procedure was also similar in the three ICUs. Nurse-to-patient ratio was different between the 3 ICUs: 1:2.5 in Rennes and St Brieuc and 1:2 in La Pitié-Salpêtrière.
Definition
ECMO-AI was considered when the infection developed during VV-ECMO run and was diagnosed 48 h or more after admission and was not incubating on admission. Diagnosis was made by treating physician. BSI was defined as a positive blood culture unless for common skin contaminants requiring 2 positives blood cultures drawn on separate occasions [15]. VAP diagnosis relied on clinical signs (fever, purulent sputum, hypoxia), radiological findings (new infiltrate on chest X-ray or CT scan), and leukocytosis in a patients intubated for more than 48 h [16]. All VAP were bacteriologically confirmed. Respiratory samples for VAP diagnosis were performed either using fiberoptic broncho-alveolar lavage or endotracheal aspiration, according to local practices. Threshold for lung samples positivity were 104 cfu/mL for BAL and 105 cfu/mL for tracheal aspirate. Each center had a nosocomial infection committee for the prevention and prospective census of acquired infections and applied the recommendations of the French Society for Hospital Hygiene for the prevention and treatment of infection (available at https://sf2h.net/publications/actualisation-precautions-standard-2017). Microorganisms responsible for infection were considered as multidrug resistant according to the European Society of Clinical Microbiology and Infectious Disease definition [17]. In all participating ICUs, patients were screened for multidrug-resistant organisms (MDRO) rectal carriage at admission, weekly afterwards and at discharge on rectal swabs. As described elsewhere, patients with no prior colonization (no colonization at admission) who tested positive for MDRO on either rectal screening or on a blood or respiratory sample were considered as having MDRO acquisition [18].
Primary and secondary endpoints
The primary endpoint was the incidence of ECMO-AI, and secondary endpoints were antimicrobial agent consumption for treatment of AI, specific ECMO-associated VAP and BSI incidences, MDRO acquisition, as well as survival during ECMO support.
Antimicrobial agent consumption was calculated for each patient and corresponds to the number of day with antimicrobial treatment. Then, the number of days alive and without antimicrobial treatment during the 60 days following cannulation was also reported.
Ethical statement
Patients or closest relative were informed of the anonymous prospective collection of their data for constitution of a cohort and had the possibility not to participate in the study. In case of refusal, the data were not collected accordingly. This manuscript follows the STROBE statement for reporting cohort studies. The study protocol received approval from the ethical committee of the French Intensive Care Society (CE 21-14).
Statistical analysis
Statistical analysis was performed with the statistical software R 4.1.1. Categorical variables were expressed as percentages and continuous variables as median and interquartile range (IQR). The Chi-square test and Fisher exact test were used to compare categorical variables and the Man–Whitney U test or the Wilcoxon for continuous variables.
Risk factors for ECMO-AI and death were estimated using bivariate and multivariable logistic regression and Kaplan–Meier survival curves with log rank test were used for survival analysis. Multivariable analyses were performed with inclusion of non-redundant variables associated with event (ECMO AI or death) with a p-value < 0.2 in the bivariate analysis. Overall, 11.8% of the data were missing (172 patients had at least one missing data). For the purpose of the multivariable analysis, missing data were considered as missing at random and were handled using chained equation, using “MICE” R package to create an imputed dataset. Incidence rate were compared using a Poisson regression model. Finally, to draw unbiased marginal estimates of exposure effect, a propensity-score matched analysis was performed. Propensity score was calculated using a non-parsimonious logistic regression model including all baseline variables listed in Table 1 (i.e., age, sex, comorbidities, period of admission, ARDS etiology, MDRO colonization at admission, non-respiratory SOFA score at cannulation, PaO2/FiO2 ratio at cannulation, PaCO2 at cannulation and lactates at cannulation). Then, using the “MatchIt” package, a k-nearest neighbor algorithm was used for propensity-score matching with a 1:1 ratio. The balance between matched groups was evaluated by the analysis of the standardized mean differences after matching. A post-matching difference < 0.2 was considered as an acceptable bias reduction. All tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Population
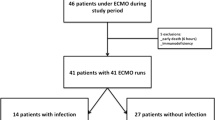
They were 301 patients with ECMO support included in the cohorts of the 3 participating ICUs. Among them, 55 received VA-ECMO only and 5 had missing data regarding ECMO-AI leaving 241 patients finally available for the study. Sixty-nine were admitted in an ICU that applied MSD and constituted the MSD group while the 172 others received standard care and constituted the SC group (Fig. 1). The mean annual MDRO prevalence rate in participating ICUs was 6.8% [6.3–7.2]) and tended to be lower in ICUs that applied MSD (6.3% [5.7–7.2]) as compared with the ICU that applied SC (10.2% [8.6–11.1], p = 0.043). The mean annual hydro-alcoholic solution consumption in participating ICUs was 106 L per 1000 patients-days [97–140], and was higher in the ICU that applied SC (180 L per 1000 patients-days [165–210]) as compared with ICUs that applied MSD (104 L per 1000 patients-days [96–108], p < 0.001).
At admission, age was 51 years [37–61], 25% of the patients were immunocompromised, SOFA score at cannulation was 12 [9–16], PaO2/FiO2 ratio on the day of cannulation was 64 mmHg [54–83] (Table 1). Baseline characteristics were not balanced between groups with a lower proportion of patients with hypertension, more patients cannulated for viral ARDS, a lower SOFA score, a higher PaO2/FiO2 ratio, a lower PaCO2 and a lower lactate level in the MSD group as compared with SC group.
Acquired infections and colonization
There were 19 ECMO-AIs (12 VAP, 7 BSI) during the 1162 ECMO-days in the MSD group as compare with 143 AIs (104 VAP, 39 BSI) in 2376 ECMO-days in the SC group, (incidence rate ratio [IRR] = 0.26, 95% CI [0.16–0.42], p < 0.001) (Table 1). Similarly, the VAP incidence rates were 10.3 per 1000 ECMO-days and 43.7 per 1000 ECMO-days, respectively (IRR = 0.23, 95% CI 0.13–0.41, p < 0.001). The BSI incidence rate was also lower in the MSD group, with incidence rates of 6.0 and 16.4 per 1000 ECMO-days, respectively (IRR = 0.36, 95% CI [0.16–0.81], p = 0.010).
In a Poisson regression model, MSD was independently associated with a lower incidence of ECMO-AI (IRR = 0.42, 95% CI [0.23–0.60] p < 0.001) (Table 2), of VAP (IRR = 0.31, 95% CI [0.17–0.55] p < 0.001) but not of BSI (IRR = 0.58, 95% CI [0.24–1.38] p = 0.216). More recent year of cannulation was associated with an increased risk of AI, whereas bacterial or other etiology of ARDS was associated with a lower rate of AI as compared with viral cause of ARDS.
There were 30 MDRO acquisition in the SC group as compared with 2 in the MSD group (IRR = 0.13, 95% CI [0.03–0.56] p = 0.001).
Microorganisms responsible for infection and antimicrobial treatment
Overall, patients in the MSD group were treated with an antimicrobial agent during 7 days [2–10] as compared with 13 days [5–30] (p < 0.001) in the SC group, corresponding to a number of day alive and without antimicrobial agent at day 60 of 42 days [8–53] and 27 [0–53], respectively (p = 0.009) (Table 1). This higher number of day alive without antimicrobial treatment was drive by more days alive without antimicrobial treatment for AI (50 days [16–60] vs 41 days [3–60] p = 0.012), whereas number of days alive without antimicrobial treatment for ARDS etiology was similar in between the two groups (46 days [7–55] vs 47 days [4–54], p = 0.289) (Additional file 1: Table S1).
Antimicrobial agents used for treatment of ARDS etiology are reported in Additional file 1: Table S1 and those used for treatment of AI are reported in Table 3. Regarding empiric therapy of AI, patients in the SC group mostly received piperacillin/tazobactam (43.1%) or carbapenems (20.7%), whereas those in the MSD group mostly received a 3rd-generation cephalosporin (38.5%) or piperacillin/tazobactam (23.1%). Regarding definitive therapy, patients in the SC group received mostly penicillin (31.0%) (14 patients treated with piperacillin, 1 with cloxacillin and 3 with amoxicillin) or an antipseudomonal cephalosporins (29.3%) while those in the DMS group received mostly a 3rd-generation cephalosporin (38.5%) or piperacillin / tazobactam (23.1%).
Details regarding type of respiratory sample performed for VAP diagnosis and microorganisms responsible for AIs and MDRO acquired colonization are reported in Table 4. There was a lower proportion of non-fermenting Gram-negative bacilli (12% vs 45%, p = 0.029) responsible for VAP in the MSD group as compared with the SC group, but the distribution of other microorganisms responsible for AI was otherwise similar. Noteworthy, none of the four Enterobacteriaceae and the two non-fermenting Gram-negative bacilli responsible for AI in the DMS group were resistant to colistin.
Antimicrobial therapy for AI differed between groups (Table 4).
Outcomes
There were no differences in other outcomes. ECMO run lasted for 7 days [4–18] and 11 days [5–24] in the SC and MSD group, respectively (p = 0.234). Twenty-six patients (38%) of the MSD group died during ECMO support as compared with 64 patients (37%) in the SC group (p = 1.00), but finally 31 (45%) and 74 (43%) patients died in ICU, in both group, respectively (p = 0.90) (Table 1 and Fig. 2).
Risk factors for death in ICU are reported in Additional file 1: Table S2. MSD was not associated with death (OR = 1.08, 95% CI [0.61–1.90] p = 0.79), even when it was forced in the multivariable model (not shown).
Propensity score matched analysis
As confirmatory analysis, patients were matched using a propensity-score. Matching process resulted in 61 patient’s pairs (Fig. 1 and Additional file 1: Table S3 for baseline characteristics of pairs). In this dataset, there were fewer ECMO-AI in the MSD group: 17 ECMO-AIs (11 VAP and 6 BSI) during 1062 ECMO-days versus 37 ECMO-AIs (28 VAP and 11 BSI) during 1063 ECMO-days in the SC group (IRR = 0.45 [0.25–0.80] p = 0.005) (p = 0.006 for VAP and p = 0.222 for BSI) (Fig. 3).
There were 2 MDRO acquisition in the MSD group as compared with 11 in the SC group (IRR = 0.18, 95% CI [0.04–0.81] p = 0.012).
Discussion
In this study, we report for the first time that MSD is independently associated with a reduced rate of ECMO-AI in patients with VV-ECMO support. This favorable result was mostly driven by a reduction of VAP and was accompanied by a reduction of MDRO acquisition, but also a lower antimicrobial agent consumption.
We confirmed a high incidence rate of ECMO-AI in VV-ECMO patients as previously reported with incidence rates from 30.1 to 50.4 per 1000 ECMO-days [2, 7]. Despite ARDS patients being particularly exposed [19,20,21], dedicated prophylaxis in this particular setting has been poorly assessed.
For decades now, selective decontamination regimens, in addition with systematic antibiotic or not, have been associated with a reduced incidence of VAP and BSI in others critically ill populations [8,9,10] in ICUs with low multidrug resistance rate. Conversely, study conducted in area with higher incidence rate reported conflicting results [22]. In the present study, participating ICUs perform epidemiological watch and have well described antimicrobial resistance rate [18, 23, 24] and MDRO colonization at admission was similar in both groups. Despite high adherence to hand hygiene, the higher rate of acquired MDRO colonization in La Pitié Salpétrière led to a higher MDRO prevalence rate. VV-ECMO and ARDS patients are at high risk of MDRO acquired infection [25] with up to 56% of AIs involving MDRO [2]. A favorable result with a decrease in the incidence of MDROs, together with a decline in the consumption of broad-spectrum antimicrobials, has been reported among patients receiving selective decontamination regimens [12, 26, 27]. In the present study either, MSD was associated with a decrease of antimicrobial agent consumption, drive by a lower consumption of antimicrobial agent for treatment of AI. However, these regimens require careful evaluation in areas with intermediate-to-high MDRO rates before definite recommendations.
As a component of MSD, the role of chlorhexidine bathing is uncertain. In areas with moderate-to-high MDRO prevalence rate, cutaneous chlorhexidine washing significantly reduces the risks of acquisition of MDROs and development of hospital-acquired bloodstream infections [28]. Conversely, in a randomized controlled trial conducted in France, chlorhexidine body-wash (combined with intra-nasal mupirocin) did not significantly reduce AI compared to placebo [10]. Interestingly, mupirocin/chlorhexidine in combination with oropharyngeal and digestive decontamination (MSD) produced a synergic preventive effect, with favorable results reported on either overall AIs, VAP, BSI, MDRO acquisition [10, 11, 26, 29]. ICU mortality was also lower with MSD than in the control group in several studies [10, 11, 29].
Attributable mortality of VAP is a matter of debate. Melsen et al. observed that the patients with moderate range severity are those with the higher VAP attributable mortality. In contrast, the outcome of those with low and high range severity, such as patients with VV-ECMO support, is minimally affected by VAP onset [30]. Conversely, an increased mortality has been previously described in patients with ECMO-AI, especially in those with VAP, but remaining confounders should be acknowledged [2, 31]. Indeed, VAP occurs in the more severe patients and especially those with persistent organ failure despite support. Finally, death may be more a consequence of treatment failure than of the VAP itself. Surprisingly, those with ECMO-AI from other infection site such as BSI did not seem to have a poorer prognosis, which contrast with the available literature in patients without ECMO support [32, 33]. Exposure of the patient’s blood to the non-epithelialized surface of tubing induces early immune changes [5]. The activation of the innate immune system increased the odds of further secondary infection and subsequent immune response [3] and may finally impact patient’s outcome. Further studies are needed in order to better understand ECMO-AI impact in critically illness.
Surprisingly, patients cannulated in the more recent years of the study were at higher risk of AI. This unexpected result can be explained not only by modifications in indication for VV-ECMO cannulation, management, but also case mix, work overload and remaining confounders. Finally, patients with bacterial cause of ARDS had a lower risk of VAP than those with a viral etiology. Indeed, viral pneumonia may induce damages to the ciliated cells, decreasing mucociliary clearance and increasing risk for bacterial colonization and proliferation in the airways.
Our study is the first to assess the benefit of MSD in VV-ECMO patients. However, some limitations should be acknowledged. First, since this was a retrospective observational design and not a randomized control trial, residual cofounders may persist despite multivariable adjustment and propensity-score matching. Second, local practices regarding ECMO and ARDS patients’ management may differ such as strategy for VAP diagnosis and treatment or annual ECMO case volume, both variables being associated with patient’s outcome [34]. Third, in La Pitié Salpétrière cannulation was mainly performed in nearby ICUs by a mobile ECMO retrieval team before admission. However, patients managed by this skilled team have a similar complications rate, including ECMO-AI, than those cannulated directly in the referral center [35]. A randomized control trial should be design to go through this issue. Fourth, the adherence to the local protocol of VAP prevention was not retrieved. Finally, our study might be underpowered to study patient important outcome such as mortality.
Conclusion
In this cohort of patients from different hospitals MSD appeared to be safe in ECMO patients and may be associated with improved outcomes including a decreased incidence of ECMO-AI. There were no differences in mortality rate in between groups, but fewer days with antimicrobial treatment and finally less MDRO acquisition among patients receiving MSD. These favorable results deserve confirmation by randomized controlled trials.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M, Dechartres A, Elbourne D. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med. 2020;46(11):2048–57. https://doi.org/10.1007/s00134-020-06248-3. (Epub 2020 Oct 6).
Grasselli G, Scaravilli V, Di Bella S, Biffi S, Bombino M, Patroniti N, Bisi L, Peri AM, Pesenti A, Gori A, Alagna L. Nosocomial infections during extracorporeal membrane oxygenation: incidence, etiology, and impact on patients’ outcome. Crit Care Med. 2017;45(10):1726–33. https://doi.org/10.1097/CCM.0000000000002652.
Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20(1):387. https://doi.org/10.1186/s13054-016-1570-4.
Al-Fares A, Pettenuzzo T, Del Sorbo L. Extracorporeal life support and systemic inflammation. Intensive Care Med Exp. 2019;7(Suppl 1):46. https://doi.org/10.1186/s40635-019-0249-y.
Frerou A, Lesouhaitier M, Gregoire M, Uhel F, Gacouin A, Reizine F, Moreau C, Loirat A, Maamar A, Nesseler N, Anselmi A, Flecher E, Verhoye JP, Le Tulzo Y, Cogné M, Roussel M, Tarte K, Tadié JM. Venoarterial extracorporeal membrane oxygenation induces early immune alterations. Crit Care. 2021;25(1):9. https://doi.org/10.1186/s13054-020-03444-x.
Nesseler N, Fadel G, Mansour A, Para M, Falcoz PE, Mongardon N, Porto A, Bertier A, Levy B, Cadoz C, Guinot PG, Fouquet O, Fellahi JL, Ouattara A, Guihaire J, Ruggieri VG, Gaudard P, Labaste F, Clavier T, Brini K, Allou N, Lacroix C, Chommeloux J, Lebreton G, Matthay MA, Provenchere S, Flécher E, Vincentelli A, ECMOSARS Investigators. Extracorporeal membrane oxygenation for respiratory failure related to COVID-19: a nationwide cohort study. Anesthesiology. 2022;136(5):732–48. https://doi.org/10.1097/ALN.0000000000004168.
Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, Scheinkestel C, Pellegrino V. Infections acquired by adults who receive extracorporeal membrane oxygenation: risk factors and outcome. Infect Control Hosp Epidemiol. 2013;34(1):24–30. https://doi.org/10.1086/668439. (Epub 2012 Nov 21).
de Smet AM, Kluytmans JA, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360(1):20–31. https://doi.org/10.1056/NEJMoa0800394.
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. 2009;2009(4):CD000022. https://doi.org/10.1002/14651858.CD000022.pub3.
Camus C, Bellissant E, Sebille V, et al. Prevention of acquired infections in intubated patients with the combination of two decontamination regimens. Crit Care Med. 2005;33(2):307–14. https://doi.org/10.1097/01.ccm.0000152224.01949.01.
Massart N, Reizine F, Fillatre P, Seguin P, La Combe B, Frerou A, Egreteau PY, Hourmant B, Kergoat P, Lorber J, Souchard J, Canet E, Rieul G, Fedun Y, Delbove A, Camus C. Multiple-site decontamination regimen decreases acquired infection incidence in mechanically ventilated COVID-19 patients. Ann Intensive Care. 2022;12(1):84. https://doi.org/10.1186/s13613-022-01057-x.
Massart N, Dupin C, Legris E, Fedun Y, Barbarot N, Legay F, Wattecamps G, Le Gall F, La Combe B, Bouju P, Frerou A, Muller L, Rieul G, Fillatre P. Multiple-site decontamination in mechanically ventilated ICU patients: a real-life study. Infect Dis Now. 2023;53(3):104666. https://doi.org/10.1016/j.idnow.2023.104666. (Epub ahead of print).
Bréchot N, Luyt CE, Schmidt M, Leprince P, Trouillet JL, Léger P, Pavie A, Chastre J, Combes A. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med. 2013;41(7):1616–26. https://doi.org/10.1097/CCM.0b013e31828a2370.
Flecher E, Anselmi A, Corbineau H, Langanay T, Verhoye JP, Felix C, Leurent G, le Tulzo Y, Malledant Y, Leguerrier A. Current aspects of extracorporeal membrane oxygenation in a tertiary referral centre: determinants of survival at follow-up. Eur J Cardiothorac Surg. 2014;46:665–71.
Tabah A, Koulenti D, Laupland K, Misset B, Valles J. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38(12):1930–45.
Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society [published correction appears in Clin Infect Dis. 2017;64(9):1298][published correction]
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
Massart N, Camus C, Benezit F, Moriconi M, Fillatre P, Le Tulzo Y. Incidence and risk factors for acquired colonization and infection due to extended-spectrum beta-lactamase-producing Gram-negative bacilli: a retrospective analysis in three ICUs with low multidrug resistance rate. Eur J Clin Microbiol Infect Dis. 2020;39(5):889–95. https://doi.org/10.1007/s10096-019-03800-y.
Forel JM, Voillet F, Pulina D, Gacouin A, Perrin G, Barrau K, Jaber S, Arnal JM, Fathallah M, Auquier P, Roch A, Azoulay E, Papazian L. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit Care. 2012;16(2):R65. https://doi.org/10.1186/cc11312.
Markowicz P, Wolff M, Djedaini K, Cohen Y, Chastre J, Delclaux C, Merrer J, Herman B, Veber B, Fontaine A, Dreyfuss D. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome. Incidence, prognosis, and risk factors. ARDS Study Group. Am J Respir Crit Care Med. 2000;16:1942–8.
Luyt CE, Bouadma L, Morris AC, et al. Pulmonary infections complicating ARDS. Intensive Care Med. 2020;46(12):2168–83. https://doi.org/10.1007/s00134-020-06292-z.
Wittekamp BH, Plantinga NL, Cooper BS, et al. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA. 2018;320(20):2087–98. https://doi.org/10.1001/jama.2018.13765SanchezRamirezCC2018.
Luyt CE, Hékimian G, Bonnet I, et al. Usefulness of point-of-care multiplex PCR to rapidly identify pathogens responsible for ventilator-associated pneumonia and their resistance to antibiotics: an observational study. Crit Care. 2020;24(1):378. https://doi.org/10.1186/s13054-020-03102-2.
Luyt CE, Sahnoun T, Gautier M, et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann Intensive Care. 2020;10(1):158. https://doi.org/10.1186/s13613-020-00775-4.
Depuydt P, Benoit D, Vogelaers D, et al. Systematic surveillance cultures as a tool to predict involvement of multidrug antibiotic resistant bacteria in ventilator-associated pneumonia. Intensive Care Med. 2008;34(4):675–82. https://doi.org/10.1007/s00134-007-0953-z.
Camus C, Sauvadet E, Tavenard A, Piau C, Uhel F, Bouju P, Letheulle J, Dollo G, Gacouin A, Lavoué S, Le Tulzo Y. Decline of multidrug-resistant Gram negative infections with the routine use of a multiple decontamination regimen in ICU. J Infect. 2016;73(3):200–9. https://doi.org/10.1016/j.jinf.2016.06.007. (Epub 2016 Jun 30).
de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362(9389):1011–6. https://doi.org/10.1016/S0140-6736(03)14409-1.
Climo MW, Yokoe DS, Warren DK, Perl TM, Bolon M, Herwaldt LA, Weinstein RA, Sepkowitz KA, Jernigan JA, Sanogo K, Wong ES. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–42. https://doi.org/10.1056/NEJMoa1113849. (Erratum In: N Engl J Med. 2013 Jun13;368(24):2341).
Massart N, Fillatre P, Wattecamps G, Camus C. Multiple-site decontamination regimen without systemic antibiotics associated with reduced mortality in intubated patients. Infect Dis (Lond). 2020;52(7):513–6. https://doi.org/10.1080/23744235.2020.1754457. (Epub 2020 Apr 20).
Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–71. https://doi.org/10.1016/S1473-3099(13)70081-1.
Schmidt M, Bréchot N, Hariri S, Guiguet M, Luyt CE, Makri R, Leprince P, Trouillet JL, Pavie A, Chastre J, Combes A. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55(12):1633–41. https://doi.org/10.1093/cid/cis783. (Epub 2012 Sep 18).
Prowle JR, Echeverri JE, Ligabo EV, Sherry N, Taori GC, Crozier TM, Hart GK, Korman TM, Mayall BC, Johnson PD, Bellomo R. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care. 2011;15(2):R100. https://doi.org/10.1186/cc10114. (Epub 2011 Mar 21).
Massart N, Wattecamps G, Moriconi M, Fillatre P. Attributable mortality of ICU acquired bloodstream infections: a propensity-score matched analysis. Eur J Clin Microbiol Infect Dis. 2021;40(8):1673–80. https://doi.org/10.1007/s10096-021-04215-4. (Epub 2021 Mar 10).
Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, Annich GM. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191(8):894–901. https://doi.org/10.1164/rccm.201409-1634OC.
Bréchot N, Mastroianni C, Schmidt M, Santi F, Lebreton G, Hoareau AM, Luyt CE, Chommeloux J, Rigolet M, Lebbah S, Hekimian G, Leprince P, Combes A. Retrieval of severe acute respiratory failure patients on extracorporeal membrane oxygenation: any impact on their outcomes? J Thorac Cardiovasc Surg. 2018;155(4):1621–9. https://doi.org/10.1016/j.jtcvs.2017.10.084. (Epub 2017 Nov 8).
Acknowledgements
None.
Funding
No external funding was received.
Author information
Authors and Affiliations
Contributions
All authors participated to acquisition of data. NM, CEL, LL, NN and PF participated to the conception and design of the study and NM performed the analysis and interpretation of data.NM, CEL, PF, CC and NN drafted the article. All authors finally approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patients or closest relative were informed of the anonymous prospective collection of their data for constitution of a cohort and had the possibility not to participate in the study. In case of refusal, the data were not collected accordingly. The study protocol received approval from the ethical committee of the French Intensive Care Society (CE 21-14).
Consent for publication
Patients or closest relative were informed of the anonymous prospective collection of the data and had the possibility not to participate in the study. In case of refusal, the data were not collected accordingly.
Competing interests
CEL received lecture fees from Merck and AdvanzPharma outside the scope of the present work. The other authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Data regarding antimicrobial agent in each group. Table S2. Risk factors for death in ICU (logistic regression). Table S3. Baseline characteristics and outcomes of matched pairs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Massart, N., Camus, C., Nesseler, N. et al. Multiple-site decontamination to prevent acquired infection in patients with veno-venous ECMO support. Ann. Intensive Care 13, 27 (2023). https://doi.org/10.1186/s13613-023-01120-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-023-01120-1