Abstract
Background
Among strategies that aimed to prevent acquired infections (AIs), selective decontamination regimens have been poorly studied in the COVID-19 setting. We assessed the impact of a multiple-site decontamination (MSD) regimen on the incidence of bloodstream infections (BSI) and ventilator-associated pneumonia (VAP) in COVID-19 patients receiving mechanical ventilation.
Methods
We performed an ancillary analysis of a multicenter retrospective observational study in 15 ICUs in western France. In addition to standard-care (SC), 3 ICUs used MSD, a variant of selective digestive decontamination, which consists of the administration of topical antibiotics four times daily in the oropharynx and the gastric tube, chlorhexidine body wash and a 5-day nasal mupirocin course. AIs were compared between the 3 ICUs using MSD (MSD group) and the 12 ICUs using SC.
Results
During study period, 614 of 1158 COVID-19 patients admitted in our ICU were intubated for at least 48 h. Due to missing data in 153 patients, 461 patients were finally included of whom 89 received MSD. There were 34 AIs in the MSD group (2117 patient-days), as compared with 274 AIs in the SC group (8957 patient-days) (p < 0.001). MSD was independently associated with a lower risk of AI (IRR = 0.56 [0.38–0.83]; p = 0.004) (Table 2). When the same model was used for each site of infection, MSD remained independently associated with a lower risk of VAP (IRR = 0.52 [0.33–0.89]; p = 0.005) but not of BSI (IRR = 0.58, [0.25–1.34], p = 0.21). Hospital mortality was lower in the MSD group (16.9% vs 30.1%, p = 0.017).
Conclusions
In ventilated COVID-19 patients, MSD was independently associated with lower AI incidence.
Similar content being viewed by others
Background
Despite increased knowledge regarding severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic, intensivists have to face a surge of critically ill patients worldwide and mechanical ventilation remained an inescapable lifesaving therapy. As it has been commonly described for other critically ill patients, those admitted in ICU with SARS-CoV-2 infectious disease (COVID-19) are at high risk for developing ventilator-associated infection (VAP) [1,2,3] and bloodstream infection (BSI) [4, 5] with implications for outcome. For four decades now, various selective oropharyngeal/digestive decontamination regimens have been reported to decrease the incidence of VAP, BSI and to some extent mortality [6,7,8] without increasing the risk of in multi-drug resistant (MDR) bacteria acquisition. To our knowledge, selective digestive decontamination has been rarely studied in the COVID-19 setting [9,10,11]. Of note, two studies reported survival benefit [10, 11]. Therefore, we conducted an observational study to assess the impact of a selective digestive decontamination regimen on acquired infections and survival in ICU COVID-19 patients. We hypothesized that this strategy could be associated with a reduction of the incidence of VAP and BSI but also with a reduced mortality rate.
Methods
Setting and patients
We performed a retrospective analysis mostly using the COCOREVAP cohort patients and some more patients of the Vannes and Saint–Brieuc centers. The COCOREVAP cohort is a multicenter retrospective observational study in 15 ICUs from 11 centers in western France. All adults admitted with COVID-19 from February 1st 2020 until December 31th 2021 who required mechanical ventilation were eligible. Additional patients in the Vannes and Saint–Brieuc centers were included between June the 1st and December 31th 2021. Patients under liberty deprivation (i.e., are under individual protection measure, such as tutorship and curatorship), pregnant women and patients younger than 18 years were excluded from the study. In addition to standard care (SC), three ICUs used a multiple-site decontamination regimen (MSD) for the prevention of acquired infections in intubated patients. MSD was used in all patients in one center (Rennes) and since May 5, 2021 in the two others (Saint–Brieuc and Vannes). Multiple-site decontamination is a variant of selective digestive decontamination, which consists of the administration of topical antibiotics including an aminoglycoside (tobramycin, 300 mg per day, in Rennes or gentamicin, 543 mg per day, in the two others centers), colistin sulfate (400 mg per day) and amphotericin B (2 g per day), four times daily in the oropharynx and the gastric tube, chlorhexidine body washing once daily and a 5-day nasal mupirocin course in patients who had an expected intubation duration of 24 h or more throughout the duration of intubation. Full details about the MSD regimen have been reported elsewhere [12]. Patients in the others ICUs received standard care alone. Patients who required intubation for an expected duration greater than 2 days were eligible for study and divided into two groups: MSD group and SC group.
Each center had a nosocomial infection committee for the prevention and prospective census of acquired infections and applied the recommendations of the French Society for Hospital Hygiene for the prevention and treatment of infection (available at https://sf2h.net/publications/actualisation-precautions-standard-2017).
The study protocol received approval from the ethical committee of the French Intensive Care Society (CE 21–56). Patients or closest relative were informed of the anonymous prospective collection of the data and had the possibility not to participate in the study. In case of refusal, the data were not collected accordingly. This manuscript follows the STROBE statement for reporting cohort studies.
Definition
Infection was considered acquired in the ICU when it was diagnosed 48 h after admission and was not incubating on admission. BSI was defined as a positive blood culture occurring 48 h or more after admission. Regarding common skin contaminants, 2 positives blood cultures drawn on separate occasions were required [4]. The diagnosis of VAP was considered in patients ventilated for 48 h or more and was based on clinical signs (fever, purulent sputum, hypoxia), radiological findings (new infiltrate on chest-X-ray or CT scan), and leukocytosis [13]. Microorganisms responsible for infection were considered as multi-drug resistant (MDR) according to the European Society of Clinical Microbiology and Infectious Disease definition [14]. Respiratory samples used for VAP diagnosis were performed either with broncho-alveolar lavage, endotracheal aspiration or distally protected samples according with local protocols. To take into account the diagnosis heterogeneity of VAP among centers, the variable “Strategy for VAP diagnoses in center of admission” was created. It corresponds to the more frequently used pulmonary sample for VAP diagnosis in the center in which the patients was admitted. Of note, 7 centers mainly used endotracheal aspiration, 4 performed a majority of distally protected samples and the others performed broncho-alveolar lavage.
Primary and secondary endpoints
The primary endpoint was the incidence of ICU-acquired infections, and secondary endpoints were specific VAP and BSI incidences as well as in hospital mortality.
Statistical analysis
Statistical analysis was performed with the statistical software R 4.1.1. Categorical variables were expressed as percentages and continuous variables as median and interquartile range (IQR). The chi-square test and Fisher exact test were used to compare categorical variables and the Mann–Whitney U test or the Wilcoxon for continuous variables. Overall, 6.1% of the data were missing (192 patients had at least one missing data). For the purpose of the multivariable analysis, missing data were considered as missing at random and were handled using chained equation, using “MICE” R package to create an imputed data set.
Incidence rate and risk factors for acquired infections were analyzed using a univariate and multivariable Poisson regression model. Survival analysis were conducted with Kaplan–Meier survival curves with log-rank test and logistic regression with a stepwise backward regression using Akaike criteria as a stopping rule. Non-redundant variables associated with event (acquired infection or death) with a p value < 0.2 in the univariate analysis were included in the multivariable analysis.
To draw unbiased marginal estimates of exposure effect, a propensity-score matched analysis was performed. Propensity score was calculated using a non-parsimonious model (including all available baseline characteristics: age, male sex, body mass index, comorbidities, period of admission, inter-hospital transport, localization before admission, simplified acute physiology score II, bacterial co-infection at admission, biological parameters at admission, strategy for VAP diagnoses in center of admission and early management) and correspond for each patient to his probability to be admitted in an ICU, where MSD is implemented. Because of the non-parsimonious design, interaction effects between variables were not taken into account (e.g., between age and SAPS II scores). Using the “MatchIt” package, a k-nearest neighbor algorithm was used for propensity-score matching with a 1:1 ratio. The balance between matched groups was evaluated by the analysis of the standardized mean differences after weighting.
All tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Population
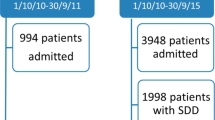
During the study period, 1158 patients were admitted in our ICUs with a COVID-19 diagnosis confirmed with a PCR, of whom 614 were intubated for at least 48 h. Due to missing data regarding AIs in 153 patients, 461 patients were included in the final study, of whom 89 received MSD (Fig. 1). Data regarding excluded patients are available in Supplementary materials. At admission, the simplified acute physiology score II was 36 [29–45], PaO2/FiO2 ratio was 100 [70–145] and 30.0% of patients were transferred from another ICU. Most baseline characteristics of MSD and SC patients were similar except a lower age (62 years [55–71] vs 68 [61–73], p < 0.05), a lower body mass index (27.46 [24.39–31.40] vs 28.76 [25.38–32.16], p < 0.05), a lower proportion of patients admitted during fall 2020 (31.5% vs 48.1%, p = 0.001) and a higher proportion of patients receiving antibiotic at admission (93.2% vs 82.3%, p = 0.018) in the MSD group (Table 1).
Acquired infections
In the MSD group, compared with the SC group, there were 34 AIs (26 VAP, 8 BSI) in 2117 patients-days and 274 AIs (212 VAP, 62 BSI) in 8957 patients-days, respectively (incidence rate ratio [IRR] = 0.53, 95% CI 0.37–0.75, p < 0.001) (Table 1). Similarly, the VAP incidence rates were 14.3 per 1000 ventilatory-days and 28.3 per 1000 ventilatory-days, respectively (IRR = 0.51 [0.34–0.76], p < 0.001). There were numerically less BSI in the MSD group, with incidence rates of 3.79 and 6.9 per 1000 patients-days, respectively (IRR = 0.55, [0.26–1.14]; p = 0.10). In a multivariable Poisson regression model, MSD administration was associated with a lower risk of AI (IRR = 0.56 [0.38–0.83]; p = 0.004) (Table 2). When the same model was used for each site of infection, MSD remained independently associated with a lower risk of VAP (IRR = 0.52 [0.33–0.89]; p = 0.005) but not with a lower risk of BSI (IRR = 0.58, [0.25–1.34], p = 0.21) (not shown). Other risk factors for AI were male sex (IRR = 1.38 [1.03–1.86] p = 0.034), cirrhosis (IRR = 1.81 [1.17–2.82] p = 0.007), inter-hospital transport (IRR = 1.31 [1.02–1.69] p = 0.038) and higher simplified acute physiology score II (IRR = 1.01 per supplementary point [1.00–1.02] p = 0.032).
Microorganisms responsible for infection
Microorganisms responsible for infection are reported in Table 3. There were no differences between groups regarding either the responsible microorganisms or clinical presentation at VAP onset. There were 5 and 43 infections due to MDR microorganisms in the MSD and the SC group, respectively (p = 0.765).
Outcomes
Fifteen patients (16.9%) in the MSD group died during hospital stay as compared with 112 patients (30.1%) in the SC group (p = 0.017; Table 1 and Fig. 2). In a multivariable logistic regression analysis, MSD remained independently associated with a lower risk for death (OR = 0.49 [0.24–0.99]; p = 0.049) (Additional file 1: Table S2). Others independent risk factors for in-hospital death were a higher age (OR = 1.06 per supplementary year, [1.03–1.09] p < 0.001), inter-hospital transport (0.40 [0.22–0.74] p = 0.03), higher SAPS II (OR = 1.05 per supplementary point, [1.03–1.07] p < 0.001) and systemic antibiotic at admission (OR = 0.47 [0.25–0.88] p = 0.018).
Propensity score matched analysis
Then, patients who received MSD where matched with similar patients who received standard care using a non-parsimonious propensity score matching. Matching process resulted in 89 matched patient’s pairs with well-balanced baseline characteristics (Table 4). In this data set, patients of the MSD group still had a lower incidence of AI as compared patients receiving standard care (32.6% vs 57.3% p = 0.002), mainly because of a lower incidence of VAP (29.2% vs 53.9% p = 0.001), while BSI incidence was not statistically different (7.9% vs 11.2% p = 0.61). Finally, hospital mortality rate remained lower in the MSD group as compared with standard care group (16.9% vs 32.6% p = 0.024).
Discussion
In this observational study conducted in ICUs with low multi-drug resistance rate [15], we have shown that MSD is associated with a decreased risk of AI but also with a better survival in COVID-19 critically ill patients. To our knowledge, only one study suggested a survival benefit with selective decontamination regimen in this setting [10], but our study is the first that specifically investigate relationship between selective digestive decontamination regimen and AI.
Prolonged mechanical ventilation, immunosuppressive treatments (i.e., corticosteroids and/or IL-6 signaling blockade) [16, 17] and immune dysfunctions [18] observed in severe COVID-19 patients might be responsible for their higher risk of nosocomial infections [1, 3, 5]. Moreover, viral pneumonia may induce damages to the ciliated cells, leading to impaired mucociliary clearance and increased risk for bacterial adhesion and colonization of the airways. While there is still a controversy on the degree of VAP attributable mortality, the occurrence of VAP seems to have a significant impact on the outcome of COVID-19 patients [3, 19] making the evaluation of preventive effective therapies a priority. Of note, the high incidence of VAP in our control group (57%) corresponds with the high incidence reported in the COVID–ICU study (58%) [1]. However, BSI incidence in the present study (12.5%) was lower than previously observed (19.5% to 29.6%) [5, 21] and our study might be underpowered to explore this specific site of AI.
Until now, attributable mortality of AI was mainly recognized for surgical patients but not for medical ones [20]. In contrast, Rouze et al. observed that VAP onset was independently associated with a poorer outcome in COVID-19 patients, whereas patients infected with other viruses had similar outcomes whether they had VAP or not [3]. This result supports a potential benefit for infection prevention strategy in this particular setting. Although, its benefit was not confirmed in study conducted in area with higher resistance rate [22], selective digestive decontamination regimens have been associated with reduced mortality and lower BSI and VAP rates in areas with low levels of antibiotic resistance. A major concern regarding decontamination regimen is the application of systematic antibiotic at admission. It is noteworthy that de Jonge et al. showed that selective digestive decontamination (including 4-day intravenous cefotaxime) was associated with higher levels of antibiotic susceptibility of Gram negative bacteria to ceftazidime, ciprofloxacin, imipenem and tobramycin [23]. Assuming a proportion of patients with a bacterial infection on admission between 10 and 25%, the systematic use of short-term antibiotic therapy to treat incubating pneumonia prevent early VAP seems reasonable [24, 25]. Finally, when analyzing the bacteria recovered from respiratory samples, the proportion of MDR bacteria was low and not statistically different between MSD and standard care groups.
To our knowledge, our study is the largest to evaluate the effects of selective digestive decontamination on ICU acquired infections in SARS-CoV-2 patients. Nevertheless, some limitations should be pointed out. First, our study was conducted on adult ICU in the west of France only, where MDR are not endemic. Hence, the effect of MSD observed here might not be generalizable to the whole population of COVID-19 ventilated patients. Second, as mentioned before, our study was retrospective that implies missing data. Third, due to a marked disproportion between groups with a relatively small sample size in the MSD group, our study might not be adequately powered to detect some differences between groups (such as differences in age or severity score). Fourth, VAP is a subjective endpoint and physicians may have been influenced in their diagnosis because of the non-blind nature of the study. Finally, other residual confounders, such as COVID-19-associated ICU surge, ARDS management but also heterogeneity between center for AI diagnosis and prevention were not assessed in our study.
To conclude, the incidence of AIs, especially VAP, was significantly lower in patients receiving a multiple-site decontamination regimen without significant impact on MDR bacteria acquisition.
Availability of data and materials
The data sets generated during the current study are available from the corresponding author on reasonable request.
References
COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. https://doi.org/10.1007/s00134-020-06294-x.
Martin-Loeches I, Povoa P, Rodríguez A, Curcio D, Suarez D, Mira J-P, et al. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med. 2015;3(11):859–68.
Rouzé A, Martin-Loeches I, Povoa P, Makris D, Artigas A, Bouchereau M, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med févr. 2021;47(2):188–98.
Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38(12):1930–45.
Massart N, Maxime V, Fillatre P, et al. Characteristics and prognosis of bloodstream infection in patients with COVID-19 admitted in the ICU: an ancillary study of the COVID-ICU study. Ann Intensive Care. 2022;11(1):183. https://doi.org/10.1186/s13613-021-00971-w.
de Smet AM, Kluytmans JA, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360(1):20–31. https://doi.org/10.1056/NEJMoa0800394.
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. 2009. https://doi.org/10.1002/14651858.CD000022.pub3.
Camus C, Bellissant E, Sebille V, et al. Prevention of acquired infections in intubated patients with the combination of two decontamination regimens. Crit Care Med. 2005;33(2):307–14. https://doi.org/10.1097/01.ccm.0000152224.01949.01.
van der Meer SB, Figaroa G, van der Voort PHJ, Nijsten MW, Pillay J. Ventilator-associated pneumonia in critically-ill patients with COVID-19 in a setting of selective decontamination of the digestive tract. Crit Care. 2021;25(1):445. https://doi.org/10.1186/s13054-021-03869-y.
Peñuelas O, Del Campo-Albendea L, de Aledo ALG, et al. Long-term survival of mechanically ventilated patients with severe COVID-19: an observational cohort study. Ann Intensive Care. 2021;11(1):143. https://doi.org/10.1186/s13613-021-00929-y.
Luque-Paz D, Tattevin P, Jaubert P, Reizine F, Kouatchet A, Camus C. Selective digestive decontamination to reduce the high rate of ventilator-associated pneumonia in critical COVID-19. Anaesth Crit Care Pain Med. 2022;41(1): 100987. https://doi.org/10.1016/j.accpm.2021.100987.
Camus C, Salomon S, Bouchigny C, et al. Short-term decline in all-cause acquired infections with the routine use of a decontamination regimen combining topical polymyxin, tobramycin, and amphotericin B with mupirocin and chlorhexidine in the ICU: a single-center experience. Crit Care Med. 2014;42(5):1121–30. https://doi.org/10.1097/CCM.0000000000000140.
Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis. 2017;64(9):1298.
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
Massart N, Camus C, Benezit F, Moriconi M, Fillatre P, Le Tulzo Y. Incidence and risk factors for acquired colonization and infection due to extended-spectrum beta-lactamase-producing Gram-negative bacilli: a retrospective analysis in three ICUs with low multidrug resistance rate. Eur J Clin Microbiol Infect Dis. 2020;39(5):889–95. https://doi.org/10.1007/s10096-019-03800-y.
RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704. https://doi.org/10.1056/NEJMoa2021436.
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–45. https://doi.org/10.1016/S0140-6736(21)00676-0.
Kuri-Cervantes L, Pampena MB, Meng W, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020. https://doi.org/10.1126/sciimmunol.abd7114.
Nseir S, Martin-Loeches I, Povoa P, Metzelard M, Du Cheyron D, Lambiotte F, et al. Relationship between ventilator-associated pneumonia and mortality in COVID-19 patients: a planned ancillary analysis of the coVAPid cohort. Crit Care déc. 2021;25(1):177.
Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–71. https://doi.org/10.1016/S1473-3099(13)70081-1.
Ippolito M, Simone B, Filisina C, et al. Bloodstream infections in hospitalized patients with COVID-19: a systematic review and meta-analysis. Microorganisms. 2021;9(10):2016. https://doi.org/10.3390/microorganisms9102016.
Wittekamp BH, Plantinga NL, Cooper BS, et al. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA. 2018;320(20):2087–98. https://doi.org/10.1001/jama.2018.13765.
de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362(9389):1011–6. https://doi.org/10.1016/S0140-6736(03)14409-1.
Rouze A, Martin-Loeches I, Povoa P, et al. Early bacterial identification among intubated patients with COVID-19 or influenza pneumonia: a European multicenter comparative cohort study. Am J Respir Crit Care Med. 2021;204(5):546–56. https://doi.org/10.1164/rccm.202101-0030OC.
Contou D, Claudinon A, Pajot O, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10(1):119. https://doi.org/10.1186/s13613-020-00736-x.
François B, Laterre PF, Luyt CE, Chastre J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit Care. 2020;24(1):289. https://doi.org/10.1186/s13054-020-03013-2.
Massart N, Dupin C, Mari A, et al. Clinician involvement for ventilator-associated pneumonia surveillance resulted in higher than expected incidence rate reported with implication for attributable mortality. Infect Dis (Lond). 2021;53(2):154–7. https://doi.org/10.1080/23744235.2020.1839129.
Acknowledgements
The author’s thanks all the care givers involved in patients care during this grueling epidemics.
Funding
This study was funded by the Foundation AP–HP and the Direction de la Recherche Clinique et du Development and the French Ministry of Health. The REVA network received a 75,000 € research grant form Air Liquide Healthcare. The funder had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
All authors participated to, or acquisition of data, NM, AD, PF and EC participated to the conception and design of the study and NM performed the analysis and interpretation of data, NM, AD, PF, FR and CC drafted the article, all authors finally approved the submitted version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study respects the French reference method MR004. Patients or closest relatives were informed of the retrospective collection of the data and only those who did not provide opposition to their participation in the study were enrolled. The French intensive care society ethical committee (comité d’éthique de la SRLF) approved the study protocol CE 21.56.
Consent for publication
Patients or closest relative were informed of the anonymous prospective collection of the data and had the possibility not to participate in the study. In case of refusal, the data were not collected accordingly.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Baseline characteristics and outcomes of patients included and not included. Table S2. Risk factors for hospital death. Figure S1. Survival curves in patients receiving MSD or not in whole population (inclusion of the 153 patients with missing data regarding AI).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Massart, N., Reizine, F., Fillatre, P. et al. Multiple-site decontamination regimen decreases acquired infection incidence in mechanically ventilated COVID-19 patients. Ann. Intensive Care 12, 84 (2022). https://doi.org/10.1186/s13613-022-01057-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-022-01057-x