Abstract
Objective
To assess prediction of multidrug resistant (MDR) pathogens in ventilator-associated pneumonia (VAP) by systematic surveillance cultures (SC) and to assess the contribution of SC to initial antibiotic therapy.
Design
Prospective cohort study of patients with microbiologically confirmed VAP. Comparison of actual early antibiotic coverage with three hypothetical empirical schemes.
Setting
A 50-bed university hospital ICU. SC consisted of oral, nasal, urinary and rectal samples upon admission, 3-weekly urinary and 1-weekly oral, nasal, and rectal samples in all patients, 3-weekly tracheal aspirates in intubated patients.
Results
MDR pathogens were found in 86 of 199 VAP episodes. Sensitivity of SC to predict MDR pathogens was 69% (tracheal SC) and 82% (all SC); specificity was 96% (tracheal) and 91% (all), respectively. Appropriate antibiotic coverage within 24 h and 48 h following MDR VAP was 77% and 89%, respectively. A carbapenem-based empirical scheme would have been equally appropriate (83% vs. 77% at 24 h; 83% vs. 89% at 48 h), but a β-lactam-fluoroquinolone empirical therapy would have been less (59% vs. 77% at 24 h; 59% vs. 89% at 48 h) as would have been β-lactam-aminoglycoside therapy (68% vs. 77% at 24 h; 68% vs. 89% at 48 h). Empirical comparators would have resulted in significantly more prescription of broad-spectrum antibiotics within the first 48 h.
Conclusions
With MDR pathogens highly prevalent, systematic SC predicted MDR pathogens causing VAP in 69% to 82% and may have contributed to high rates of early appropriate antibiotic therapy with limited use of broad-spectrum antimicrobials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Timely administration of appropriate antimicrobials improves survival in patients with ventilator-associated pneumonia (VAP) [1–4]. VAP caused by multidrug antibiotic resistant (MDR) pathogens carries an increased risk for inappropriate empirical therapy and consequently for adverse outcome [5–9]. To maintain a high rate of initially appropriate antibiotic therapy in these infections and to limit empirical use of broad-spectrum antimicrobials where possible, clinical predictors for MDR VAP have been used for risk stratification [4, 10–12]. Microbiological results available 48–72 h following sampling may subsequently permit narrowing the spectrum of the antimicrobial therapy (“deescalation”) in order to limit selection of resistant strains [13–17]. Alternative to this clinically stratified empirical prescription combined with subsequent, microbiologically guided deescalation, surveillance cultures (SC) may provide microbiological guidance to initial antibiotic prescription [18, 19]. Previously we found that regular SC predicted 70% of bacteremic MDR microbial isolates in late-onset pneumonia at our ICU [18]. In addition, prediction of microbial cause by SC was associated with more frequently appropriate antimicrobials within 24 h of bacteremia and with better survival [20]. However, the added value of guiding initial antibiotics by SC as compared to a purely empirical broad-spectrum therapy as recommended by the updated guidelines of the American Thoracic Society was questioned [21]. In this study we assessed prospectively the prediction of MDR microbial cause of VAP by systematic SC. Additionally, we compared actual, SC-guided, initial antibiotic prescription with a hypothetical, empirical prescription based upon clinical risk stratification for MDR VAP.
Materials and methods
Clinical setting and design
We conducted a prospective observational cohort study between 1 April 2004 and 30 November 2006 in the 50-bed medical and surgical ICU of the 1,056-bed Ghent University Hospital. Patients aged over16years and intubated at least 48 h were daily evaluated for presence of pneumonia, and all consecutive microbiologically confirmed VAP episodes were retained for analysis. During the study period 267 VAP episodes (in 220 patients) were suspected and 199 episodes (in 160 patients) microbiologically confirmed (26 by BAL, 173 by endotracheal aspirate) and included for study. The median age of patients was 60 years (range 50–72), and 75% were men. Their mean Acute Physiology and Chronic Health Evaluation II score upon admission was 20.3 ± 8.8; 60 patients were admitted for a primary medical reason, 79 for unscheduled and 21 for scheduled surgery. ICU mortality and in-hospital mortality were 27.7% and 37.1%, respectively. The study was approved by the Ethics Committee of Ghent University Hospital.
In a first part of the study we determined sensitivity and specificity of systematic SC to predict MDR pathogens causing VAP. Therefore we considered only VAP episodes with at least one prior SC. Sensitivity was calculated by division of the number of predicted causative MDR pathogens (true predicted MDR) by the number of all causative MDR pathogens. Specificity was calculated by division of the number of MDR pathogens in SC not causing VAP (false predicted MDR) by the total number of causative pathogens subtracting this ratio from 1. In the second part of the study antibiotic therapy within the first 48 h following diagnosis of VAP was assessed in terms of rate of appropriate coverage and consumption as expressed as number of daily defined doses (DDD) per antibiotic class. To determine the added value of SC-guided early antibiotic therapy we compared actual antibiotic prescription with three hypothetical empirical schemes (comparators).
Microbiology
The following pathogens were considered as MDR: methicillin-resistant Staphylococcus aureus (MRSA), extended spectrum β-lactamase (ESBL) producing Enterobacteriaceae, MDR nonfermenting organisms such as Acinetobacter baumannii and Stenotrophomonas maltophilia, and Pseudomonas aeruginosa resistant for at least one of the following antipseudomonal antibiotics: ceftazidime, piperacillin, and imipenem [18, 20, 22]. During the study period no MDR outbreaks were identified at our ICU, and overall antibiotic susceptibility was constant between 2003 and 2006. In 2006 overall susceptibility of Gram-negative rods other than P. aeruginosa was 75%, 76%, and 95% for ceftazidime, piperacillin-tazobactam, and meropenem, respectively, 78% for fluoroquinolones and 93% for aminoglycosides. Susceptibility of P. aeruginosa was 78%, 79%, and 75% for ceftazidime, piperacillin-tazobactam, and meropenem, respectively, 73% for fluoroquinolones (ciprofloxacin) and 85% for aminoglycosides (amikacin). Susceptibility of S. aureus for methicillin was 72%.
Systematic SC consisted of oral, nasal, and rectal swabs and urinary cultures upon admission, followed by thrice weekly urinary (Monday, Wednesday, Friday) and once weekly oral, nasal, and rectal samples (Monday) in all patients, as well as thrice weekly tracheal aspirates (Monday, Wednesday, Friday) in intubated patients. SC were processed as described previously [18, 20]. All tracheal aspirates were processed semiquantitatively as a routine and quantitatively upon request. Semiquantitative scoring was derived from streaking and diluting the specimen in three segments, scored as few (+/-) for less than ten colonies, light (+), moderate (++), and heavy (+++) growth when moderate to heavy growth was observed in first, second, and third streaks, respectively. Simultaneous semiquantitative and quantitative analysis of 114 samples showed good concordance of ++ and +++ growth with more than 105 CFU/ml (100%), and of ± or no growth with less than 104 CFU/ml (93%). To evaluate the presence of MDR pathogens for surveillance purposes all SC (including tracheal cultures) were judged qualitatively.
VAP definitions
VAP was considered clinically likely if a new or progressive and persistent chest radiography infiltrate was present together with a clinical pulmonary infection score of at least six [23–26]. In our ICU bronchoalveolar lavage (BAL) is not performed systematically but is limited to patients with suspected VAP and (a) negative endotracheal aspirates, (b) infiltrates limited to upper lobes or the left lung, or (c) acute respiratory distress syndrome. BAL is performed under bronchoscopic guidance and is directed at the area with predominant infiltrates and/or the segmentar bronchi with purulent secretions.
In agreement with national guidelines [27], microbiological confirmation of clinically likely VAP required a pathogen to show ++ or +++ semiquantitative or more than 105 CFU/ml quantitative growth (> 104 CFU/ml on BAL fluid) in a good quality endotracheal aspirate (with identification of more than 25 polymorphonuclear and fewer than 10 epithelial cells per low-resolution field). Additionally, clinically likely VAP with + growth on endotracheal aspirate was considered as microbiologically confirmed if Gram-staining showed predominant presence of a pathogen and antibiotic therapy had been started or changed within 72 h. VAP was considered polymicrobial if more than one pathogen grew above these thresholds. Early-onset and late-onset VAP were defined as VAP diagnosed 5 or days or fewer as opposed to more than 5 days following intubation. Antimicrobial therapy was considered appropriate if it included at least one antimicrobial drug with in vitro activity against the causative pathogen.
Surveillance-guided antibiotic therapy and empirical comparators
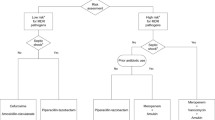
In the absence of SC and in patients with negative SC the MDR risk stratification as suggested by Trouillet et al. [10] was used to guide early antibiotic prescription. If SC identified MDR pathogens, initial antibiotic therapy was SC guided. Antibiotic prescription strategy is detailed in Fig. 1. Three empirical schemes were used as hypothetical comparators: a carbapenem-based scheme and two β-lactam-based schemes (a β-lactam-fluoroquinolone and a β-lactam-aminoglycoside scheme). The carbapenem scheme reflected the recommendations of the updated American Thoracic Society (ATS) guidelines [4] and was defined as a limited spectrum β-lactam antibiotic for early-onset VAP with no prior antibiotic exposure, and of a combination of a carbapenem plus a fluoroquinolone plus a glycopeptide for VAP with risk factors for MDR pathogens. The β-lactam-fluoroquinolone and β-lactam-aminoglycoside schemes were based upon the Trouillet et al. [10] risk classification and were defined as prescription of a non-antipseudomonal β-lactam antibiotic in early-onset VAP with no prior antibiotic exposure, an antipseudomonal β-lactam antibiotic in VAP with one risk factor for MDR (late-onset VAP and prior antibiotic exposure) and a glycopeptide added to double antipseudomonal coverage in VAP with both risk factors for MDR; double antipseudomonal coverage consisted of an antipseudomonal β-lactam plus a fluoroquinolone in the β-lactam-fluoroquinolone scheme and of an antipseudomonal β-lactam plus an aminoglycoside in the β-lactam-aminoglycoside scheme.
Surveillance-guided prescription and empirical prescription (in the absence of surveillance cultures (SC) or with negative SC). 1If previous exposure to antipseudomonal β-lactam; 2if Gram-positive cocci on Gram-staining and (other) MRSA-colonized patient at the ICU unit; 3if documented susceptibility on SC; 4in the absence of septic shock; 5if P. aeruginosa resistant to both β-lactam and carbapenem
Statistics
Continuous variables are described as mean (± standard deviation) or median (interquartile range) for normal or nonnormal distribution, respectively. Categorical variables are described as absolute numbers (and percentages). For comparative tests on continuous variables the Mann–Whitney U test and t test were used as appropriate, depending on variable distribution. For categorical variables Pearson's χ2 test or Fisher's exact test were used as appropriate. Statistical analyses are executed with SPSS 11.0 (SPSS, Chicago, Ill., USA). All used tests were two-tailed and statistical significance was defined as p < 0.05.
Results
Microbiology of VAP and prediction by SC
VAP was monomicrobial in 170 and polymicrobial in 29. Gram staining of endotracheal aspirate showed predominant growth of a pathogen in 76%. In 86 episodes (43%) at least one MDR pathogen was identified (Table 1). In total 93 of 230 identified microbial pathogens were MDR: 13 MRSA, 45 ESBL-producing Enterobacteriaceae, 24 MDR P. aeruginosa and 11 MDR nonfermenters other than P. aeruginosa. Availability of SC and prediction of MDR by SC in the different clinical risk categories for MDR infection are detailed in Table 2. Overall sensitivity of MDR prediction by SC was 69% for tracheal SC and 82% for all SC. Specificity of MDR prediction by SC was 96% (tracheal SC) and 91% (all SC). Assuming a prevalence of 43% MDR VAP, positive predictive values of tracheal and all SC to the type of MDR pathogen in VAP were 90% and 87%, respectively, and negative predictive values of tracheal and all SC were 80% and 87%, respectively.
Early antibiotic therapy
Within 24 h and 48 h of VAP diagnosis antimicrobial therapy was appropriate in 86% and 93% in the whole cohort, respectively, and in 77% and 89% in patients with MDR VAP. Combination antibiotic therapy was prescribed in 49 patients (25%). Appropriate antimicrobial coverage by the empirical comparators is shown in Table 3. In MDR VAP appropriate coverage by the carbapenem scheme would not have differed from actual coverage (83% vs. 77% at 24 h, p = 0.4; 83% vs. 89% at 48 h, p = 0.27), but appropriate coverage of the β-lactam-fluoroquinolone scheme would have been significantly less at both time points (59% vs. 77% at 24 h, p = 0.01; 59% vs. 89% at 48 h, p < 0.001). In contrast, appropriate coverage by the β-lactam-aminoglycoside scheme would only have been less than actual coverage at 48 h (68% vs. 77% at 24 h, p = 0.14; 68% vs. 89% at 48 h, p = 0.001). Total antibiotic prescription for 48 h following diagnosis of VAP was lower than that of empirical comparators (Table 3).
Discussion
In our prospective study in patients with VAP sensitivity of systematic SC to predict MDR causative pathogens (69–82%) was similar to that reported in our retrospective study in bacteremic VAP [18] but with a much higher specificity (81–96%). In a setting of high prevalence of MDR pathogens this resulted in a high positive predictive value.
Our study is to be compared with four others assessing the potential of SC in predicting microbial cause of VAP. In a prospective study of VAP following cardiac surgery Bouza et al. [26] found SC to predict only one single episode. However, only 28 episodes (of which only 8 or 9 late-onset) of VAP were analyzed, no information regarding MDR occurrence was provided, and SC were taken only once weekly in patients ventilated longer than 1 week. A second study by Hayon et al. [27] reported that 42% of 165 episodes of microbiologically confirmed VAP were predicted by prior cultures (including both SC and diagnostic cultures). Prediction increased with longer duration of mechanical ventilation (49% vs. 18% prediction of at least 15 days vs. shorter than 15 days), which the authors attributed to the higher number of available cultures preceding VAP. Similarly, prediction was significantly better if less then 72 h had elapsed between the last tracheal culture preceding VAP and the bronchoscopy performed at clinical suspicion of VAP (52% of 33 episodes vs. 28% of 69 episodes, p < 0.03). Systematic nasal, rectal, and urinary SC taken once weekly represented only 13% of all available cultures; no tracheal aspirates were obtained at predetermined times. In contrast to these two studies Michel et al. [19] reported 83% prediction of VAP microbial cause by routine endotracheal SC twice weekly until extubation. A fourth study, published in abstract form, found 80% prediction of MDR pathogens in VAP by tracheal SC obtained twice weekly [28]. As these “positive” surveillance studies share a higher SC sampling frequency than the “negative” studies, a sampling frequency of at least twice a week might be essential to obtain a good sensitivity.
Apart from evaluating a surveillance-guided antibiotic strategy, our study is the first report assessing the coverage rate of the updated 2005 ATS guidelines for nosocomial pneumonia [4] in an ICU setting with high prevalence of MDR. A carbapenem-fluoroquinolone-glycopeptide combination scheme would have allowed 81% early appropriate therapy in MDR VAP. Strictly applied, this scheme would have required triple combination antibiotic prescription for at least 48 h in the majority (± 86%) of VAP episodes (including MDR and non-MDR episodes). It could be argued to use results of Gram staining to limit the use of combination therapy [29]. However, relying on Gram staining of endotracheal aspirate potentially could significantly have increased erroneous therapeutic decisions in our cohort, as Gram staining was negative in 24% of microbiologically confirmed VAP and in addition revealed only one type of pathogen in 11 episodes of polymicrobial VAP caused by Gram-positive and Gram-negative pathogens. Michel et al. [19] have reported significantly better coverage rates of their surveillance based antibiotic prescription than empirical schemes based upon the ATS guidelines of 1996. It should be noted, however, that although the 2005 update of the ATS guideline was not available at the time of publication of the Michel et al. findings, the 1996 empirical scheme may not have been an adequate comparator as late as in 2004. Moreover, in their study the empirical schemes (ATS-based or “Trouillet-based”) were not detailed in terms of β-lactam or carbapenem preference or use of combination therapy with, for example, a fluoroquinolone or glycopeptide.
The different coverage rates of our three hypothetical comparators stress the importance of tailoring empirical schemes such as provided in general guidelines to the local microbial flora to obtain acceptable rates of appropriate coverage [30]. Two other studies may be mentioned here for comparison with ours. Ionas et al. [31] found 79% and 80% appropriate empirical therapy based upon the Trouillet and the 1996 ATS recommendations, respectively, in a cohort of 71 ICU patients. A 2003 study assessing the potential adequacy of selected empirical antimicrobial combinations found rates of appropriate therapy all below 70% in patients with hospital-acquired pneumonia either of early onset with specific risk factors or of late onset and prior antimicrobial therapy [32].
Some important limitations of our study should be mentioned. Firstly, although overall SC guided, part of our antibiotic prescription was empirical and was left at the discretion of the attending physician in case of no (19%) or negative SC. As our SC predicted only 69–82% of MDR cause, a strictly surveillance-based prescription may have resulted in a lower rate of appropriate therapy than actually observed as a negative SC is not an absolute argument in favor of limited spectrum empirical antibiotic therapy in the presence of risk factors for MDR. Secondly, the design of the present study does not allow testing whether outcome was positively affected by the use of SC. A randomized controlled trial comparing an SC guided antibiotic strategy to a purely empirical (e.g., deescalation) strategy is necessary to address the issue of outcome and should be preferably multicentric as resistance patterns both are highly variable between ICUs and directive of appropriate empirical schemes.
Cost resulting from the high microbiological workload is probably the most important barrier to a more widespread use of systematic SC [18] although some gains could result from limiting empirical antibiotic prescription. To contain this workload SC may be restricted to patient categories where the risk for MDR infection is highest, such as patients admitted from the ward after 48 h of hospital admission, patients referred from other ICUs, and patients with prolonged (> 48–72 h) mechanical ventilation. Furthermore, for the purpose of guiding antibiotics in VAP it could be argued to concentrate efforts on frequent surveillance of the airways only [19, 28]. A decision whether to try systematic SC as a strategy to improve empirical prescription should finally depend on local availability of microbiological laboratory resources, endemicity and susceptibility patterns of MDR pathogens, and urgency to curtail the use of certain classes of broad-spectrum antibiotics [33–35]. Tracheal surveillance in patients ventilated for at least 72 h is probably the more cost-effective part of a systematic SC program and could be assessed in a randomized trial.
References
Luna C, Vujachich P, Niederman MS, Vay C, Gherardi C, Matera J, Jolly EC (1997) Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 160:608–613
Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH (2002) Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 122:262–268
Kollef MH, Sherman G, Ward S, Fraser VJ (1999) Inadequate antimicrobial treatment of infections. A risk factor for hospital mortality among critically ill patients. Chest 115:462–474
American Thoracic Society (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and health-care associated pneumonia. Am J Respir Crit Care Med 171:388–416
Rello J, Ausina V, Ricart M, Castella J, Prats G (1993) Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest 104:1230–1235
Kollef MH (2000) Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis 31:S131–138
Cosgrove SE, Kaye KS, Eliopoulous GM, Carmeli Y (2002) Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch Intern Med 162:185–190
Alvarez-Lerma F, Pellus AM, Sanchez BA, Ortiz EP, Jorda R, Barcenilla F, Maravi E, Galvan B, Palomar M, Serra J, Bermejo B, Mateu A, Quintana E, Palacios MS, Giral R, Gonzalez V, Lerma FA, Mesa JL, Melgarejo JA, Martinez J, Insausti J, Olaechea P, Chanovas M, Gilabert A, Junquera C, Valles J, Palacios F, Calvo R, Mesalles E, Nava J, Santos A, Armengol S, Marzo D (1996) Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive Care Med 22:387–394
Combes A, Luyt CE, Trouillet JL (2006) Impact of antibiotic-resistant bacteria on the outcome of ventilator-associated pneumonia. Semin Respir Crit Care Med 27:23–28
Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, Gibert C (1998) Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med 157:531–539
Kollef MH, Fraser VJ (2001) Antibiotic resistance in the intensive care unit. Ann Intern Med 134:298–314
Wunderink RG (1993) Mortality and ventilator-associated pneumonia. The best antibiotics may be the least antibiotics. Chest 104:993–995
Kollef MH, Ward S (1998) The influence of mini-BAL cultures on patient outcomes—Implications for the antibiotic management of ventilator-associated pneumonia.Chest 113:412–420
Hoffken G, Niederman MS (2002) Nosocomial pneumonia—the importance of a de-escalating strategy for antibiotic treatment of pneumonia in the ICU. Chest 122:2183–2196
Rello J, Vidaur L, Sandiumenge A, Rodriguez A, Gualis B, Boque C, Diaz E (20024) De-escalation therapy in ventilator-associated pneumonia. Crit Care Med 32:2183–2190
Kollef MH, Kollef KE (2005) Antibiotic utilization and outcomes for patients with clinically suspected ventilator-associated pneumonia and negative quantitative BAL results. Chest 128:2706–2713
Kollef MH (2006) Diagnosis of ventilator-associated pneumonia. N Engl J Med 355:2691–2693
Depuydt P, Blot S, Benoit D, Claeys G, Verschraegen G, Vandewoude K, Vogelaers D, Decruyenaere J, Colardyn F (2005) Antimicrobial resistance in nosocomial bloodstream infection associated with pneumonia and the value of systematic surveillance cultures in an adult intensive care unit. Crit Care Med 34:653–659
Michel F, Franceschini B, Berger P, Arnal JM, Gainnier M, Sainty JM, Papazian L (2005) Early antibiotic treatment for BAL-confirmed ventilator-associated pneumonia: a role for routine endotracheal aspirate cultures. Chest 127:589–597
Depuydt P, Benoit D, Vogelaers D, Claeys G, Verschraegen G, Vandewoude K, Decruyenaere J, Blot S (2006) Outcome in bacteremia associated with nosocomial pneumonia and the impact of pathogen prediction by tracheal surveillance cultures. Intensive Care Med 11:1773–1781
Kollef MH (2005) Providing appropriate therapy in the intensive care unit: surveillance vs. de-escalation. Crit Care Med 34:903–905
Carmeli Y, Troillet N, Karchmer AW, Samore MH (1999) Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch Intern Med 159:1127–1232
Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM (1991) Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 143:1121–1129
Calandra T, Cohen J (2005) The International Sepsis Forum Consensus Conference on Definitions of Infection in the Intensive Care Unit. Crit Care Med 33:1538–1548
Infectious Disease Advisory Board (2002) Diagnose en antibacteriële behandeling van ventilator geassocieerde pneumonie (VAP) bij volwassenen. IDAB Symposium, 14 Sept Brussels. Belgium. MAPU
Bouza E, Perez A, Munoz P, Perez MJ, Rincon C, Sanchez C, Martin-Rabadan P, Riesgo M (2003) Ventilator-associated pneumonia after heart surgery: a prospective analysis and the value of surveillance. Crit Care Med 31:1964–1970
Hayon J, Figliolini C, Combes A, Trouillet JL, Kassis N, Dombret MC, Gibert C, Chastre J (2002) Role of serial routine microbiologic culture results in the initial management of ventilator-associated pneumonia. Am J Respir Crit Care 165:41–46
Bagnulo H, Godino M, Galiana A, Bertulo M, Pedreira W (2007) Are routine endotracheal aspirates predictive of the etiology of ventilator-associated pneumonia? Crit Care 11 (in press)
Blot F, Raynard B, Chachaty E, Tancrede C, Antoun S, Nitenberg G (2000) Value of gram stain examination of lower respiratory tract secretions for early diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med 162:1731–1737
Rello J, Sa-Borges M, Correa H, Leal SR, Baraibar J (1999) Variations in etiology of ventilator-associated pneumonia across four treatment sites. Am J Respir Crit Care Med 160:608–613
Ioanas M, Cavalcanti M, Ferrer M, Valencia M, Agusti C, de la Bellacasa JP, Torres A (2003) Hospital-acquired pneumonia: coverage and treatment adequacy of current guidelines. Eur Respir J 22:876–882
Leroy O, Giradie P, Yazdanpanah Y, Georges H, Alfandari S, Sanders V, Devos P, Beaucaire G (2002) Hospital-acquired pneumonia: microbiological data and potential adequacy of antimicrobial regimens. Eur Respir J 20:432–439
Lee SO, Kim NJ, Choi SH, Kim TH, Chung JW, Woo JH, Ryu J, Kim YS (2004) Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: a case-control study. Antimicrob Agents Chemother 48:224–228
Nouer SA, Nucci M, de-Oliveira MP, Pellegrino P, Moreira B (2005) Risk factors for acquisition of multidrug-resistant Pseudomonas aeruginosa producing SPM metallo-beta-lactamase. Antimicrob Agents Chemother 49:3663–3667
Falagas ME, Kopterides P (2006) Risk factors for the isolation of multi-drug-resistant Acinetobacter baumanii and Pseudomonas aeruginosa: a systematic review of the literature. J Hosp Infect 64:7–15
Acknowledgements
P. Depuydt was supported by a clinical doctoral grant Fund for Scientific Research Flanders (1.7.201.07.N.00).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Depuydt, P., Benoit, D., Vogelaers, D. et al. Systematic surveillance cultures as a toolto predict involvement of multidrug antibiotic resistant bacteria in ventilator-associated pneumonia. Intensive Care Med 34, 675–682 (2008). https://doi.org/10.1007/s00134-007-0953-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0953-z