Abstract
Background
Estimation of the composition and densities of mosquito species populations is crucial for monitoring the epidemiology of mosquito-borne diseases and provide information on local vectors to public health officials and policy-makers. The aim of this study was to evaluate malaria vector bionomics in ecologically distinct sites in Taita-Taveta County, Kenya.
Methods
Adult mosquitoes were collected using backpack aspirators and paired indoor/outdoor CDC light traps in 10 randomly selected households in six villages with distinct ecologies over a study period of 3 years. All Anopheles mosquitoes were morphotyped, and sibling species of Anopheles gambiae sensu lato (An. gambiae s.l.) were identified and separated by PCR analysis of extracted ribosomal DNA. All female anophelines were tested for sporozoite infectivity, with engorged females screened for blood-meal sources using the enzyme-linked immunosorbent assay technique. A subsample of those testing positive and those testing negative for Plasmodium in the ELISA were subjected to PCR assay.
Results
A total of eight different Anopheles species were collected both indoors and outdoors. Anopheles gambiae s.l. (82.6%, n = 5252) was the predominant species sensu lato, followed by Anopheles coustani sensu lato (An. coustani s.l.; (10.5%, n = 666) and Anopheles funestus sensu lato (An. funestus s.l.; 5.6%, n = 357). A subset of 683 mosquito samples representing An. gambiae s.l. (n = 580, approx. 11.0%) and An. funestus s.l. (n = 103, approx. 28.9%) were identified by molecular diagnostic assays into sibling species. The An. gambiae s.l. complex was composed of Anopheles arabiensis (62.5%, n = 363/580), An. gambiae sensu stricto (An. gambiae s.s.; 0.7%, n = 4/580), Anopheles merus (0.7%, n = 4/580) and Anopheles quadriannulatus (0.2%, n = 1/580), with the remaining samples (35.5%, n = 206/580) unamplified. Anopheles funestus s.l. was composed of An. rivulorum (14.6%, n = 15/103) and An. leesoni (11.6%, n = 12/103); the remaining samples were unamplified (73.8%, n = 76/103). A total of 981 samples were subjected to PCR analysis for malaria parasite detection; of these 16 (1.6%) were confirmed to be positive for Plasmodium falciparum. The overall human blood index was 0.13 (32/238).
Conclusions
Anopheles gambiae, An. funestus and An. coustani are key malaria vectors in the Taveta region of Kenya, showing concurrent indoor and outdoor transmission. All of the vectors tested showed a higher propensity for bovine and goat blood than for human blood.
Graphical Abstract

Similar content being viewed by others
Background
Despite significant advances in the past two decades nearly half of the global population continues to be at risk for malaria transmission. According to WHO, over 200 million cases and 409,000 deaths were reported in 2020 [1], with the most burdened countries located in WHO’s African Region. A few African countries, such as Algeria and Morocco, have been certified as malaria-free, while others, such as Zimbabwe, Botswana, Swaziland and South Africa, are listed among those on the verge of malaria elimination. However, high-burden African countries, such as Nigeria, Democratic Republic of Congo (DRC), Mozambique, Ivory Coast, Tanzania and Uganda, reported an increase in malaria cases [1, 2], with > 90% of the malaria cases reported in 2020 in these countries [1]. The elimination and reduction of malaria cases has been attributed to widespread use of interventions, such as prompt diagnosis, treatment and vector control strategies [2, 3].
Malaria is endemic in Kenya, with the majority of affected populations living in the western and coastal areas of Kenya. Epidemiology is governed by rainfall patterns, temperature and altitude, and is driven by distinct vectors that are dependent on specific ecological conditions. Vector control is an essential component and an important cornerstone of malaria prevention, control and elimination strategies by effectively reducing vector-human contacts and thereby reducing disease transmission [4, 5]. Malaria reduction in coastal Kenya has been mainly due to coverage and widespread use of long-lasting insecticide-treated nets (LLINs), while in western Kenya the combination of LLINs and indoor residual spraying have been the drivers of malaria reduction [4, 6,7,8,9]. It has been noted that this reduction in disease burden was accompanied by changes in the vector populations, potentially due to selective pressure, with the changes including diversity, role as vectors and behaviour. Species with greater behavioural plasticity were favoured, particularly those that bite both outdoors and outside regular sleeping hours as well as biting both humans and peri-domestic animals [10,11,12,13,14].
In Kenya, the composition and distribution of malaria vector species are highly heterogenous. Anopheles funestus is described an emerging main malaria vector in the highland regions of Kenya. However, in the low-altitude arid and semi-arid areas of the coastal region, Anopheles arabiensis is the predominant vector [15]. In some areas of coastal and western Kenya, Anopheles coustani, a secondary vector, is now becoming a major vector and contributing substantially to malaria transmission [10, 16]. In much of coastal Kenya, An. arabiensis has replaced Anopheles gambiae sensu stricto (An. gambiae s.s), and An. funestus sensu stricto (s.s.) has become the dominant malaria vector [15]. Moreover, several secondary zoophillic, exophagic, and exophilic vectors may also play a role in transmission, including An. coustani sensu lato (s.l.) Anopheles pharoensis sensu lato (An. pharoensis s.l.), Anopheles rivulorum, Anopheles parensis, Anopheles merus and Anopheles quadriannulatus [10, 14, 16,17,18,19]. Most vectors that evade control measures also present with diverse trophic preferences, including humans but also cattle, goats, chicken, dogs and pigs [15, 20,21,22], thus allowing vector populations to persist and increase in number. The changes in species composition, increasing role of secondary vectors, feeding patterns, heterogeneity in transmission focal points and geographic expansion of vectors has implications for the control of malaria and disease elimination efforts. Furthermore, the geographic expansion of the invasive urban malaria vector Anopheles stephensi into the Horn of Africa calls for improved surveillance strategies not only in rural areas but also in urban environments [23].
Surveillance and control strategies require prior knowledge on the vector bionomics, species composition, biting behaviours and sporozoite rates of local vector species that transmit malaria. Hence, a 3-year entomological surveillance was undertaken in Taita-Taveta County, Kenya to gain an understanding of malaria transmission dynamics and gather information on local vector species diversity, trophic preferences and plasmodial infection status.
Methods
Study site
The study was carried out in the subcounty of Taveta, Taita-Taveta County (3°24′00″ S, 37°41′00″ E), coastal Kenya. Taveta subcounty is located about 109 km west of Voi town, off the Nairobi-Mombasa road, and is mainly inhabited by members of the Taita and Taveta ethnic groups. The primary occupations of the residents of this subcounty are mixed farming, livestock, trade/business and casual wage labour. The area is a plateau that generally slopes towards the south and is at 752 m a.s.l. The mean annual rainfall ranges between 200 and 1200 mm, with long rains between March and May and the long dry season occurring between June and October. Mean annual temperatures range between 21 °C and 31 °C. Irrigated agricultural activities are the main source of livelihood, with water for irrigation obtained from the Tsavo, Lumi, Njoro and Kitobo rivers, as well as from spring water at the foot of Mount Kilimanjaro.
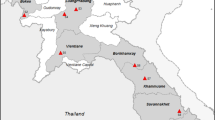
Taita-Taveta County is malaria endemic [8, 24], with perennial transmission at the end of the long rainy season [25,26,27]. Prevalence rates are rising, as evidenced from clinical malaria data reported by the Kenyan National Malaria Control Programs (NMCP) [24]. In 2011, the annual entomological inoculation rate in Taita-Taveta County was about 31.13 infective bites per person per year [10]. In the present study, six villages representing different ecological and hydrological (irrigated vs non-irrigated) set-ups were targeted for mosquito surveillance: Kiwalwa, Mwarusa, Njoro, Kimundia, Kingwareni and Chala villages (Fig. 1).
Entomological sampling
Adult mosquitoes were collected using the Centers for Disease Control and Prevention (CDC) Backpack Aspirator and paired indoor/outdoor CDC light traps as previously described [10]. Ten houses were randomly selected in each village for mosquito collection. Paired CDC light mosquito trapping was done, with one trap set indoors and the second trap located outdoors (a few meters from an animal enclosure), from 1800 hours to 0600 hours. Indoor resting mosquitoes were collected from five randomly selected houses in each village using the CDC Backpack Aspirator between 0600 hours and 1000 hours. All samples collected were transported to the field laboratory at Taveta station for sorting and preservation before being transported to the laboratories of the Kenyan Medical Research Institute Wellcome Trust Research Programme (KWTRP) in Kilifi for further processing. Samples were collected in 2016 (November), 2017 (February, March) and 2018 (March, April, October).
Morphological identification and processing of mosquitoes
Female Anopheles mosquitoes were identified to species level using dichotomous morphological keys [28], following which they were separated according to physiological status. The mosquitoes were then dissected into legs and wings, head and thorax, and abdomen (blood fed) compartments, and placed individually in well-labelled sterile 1.5-ml Eppendorf tubes.
Molecular identification of the main malaria vectors
DNA was extracted from the legs and wings and subsequently used for sibling species identification by PCR as previously described [29], with slight modification. Briefly, 70 µl of 5% Chelex 100 chelating resin was added to the 1.5-ml vial containing the legs and wings of one mosquito, followed by the addition of one steel bead (diameter: 5 mm) and subsequent homogenization using a tissue lyser (1 cycle; frequency: 30 Hz; duration: 1 min) [30]. The homogenate was then incubated at 95 °C for 10 min, followed by centrifugation at 15,000 rpm for 10 min. The supernatant was then transferred into a sterile 1.5-ml vial. To ensure complete removal of the Chelex from the DNA, another centrifugation at 15,000 rpm for 10 min followed. Finally, the supernatant was transferred into a sterile 1.5-ml vial and stored at − 80 °C until further processing. For An. gambiae sensu lato (An. gambiae s.l.), genomic DNA was amplified using specific primers targeting the intergenic spacer region (IGS2) of the ribosomal DNA [31]. We screened for An. gambiae s.s., An. arabiensis, An. merus and An. quadriannulatus [31]. The mosquito genomic DNA for An. funestus sensu lato (An. funestus s. l.) was amplified using specific primers targeting the internal transcribed region 2 (ITS2) from the 5.8S and 28S coding region flanking the variable ITS2 region [32]. The reaction volume of 15 µl contained 5 µl of 2× GoTaq Green Master Mix (Promega Corp., Madison, WI, USA), 0.5 µl of each primer (concentration: 10 µM), 4.5 µl of nuclease-free water and 3 µl of genomic DNA. The thermocycling conditions were: an initial denaturation at 95 °C for 2 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 45 s and 72 °C for 30 s, with a final elongation at 72 °C for 10 min. The resulting amplicons were visualized in 1.5% agarose with RedSafe™ Nucleic Acid Staining Solution (magnification: 20,000×) (iNtRON Biotechnology, Sangdaewon-Dong, Gyeonggi-do, South Korea) using a UV photographer (Bio-Rad Laboratories, Hercules, CA, USA).
Plasmodium screening in mosquitoes
All anopheline mosquitoes collected were tested for sporozoite infectivity using a specific Plasmodium falciparum sporozoite enzyme-linked immunosorbent assay (ELISA) as previously described [15]. The head and thorax compartment of individual mosquitoes were homogenized in 250 µl of grinding buffer using a sterile pestle. The microtiter plate was first coated with the primary P. falciparum capture monoclonal antibodies (mAb) followed by blocking of any unbound sites using blocking buffer. A 50-μl sample of the mosquito homogenate was then put added to wells of the microtiter plate and incubated for 2 h. The plate was then rinsed, followed by the addition of 100 µl anti-sporozoite monoclonal peroxidase-labelled conjugate antibody (secondary mAb). After a second rinsing of the microtiter plate, 100 µl of 2,2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) substrate added to each well. The results were read qualitatively (visually) based on colour change, with a homogenous greenish-blue colour considered to indicate positivity and a clear sample without any colour change considered to indicate negativity. For quality assurance purposes, each microtiter plate was loaded with a positive control (BEI Resources, Manassas, VA, USA) and a negative control (naïve females of An. gambiae s.s (obtained from insectary colony) that had never obtained an infectious blood meal). The P. falciparum Sporozoite ELISA Reagent Kit (MRA-890) was obtained through BEI Resources, US National Institute of Allergy and Infectious Diseases (NIAID) and US National Institutes of Health (NIH) (both Bethesda, MD, USA), contributed by Robert A. Wirtz (CDC, Atlanta, GA, USA) [15].
A subsample of those samples testing positive and negative, respectively, in the ELISA were analysed by PCR as previously described [33]. Molecular detection by PCR was conducted in 2022, several years after sampling. DNA was extracted from the samples by mixing 100 µl of the remaining homogenate (lysate) with an equal volume of 5% Chelex solution (w/v). The mixture was thoroughly vortexed to ensure complete mixing, followed by incubation at 95 °C for 10 min. This was followed by centrifugation at 15,000 rpm for 10 min. The supernatants were transferred into respective sterile 1.5-ml vials. To ensure complete removal of the Chelex from the DNA, another centrifugation at 15,000 rpm for 10 min was performed. Finally, the supernatants were transferred into respective sterile 1.5-ml vials and stored at − 80 °C until further processing. The obtained DNA was subjected to PCR analysis. The primers used target a subunit of the cytochrome c oxidase I gene (COX1) located in the plasmodium mitochondrial genome. The set of primers used were COX-IF (forward primer: 5′-AGAACGAACGCTTTTAACGCCTG-3′) and COX-IR (reverse primer: 3′-ACTTAATGGTGGATATAAAGTCCATCCwGT-5′). The reaction volume of 20 µl contained 10 µl of 2× GoTaq Green Master Mix (Promega Corp.), 0.5 µl of each primer (concentration: 10 µM), 5 µl of PCR water (nuclease free) and 4 µl of genomic DNA. The PCR cycling conditions were: an initial denaturation at 95 °C for 2 min, followed by 40 cycles of 95 °C for 30 s, 56 °C for 45 s and 72 °C for 30 s, with a final elongation at 72 °C for 2 min. The resulting amplicons were visualized in a UV photographer after gel electrophoresis (Bio-Rad Laboratories). To quality check if the sample DNA was degraded or not (in the unamplified samples), we performed species identification as described above [31].
Blood-meal analysis
Blood-meal analyses to determine mosquito feeding preferences were conducted using a previously described protocol [20, 34, 35]. Direct ELISA assay was used to discriminate blood-meal sources by using anti-host (immunoglobulin G) conjugate against humans, bovines, goats and chickens. Briefly, the abdomens of individual mosquitoes were homogenized in 1000 µl of 1× phosphate-buffered saline (PBS) using sterile pestles. A 50-µl sample of the homogenates was coated to the wells of the microtiter plate and incubated at room temperature for 30 min. The contents were then aspirated out, the wells washed and 50 µl of conjugated mAbs was added. Lastly, 100 µl of ABTS substrate was added to the well. Each step was followed by incubation at room temperature as well as rinsing of the plate. The results were read qualitatively (visually) through colour change, with a homogenous greenish-blue colour considered to indicate positivity and a clear sample without colour change considered to indicate negativity, as previously described [20, 36]. The positive controls were serum from human, bovine, goat, and chicken, whereas PBS was used as negative control.
Data analysis
Data collected were entered into Microsoft Excel (Microsoft Corp., Redmond, WA, USA) and analysed using R studio statistical software version 4.0.2 [37]. The human blood index (HBI), defined as the proportion of mosquitoes that obtained blood from human hosts, was calculated by dividing the number of mosquitoes that obtained their meals from humans (including mixed blood-meal origins) by the total number of blood-fed Anopheles mosquitoes analysed. Human biting rate (HBR) by CDC light trap was calculated by dividing the number of female anopheline mosquitoes captures by the number of collection events.
We described discrete (An. gambiae and An. funestus) and categorical variables by calculating medians and proportions, respectively. Mosquito densities (An. gambiae and An. funestus) in each location over time was summarized using box and whiskers plot. We fitted a generalized linear model of negative binomial distribution with a log function to determine the association between the outcomes and independent variables (location, roof type, wall type and the presence of eaves). The estimated coefficients and 95% confidence interval in both models were reported, and all predictors with P < 0.05 were significantly associated with outcome variables (An. gambiae and An. funestus). All statistical analyses were performed using Stata version 15.0 (Stata Corp., College Station, TX, USA).
Results
Spatial and temporal distribution of malaria vectors
A total of 6361 Anopheles mosquitoes were collected comprising of eight Anopheles species. Table 1 summarizes the malaria vector species and their distribution in the six villages between 2016 and 2018. Anopheles gambiae s.l. (82.57%, n = 5252) was the predominant species followed by An. coustani (10.47%, n = 666), An. funestus (5.61%, n = 357), Anopheles pretoriensis (0.41%, n = 26), An. pharoensis (0.88%, n = 56), Anopheles squamosus (0.0.03%, n = 2), Anopheles maculipalpis (0.02%, n = 1) and Anopheles moucheti (0.02%, n = 1). Overall, significantly high densities of Anopheles mosquitoes were recorded during the wet season (n = 5867, 92.2%) compared to the dry season (n = 494, 7.8%). Anopheles gambiae s.l. accounted for > 96% of mosquitoes collected during the wet season while An. coustani accounted for 84.8% (n = 565) of mosquitoes collected during the same period. However, An. funestus s.l. were most abundant during the dry season (n = 187, 52.4%) as opposed to the wet season (n = 170, 47.6%) (Table 1). An equilibrium was observed in the densities of anophelines/Anopheles captured indoors (n = 3388, 53.3%) and outdoors (n = 2973, 46.7%). Among the An. gambiae and An. funestus s.l., a tendency towards endophily was observed. Approximately two-thirds of An. gambiae (58.7%) were captured indoors compared to 2171 (41.3%) captured outdoors. A slight majority of An. funestus s.l. were collected indoors (53%) compared to indoors (47%). A minor malaria vector, An. coustani was recorded to have a significantly higher density outdoors (n = 559, 83.9%) than indoors (n = 107, 16.1%).
The highest number of mosquitoes were collected in Kimundia (43.9%, n = 2795) followed by Kiwalwa (24.4%, n = 1549), Mwarusa (13.1%, n = 834), Kingwareni (10.0%, n = 637), Njoro (8.2%, n = 524) and Chala (0.35%, n = 22). Anopheles gambiae s.l. was reported in all the sites in varying densities, with higher numbers in Kimundia (n = 2218) followed by Kiwalwa (n = 1372), Mwarusa (n = 756), Kingwareni (n = 566), Njoro (n = 322) and Chala (n = 18). Anopheles funestus was analogously reported in all the sites in varying densities, with higher numbers in Njoro (n = 126) followed by Kiwalwa (n = 103), Kimundia (n = 65), Kingwareni (n = 36), Mwarusa (n = 24) and Chala (n = 3). Anopheles coustani (a secondary vector), on the other hand, was reported in all the sites except Chala, and still at varying densities across the different sites, as demonstrated in Table 1. Compared to all other sites, the highest number (n = 487) of An. coustani samples was collected in Kimundia. Anopheles pharoensis was reported in varying densities in all the sites except Chala (Table 1). Other Anopheles mosquitoes collected include An. maculipalpis (n = 1) in Chala, An. moucheti (n = 1) and An. pretoriensis (n = 26) in Njoro and An. squamosus (n = 2) in Kimundia (Table 1). Overall, individuals in Taita-Taveta County received about 16.89 bites per person per night (b/p/n). A significantly majority of these bites were from An. gambiae (13.9 b/p/n), with An. coustani and An. funestus contributing a marginal 2.0 and 1.0 b/p/n, respectively.
Effect of location and household characteristics on An. gambiae and An. funestus counts
Multivariate analysis indicated that the covariates roof type and eaves were positively associated with increased An. gambiae counts. Interestingly, sampling mosquitoes in outdoor locations was associated with a decrease in An. gambiae counts (Table 2). Multivariate analysis also indicated that the covariates/predictors roof type (grass), location (indoor vs outdoor) and eaves were positively associated with increased An. funestus counts. The other covariates were negatively associated with An. funestus counts (Table 2).
Molecular identification of An. gambiae and An. funestus s.l.
A subset of 683 mosquito samples representing An. gambiae s.l. (n = 580, approx. 11.0%) and An. funestus (n = 103, approx. 28.9%) were identified by molecular diagnostic assays into sibling species. The An. gambiae s.l. complex was composed of: An. arabiensis (62.5%, n = 363/580), An. gambiae s.s (0.7%, n = 4/580), An. merus (0.7%, n = 4/580) and An. quadriannulatus (0.2%, n = 1/580), with other samples (n = 35.5%, n = 206/580) unamplified. The An. funestus s.l. complex was composed of: An. rivulorum (14.6%, n = 15/103) and Anopheles leesoni (11.6%, n = 12/103), with all other samples (73.8%, n = 76/103) unamplified. The high rate of unamplified samples could be due to DNA degradation as samples were analysed 7 years after collection.
Malaria transmission foci
A total of 6361 anopheline mosquitoes were tested for P. falciparum sporozoites using circumsporozoite-ELISA. Plasmodium falciparum transmission intensity varied according to site and location (indoors vs outdoor) (Table 3). Plasmodium falciparum sporozoite-infected mosquitoes were collected both indoors and outdoors in the study area (Table 4). Anopheles gambiae and An. coustani were observed transmitting malaria both indoors and outdoors, while An. funestus was only recorded transmitting malaria outdoors. A subsample of 981 samples consisting of those which tested either positive (n = 36) or negative (n = 945) in the ELISA were subjected to PCR amplification for confirmation. Of the 36 samples that tested positive for P. falciparum sporozoites in the ELISA, positivity was confirmed in 16 samples (6 An. arabiensis, 6 An. coustani, 4 unamplified) by PCR (Table 3). This difference could be attributable to false positivity by ELISA, as has been reported previously [38, 39]. All samples which tested negative for P. falciparum sporozoites in the ELISA also tested consistently negative by PCR. Due the limitation of only analysing a subsample for confirmation purposes, no entomological endpoints were calculated. Moreover, sample analysis by PCR was conducted several years after collection, with the possibility that the DNA material in some samples could have degraded.
The feeding preference of key malaria vectors
The blood-meal sources for the mosquitoes analysed are shown in Table 4. Of the 238 (217 indoors vs 21 outdoors) Anopheles mosquitoes tested for blood-meal sources, consisting of An. gambiae s.l. (92%, n = 219), An. funestus s.l. (5.9%, n = 14) and An. coustani (2.1%, n = 5), a significant majority had fed on single hosts, namely bovine (n = 80), goat (n = 33) and, at the lowest frequency, humans (n = 24). Regarding mixed blood-meal sources, the majority of the samples tested showed blood from the following combinations: bovine-goat (n = 18), human-goat (n = 4), bovine-human (n = 3) and, at the lowest frequency, bovine-human-goat (n = 1). In the remaining samples (31.4%, n = 75) the blood-meal source could not be identified. Anopheles gambaie s.l. preferentially fed on bovine (36.4%, n = 80), followed by humans (10.9%, n = 24) and goats (10.0%, n = 22), and 10.0% (n = 22) obtained their blood meals from mixed hosts, as demonstrated in Table 4. For the 14 An. funestus tested, the majority (57.1%) preferred obtained blood meals from goat, and 21.4% obtained mixed meals from the bovine-goat combination. Anopheles coustani showed a higher propensity for feeding on goat (60%, n = 3) and bovine (20%, n = 1) and mixed meals on bovine-human (20%, n = 1). The overall HBI was 0.13, with An. gambiae s.l. having the highest HBI (0.129) followed by An. coustani (HBI: 0.004) and An. funestus (HBI: 0), as none of these was found to have obtained blood from humans (Table 4).
Discussion
This study provides additional information on malaria transmission dynamics and elucidates the spatial–temporal variation, composition and diversity of malaria vectors, trophic preferences and plasmodial infection status in Taita-Taveta County, Kenya. Understanding the distribution, species composition, infectivity and trophic preference of mosquitoes is essential for designing targeted control measures. During the course of the present study seven different species of mosquitoes were collected in the different study areas at varying densities: An. funestus, An. gambiae, An. coustani, An. maculipalpis, An. moucheti, An. pharoensis, An. pretoriensis and An. squamosus. Anopheles funestus, An. gambiae and An. coustani were collected at relatively higher densities. Anopheles funestus and An. gambiae were collected in all of the study villages. The densities of Anopheles mosquitoes were compared during the wet season and dry season and found to be overall higher in the wet season, likely attributable to the increase in larval development sites during the rainy season. Anopheles gambiae s.l. was present at significantly high numbers during the wet season. Mosquito abundance and diversity could also have been modified by agronomic activities and seasonality in the study area. Unplanned agricultural activities involving irrigation systems may be a factor that could have propagated ideal larval developmental sites, establishing adult mosquitoes and hence malaria transmission at these sites [40,41,42]. Additionally, during the rainy season there is an upsurge in the number of aquatic sites able to support larval development, thereby increasing the abundance of malaria vectors. The diversity and abundance of ideal larval developmental sites for Anopheles positively impacts on the resulting adult mosquito population and, consequently, the dynamics of malaria transmission. Agricultural practices have also been shown to influence the diversity, abundance and distribution of mosquito species [40]. However, we observed the reverse for An. funestus s.l. In Kenya, An. funestus prefers breeding in large semi-permanent habitats along river streams that are characterized by vegetation and algae [43]. In Tanzania, An. funestus have been shown to oviposit in permanent and semi-permanent small springs, natural ponds and river tributaries with clear water covered with vegetation [44]. In Taita-Taveta County, the presence of river water used for irrigation creates semi-permanent water bodies that are ideal habitats for mosquito breeding. During the dry season, water levels fall, possibly leading to reduced mosquito breeding. These results clearly indicate that vectors will have ideal developmental sites year in and year out. In different eco-epidemiological regions of Africa, these mosquitoes prefer oviposition in savannah with higher exposure to sunlight and temperature [45, 46]. Such larval developmental sites remain productive for a longer time and in most cases, An. funestus density peaks during the dry season.
The study also adds to the body of information on the role of complemental vectors in malaria transmission. Sporozoite antigens were detected in An. gambiae and An. funestus, the main malaria vectors, as well as in An. coustani. This result corroborates the findings of a previous study conducted in the same area [10], which highlighted the role of An. parensis and An. coustani in malaria transmission. Evidence has also been generated on the role of secondary vectors in malaria transmission [17, 18]. The sporozoite rate in our study was high in the wet season compared to the dry season. This could be attributed to increased densities of malaria vectors, which in turn would increase overall vector-human contact and the likelihood of some vectors surviving the extrinsic incubation period, thereby transmitting malaria. The majority of these vectors prefer biting and resting outdoors. Anopheles coustani s.l. mosquitoes have been demonstrated to be relaxed or flexible feeders with a preference for the outdoor environment. In our study, they tested positive for Plasmodium antigens, indicating their role as a secondary vector in malaria transmission. Several secondary vectors have been described as contributing to malaria transmission when interventions only target and affect the main malaria vectors. Anopheles coustani have been consistently found to harbour Plasmodium antigen in the DRC, Tanzania and Kenya [10, 19, 47]. Moreover, a study conducted in Zambia demonstrated an unexpected anthropophily in An. coustani [48]. These behaviours could be naturally acquired or they could have been induced due to the use of indoor-based interventions. Human behaviours have been shown to be one of the factors contributing to outdoor malaria transmission. A recent study indicated that human behaviour associated with social and economic activities have in some way contributed to outdoor transmission [14]. Socio-cultural activities, such as burial ceremonies, weddings, leisure activities and rituals, are among the factors that keep people outdoors and unprotected [14, 49].
Anopheles gambiae s.l. collected in the indoor environment had the highest blood-feeding indices. Amongst the mosquitoes which had fed on a single host, feeding on humans was found to be low while the goat was the most preferred single host. Anopheles funestus and An. coustani were found to prefer feeding on goats while An. gambiae preferred feeding on bovines/cows. Although some mosquitoes were found to be having mixed-feeding behaviour, combination blood-meal sources were found at only a low frequency amongst the tested mosquitoes. The high number of mosquitoes collected indoors that had also fed on an animal could be due to humans and animals sharing the house because of security issues or due to mosquitoes finding refuge indoors after feeding outdoors. Our results provide additional information on the biting behaviours of members of An. gambiae s.l. Our trophic preference analysis revealed that the majority of An. gambiae s.l. were highly zoophillic as they obtained blood from bovine and goat. These finds are consistent with the results of previous studies [10, 14]. The HBI for An. gambiae s.l. was approximately 0.14, indicating that approximately 14% of the collected An. gambiae s.l. had obtained blood meals from a human source as compared to other vertebrate sources. On the other hand, none of the An. funestus s.l. had obtained blood from human hosts, indicating that the sibling species in circulation are composed of highly zoophillic mosquitoes, such as An. parensis, An. rivulorum and An. leesoni, that had obtained meals from goat and bovine sources. A similar trend was observed for An. coustani, which showed a preference for vertebrate sources other than humans, thus indicating the zoophillic nature of this species and its accidental feeding on humans and, consequently, its transmission of parasites. These observations strongly support the urgent need to develop new and complimentary strategies which target outdoor transmission that occurs when people are unprotected. Several strategies are in the pipeline to address outdoor malaria transmission, such as spatial repellents, attractive toxic sugar bait (ATSB), ivermectin and gene drive technology, amongst others which might complement current interventions [50,51,52,53,54,55,56,57,58].
The present study is characterized by several limitations. First, molecular detection of Plasmodium and species identification were performed several years after sample collection. The first field collection was conducted in November 2016, and the mosquito samples were collected between 2016 and 2018. The samples were processed for ELISA procedures, and subsequently the ELISA homogenates/lysates were preserved in freezers at − 20 °C until DNA extraction and subsequent re-evaluation/analysis by PCR in 2022. The DNA material in some of the homogenates/lysates of the mosquito samples could have degraded, which may have resulted in the high amplification rate seen in the assays on species identification and Plasmodium detection. The degradation of homogenates/lysates and DNA in our study was not unexpected. A similar challenge was faced in a study conducted in Cameroon in which human samples collected in 2015 were screened for the presence of Plasmodium 18S ribosomal RNA. The samples were then re-evaluated in 2017, and only a fraction (62%) of the samples that initially tested positive were detected to be positive in the second evaluation [59]. Analysis/evaluation and re-evaluation of freshly collected samples are highly encouraged. Other factors that could have resulted in discordant results between the ELISA for Plasmodium circumsporozoite protein and PCR assays for Plasmodium detection could be the presence of bovine parasites in the highly zoophillic An. coustani [38, 39]. The proportionately high false positivity rate reported this study should serve as a warning to public health entomologists when interpreting ELISA results. Confirming ELISA results by PCR or other highly complementary methods in strongly recommended.
Secondly, only a subset of An. gambiae s.l. (n = 580, approx. 11.0%) and An. funestus (n = 103, approx. 28.9%) was identified by molecular tools into sibling species. The currently used surveillance tools for entomological surveillance is hampered by their high cost. Molecular identification is expensive and requires more time from DNA extraction to gel electrophoresis. Because of the limitations mentioned, entomological surveillance of wild vector populations in our study was hampered, limiting the scope of the analysis to just subpopulations of malaria vectors, thus leading to overgeneralization of the data. Assessing the role of each mosquito species in malaria transmission requires the development of robust and cost-effective methods for entomological surveillance that will inform decision-making on the composition of malaria vector species and the role of these species in malaria transmission.
Conclusion
This study provides evidence that malaria transmission is occurring in both the indoor and outdoor environment in Taita-Taveta county, coastal Kenya. Anopheles gambiae, An. funestus and An. coustani were determined to be the key malaria vectors, both indoors and outdoors, transmitting malaria in both indoor and outdoor settings. All of the vectors tested showed a higher propensity for bovine and goat blood. Only An. gambiae was found to have fed on humans as a single host. More research is required to investigate the role of secondary vectors in malaria transmission. Also, the development of new complimentary strategies addressing the spatial–temporal gaps left behind by the current interventions by targeting outdoor transmission is urgently needed to tackle the transmission that occurs when people are unprotected.
Availability of data and materials
The supporting data are under the custodianship of the KEMRI-Wellcome Trust Data Governance Committee and are accessible upon request addressed to that committee.
Abbreviations
- ABTS:
-
2,2ʹ-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)
- b/p/n:
-
Bites per person per night
- CDC:
-
US Centers for Disease Control and Prevention
- ELISA:
-
Enzyme-linked immunosorbent assay
- HBI:
-
Human blood index
- HBR:
-
Human biting rate
- LLINs:
-
Long-lasting insecticide-treated nets
- PBS:
-
Phosphate-buffered saline
References
WHO. World malaria report 2020: 20 years of global progress and challenges. 2020. https://www.who.int/publications/i/item/9789240015791. Accessed 08 Jan 2021.
WHO. World malaria report. 2019. https://www.who.int/publications/i/item/9789241565721. Accessed 05 June 2020.
WHO. Vector control technical expert group report to MPAC capacity building in entomology and vector control. 2013. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjBsuWrw4v7AhXGGzQIHXoMCS0QFnoECBcQAQ&url=https%3A%2F%2Fapps.who.int%2Firis%2Fbitstream%2Fhandle%2F10665%2F329951%2FWHO-HTM-GMP-2015-13-eng.pdf&usg=AOvVaw1esGwV_W5dy1e8M1Oj76Cx. Accessed 05 June 2020.
WHO. Guidance note on the control of residual malaria parasite transmission. 2014. https://apps.who.int/iris/handle/10665/338358. Accessed 05 June 2020.
WHO. Guidelines for malaria vector control. 2019. https://apps.who.int/iris/handle/10665/310862. Accessed 05 June 2022.
WHO. Global technical strategy for malaria 2016–2030. 2015. https://caribbean.eclac.org/publications/global-technical-strategy-malaria-2016-2030-june-2015. Accessed 05 June 2020.
WHO. Achieving the malaria MDG target: reversing the incidence of malaria 2000–2015. 2015. https://www.who.int/publications/i/item/9789241509442. Accessed 05 June 2020.
Ministry of Health. Kenya Malaria Indicator Survey 2020. 2021. https://dhsprogram.com/pubs/pdf/PR129/PR129pdf. Accessed 01 Aug 2021.
Ministry of Health. DHS program Kenya Malaria Indicator Survey 2021. 2021. https://dhsprogram.com/methodology/survey/survey-display-579.cfm. Accessed 14 May 2022.
Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors. 2013;6:114. https://doi.org/10.1186/1756-3305-6-114.
Forson AO, Hinne IA, Dhikrullahi SB, Sraku IK, Mohammed AR, Attah SK, et al. The resting behavior of malaria vectors in different ecological zones of Ghana and its implications for vector control. Parasit Vectors. 2022;15:1–14.
Sougoufara S, Diédhiou SM, Doucouré S, Diagne N, Sembène PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:1–7.
Sougoufara S, Harry M, Doucouré S, Sembène P, Sokhna C. Shift in species composition in the Anopheles gambiae complex after implementation of long-lasting insecticidal nets in Dielmo, Senegal. Med Vet Entomol. 2016;30:365–8.
Bamou R, Rono M, Degefa T, Midega J, Mbogo C, Ingosi P, et al. Entomological and anthropological factors contributing to persistent malaria transmission in Kenya, Ethiopia, and Cameroon. J Infect Dis. 2021;223:S155–70.
Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13. https://doi.org/10.1186/1475-2875-12-13.
Mustapha AM, Musembi S, Nyamache AK, Machani MG, Kosgei J, Wamuyu L, et al. Secondary malaria vectors in western Kenya include novel species with unexpectedly high densities and parasite infection rates. Parasit Vectors. 2021;14:1–11.
Lobo NF, Laurent BS, Sikaala CH, Hamainza B, Chanda J, Chinula D, et al. Unexpected diversity of Anopheles species in Eastern Zambia: implications for evaluating vector behavior and interventions using molecular tools. Sci Rep. 2015;5:17952.
Zhong D, Hemming-Schroeder E, Wang X, Kibret S, Zhou G, Atieli H, et al. Extensive new Anopheles cryptic species involved in human malaria transmission in western Kenya. Sci Rep. 2020;10:1–13.
Ayuya S, Nicholas K, Busula A, Webale MK, Omukunda EN. Detection of Plasmodium sporozoites in Anopheles coustani s.l.; a hindrance to malaria control strategies in highlands of western Kenya. bioRxiv. 2021. https://doi.org/10.1101/2021.02.10.430589.
Mwangangi JM, Mbogo CM, Nzovu JG, Githure JI, Yan G, Beier JC. Blood-meal analysis for anopheline mosquitoes sampled along the Kenyan coast. J Am Mosq Control Assoc. 2003;19:371–5.
Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62.
Lozano-Fuentes S, Kading RC, Hartman DA, Okoth E, Githaka N, Nene V, et al. Evaluation of a topical formulation of eprinomectin against Anopheles arabiensis when administered to Zebu cattle (Bos indicus) under field conditions. Malar J. 2016;15:324.
Sinka M, Pironon S, Massey N, Longbottom J, Hemingway J, Moyes C, et al. A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci USA. 2020;117:24900–8.
National Malaria Control Programme (NMCP). Kenya malaria operational plan, 2019. 2019. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiJmbSOvIv7AhU7BzQIHbB5B-QQFnoECBIQAQ&url=https%3A%2F%2Fwww.pmi.gov%2Ffy-2019-kenya-malaria-operational-plan%2F&usg=AOvVaw14jzhvjFyQtMfTMqE2CGPb. Accessed 05 June 2022.
Wilson D. Report on the Pare-Taveta Malaria Scheme, 1954–1959. East African Institute of Malaria and Vector-borne Diseases in collaboration with Colonial Pesticide Research Unit. Dar es Salaam: Government Printer; 1960.
Bradley DJ. Morbidity and mortality at Pare-Taveta, Kenya and Tanzania, 1954–66: the effects of a period of malaria control. In: Jamison DT, Feachem RG, Makgoba MW, Bos ER, Baingana FK, Hofman KJ, et al., editors. Disease and mortality in sub-Saharan Africa. Washington DC: World Bank; 1991. p. 248–63.
Dowling M. Malaria eradication scheme in Mauritius. BMJ. 1952;2:309.
Gillies MT, De Meillon B. The anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). Johannesburg: South African Institute for Medical Research; 1968.
Musapa M, Kumwenda T, Mkulama M, Chishimba S, Norris DE, Thuma PE, et al. A simple Chelex protocol for DNA extraction from Anopheles spp. J Vis Exp. 2013;71:e3281.
Laroche M, Almeras L, Pecchi E, Bechah Y, Raoult D, Viola A, et al. MALDI-TOF MS as an innovative tool for detection of Plasmodium parasites in Anopheles mosquitoes. Malar J. 2017;16:5.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Koekemoer L, Kamau L, Hunt R, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–11.
Echeverry DF, Deason NA, Makuru V, Davidson J, Xiao H, Niedbalski J, et al. Fast and robust single PCR for Plasmodium sporozoite detection in mosquitoes using the cytochrome oxidase I gene. Malar J. 2017;16:230.
Beier JC, Perkins PV, Wirtz RA, Koros J, Diggs D, Gargan TP, et al. Bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. J Med Entomol. 1988;25:9–16.
Munyao V, Karisa J, Munyao CM, Ngari M, Menza N, Peshu N, et al. Surveillance of culicine mosquitoes in six villages of Taita-Taveta County, Kenya, with host determinations from blood-fed females. J Med Entomol. 2020;57:1972-82 https://doi.org/10.1093/jme/tjaa109.
Karisa J, Muriu S, Omuoyo D, Karia B, Ngari M, Nyamwaya D, et al. Urban ecology of arboviral mosquito vectors along the Kenyan coast. J Med Entomol. 2021;58:428–38.
R Core Team. R: a language and environment for statistical computing. Version 4.0. 2 (Taking Off Again). Vienna: R Foundation for Statistical Computing; 2020.
Bashar K, Tuno N, Ahmed TU, Howlader AJ. False positivity of circumsporozoite protein (CSP)–ELISA in zoophilic anophelines in Bangladesh. Acta Trop. 2013;125:220–5.
Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, et al. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar J. 2011;10:1–9.
Mwangangi JM, Shililu J, Muturi EJ, Muriu S, Jacob B, Kabiru EW, et al. Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malar J. 2010;9:1–10.
Mutero CM, Blank H, Konradsen F, Van Der Hoek W. Water management for controlling the breeding of Anopheles mosquitoes in rice irrigation schemes in Kenya. Acta Trop. 2000;76:253–63.
Muturi EJ, Shililu J, Jacob B, Gu W, Githure J, Novak R. Mosquito species diversity and abundance in relation to land use in a riceland agroecosystem in Mwea, Kenya. J Vector Ecol. 2006;31:129–37.
Gimnig JE, Ombok M, Kamau L, Hawley WA. Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. J Med Entomol. 2001;38:282–8.
Nambunga IH, Ngowo HS, Mapua SA, Hape EE, Msugupakulya BJ, Msaky DS, et al. Aquatic habitats of the malaria vector Anopheles funestus in rural south-eastern Tanzania. Malar J. 2020;19:219. https://doi.org/10.1186/s12936-020-03295-5.
Antonio-Nkondjio C, Ndo C, Costantini C, Awono-Ambene P, Fontenille D, Simard F. Distribution and larval habitat characterization of Anopheles moucheti, Anopheles nili, and other malaria vectors in river networks of southern Cameroon. Acta Trop. 2009;112:270–6.
Ayala D, Costantini C, Ose K, Kamdem GC, Antonio-Nkondjio C, Agbor J-P, et al. Habitat suitability and ecological niche profile of major malaria vectors in Cameroon. Malar J. 2009;8:1–15.
Antonio-Nkondjio C, Kerah CH, Simard F, Awono-Ambene P, Chouaibou M, Tchuinkam T, et al. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol. 2006;43:1215–21.
Fornadel CM, Norris LC, Franco V, Norris DE. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector Borne Zoonotic Dis. 2011;11:1173–9.
Monroe A, Mihayo K, Okumu F, Finda M, Moore S, Koenker H, et al. Human behaviour and residual malaria transmission in Zanzibar: findings from in-depth interviews and direct observation of community events. Malar J. 2019;18:220.
Lyimo IN, Kessy ST, Mbina KF, Daraja AA, Mnyone LL. Ivermectin-treated cattle reduces blood digestion, egg production and survival of a free-living population of Anopheles arabiensis under semi-field condition in south-eastern Tanzania. Malar J. 2017;16:239.
Chaccour C, Hammann F, Rabinovich NR. Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety. Malar J. 2017;16:161. https://doi.org/10.1186/s12936-017-1801-4.
Chaccour C, Lines J, Whitty CJ. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J Infect Dis. 2010;202:113–6. https://doi.org/10.1086/653208.
Chaccour CJ, Hammann F, Alustiza M, Castejon S, Tarimo BB, Abizanda G, et al. Cytochrome P450/ABC transporter inhibition simultaneously enhances ivermectin pharmacokinetics in the mammal host and pharmacodynamics in Anopheles gambiae. Sci Rep. 2017;7:8535. https://doi.org/10.1038/s41598-017-08906-x.
Yao FA, Millogo A-A, Epopa PS, North A, Noulin F, Dao K, et al. Mark-release-recapture experiment in Burkina Faso demonstrates reduced fitness and dispersal of genetically-modified sterile malaria mosquitoes. Nat Commun. 2022;13:1–11.
Maia MF, Tenywa FC, Nelson H, Kambagha A, Ashura A, Bakari I, et al. Attractive toxic sugar baits for controlling mosquitoes: a qualitative study in Bagamoyo, Tanzania. Malar J. 2018;17:22.
Tenywa FC, Kambagha A, Saddler A, Maia MF. The development of an ivermectin-based attractive toxic sugar bait (ATSB) to target Anopheles arabiensis. Malar J. 2017;16:338. https://doi.org/10.1186/s12936-017-1994-6.
Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, Kravchenko VD, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar J. 2020;19:1–16.
Hammond A, Pollegioni P, Persampieri T, North A, Minuz R, Trusso A, et al. Gene-drive suppression of mosquito populations in large cages as a bridge between lab and field. Nat Commun. 2021;12:1–9.
Lloyd YM, Esemu LF, Antallan J, Thomas B, Tassi Yunga S, Obase B, et al. PCR-based detection of Plasmodium falciparum in saliva using mitochondrial cox3 and varATS primers. Trop Med Health. 2018;46:1–6.
Acknowledgements
The authors would like to acknowledge the technical assistance provided by Festus Yaah, Gabriel Nzai and Shida David in conducting the field sampling, sorting and morphological identification under the supervision and guidance of the field supervisor and principal investigator. We would also like to acknowledge the provision of the P. falciparum Sporozoite ELISA Reagent Kit (MRA-890), obtained through BEI Resources, NIAID and NIH, contributed by Robert A. Wirtz (CDC, Atlanta, GA, USA).
Funding
This study was funded by the Government of Kenya through Kenya Medical Research Institute Internal Research Grants (KEMRI-IRG) Grant Number INNOV/IRG/020/2. Jonathan Karisa is supported by UNITAID (BOHEMIA [Broad One-Health Endectocide-based Malaria Intervention in Africa]) and by the DELTAS Africa Initiative (DEL-15-003). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS) Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107769/Z/10/Z) and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
Author information
Authors and Affiliations
Contributions
JMM, JM, MR, SM and CM conceived this study. JMM and VM organized and supervised all of the field sampling of mosquitoes during the 3 years of the study. VM, JK and JMM supervised and assisted the field team in the sorting and morphological identification of the collected mosquitoes. JK supervised and performed DNA extraction and molecular identification of mosquito species into sibling species, assisted by KO, MT and ZO. The ELISA for sporozoite and blood-meal detection was performed by JK and VM. DNA extraction for sporozoite analysis by PCR was conducted by ZO and MT. Reconfirmation of Plasmodium positivity by PCR was performed by KO, ZO, BB and MT. MR and CW provided technical guidance on all the laboratory procedures. Statistical analysis was done by JK, LB, KM under the guidance of MM and JMM. JK and KO drafted the first version of the manuscript. SM, MM, and CW reviewed the first draft of this manuscript. NP, SM, JM, and CM offered scientific advisory leadership and reviewed/critiqued this manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate.
The study obtained ethical approval from Kenya Medical Research Institute (KEMRI) Scientific and Ethics Review Unit (SERU) (KEMRI/SERU/CGMRC/035/3219) for implementation. Verbal consent was obtained from the local administration including chiefs, sub-chiefs, and village elders before commencing this study. Verbal informed consent was obtained from household heads prior to setting up of traps for sample collection.
Consent for publication
All the authors have reviewed and approved the publication of this paper. This paper has been published with the permission of the Director of the Kenya Medical Research Institute (KEMRI).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Karisa, J., Ominde, K., Muriu, S. et al. Malaria vector bionomics in Taita-Taveta County, coastal Kenya. Parasites Vectors 15, 430 (2022). https://doi.org/10.1186/s13071-022-05527-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05527-w