Abstract
Background
Insecticide-based vector control interventions in combination with case management with artemisinin-based combination therapy has reduced malaria incidence and prevalence worldwide. Current control methods focus on the primary malaria vectors, Anopheles gambiae sensu lato (s.l.) and the An. funestus group; however, the impact of secondary and suspected vectors has been either sidelined or received limited attention. Defining the susceptibility of secondary, suspected vector species to different parasites in time and space is essential for efficient malaria control and elimination programs. The aim of this study was to assess the susceptibility of An. gambiae s.l., An. coustani complex and An. pharoensis to Plasmodium vivax and P. falciparum infection in Ethiopia.
Methods
Larvae of Anopheles spp. were collected from different aquatic habitats and reared to adults under laboratory conditions, with the temperature and humidity maintained at 27 ± 1 °C and 75 ± 5%, respectively. Adult female mosquitoes were identified to species as An. gambiae s.l., An. coustani complex and An. pharoensis. Females of these three Anopheles spp. were allowed to feed in parallel feeding assays on infected blood containing the same gametocytes isolated from P. falciparum and P. vivax gametocyte-positive patients by indirect membrane feeding assays. All blood-fed mosquitoes were held under laboratory conditions. After 7 days, all surviving mosquitoes were dissected to detect mid-gut oocyst and enumerated under a microscope.
Results
Of 5915 female Anopheles mosquitoes exposed to gametocyte-infected blood, 2106 (35.6%)s fed successfully in the 32 independent infection experiments. There was a significant variation in feeding rates among An. gambiae s.l., An. pharoensis and An. coustani complex (G-test = 48.43, P = 3.049e-11). All three exposed mosquito species were receptive to P. vivax and P. falciparum infection development. The percentage of infected mosquitoes following feeding on an infected blood meal was significantly different among species (G-test = 6.49, P = 0.03886). The median infection intensity (II) for An. coustani complex, An. gambiae s.l. and An. pharoensis was 1.16, 2.00 and 1.25, respectively. Although the proportion of infected mosquitoes significantly differed in terms of II, infection rate (IR) and mean oocyst density among the species, mean oocyst density and IR were highly correlated with gametocyte density in all tests (P < 0.001).
Conclusion
Primary, secondary and suspected vectors were experimentally susceptible to both P. vivax and P. falciparum infection. An effective malaria elimination program might include surveillance and control tools which target secondary and suspected vectors that might play an outdoor transmission role, possibly resulting in reduced focal malaria transmission.
Graphical Abstract

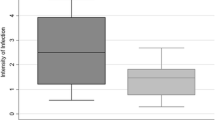
Comparison of the three species’ mean infection rates with standard deviation
Similar content being viewed by others
Background
Malaria continues to be a major public health challenge worldwide, with 241 million estimated cases and 627,000 deaths in 2020, of which 96% were reported from the WHO African region [1]. In Ethiopia, despite a considerable decline of malaria morbidity in recent years [1,2,3], the disease remains a focus of public health concern, with 1.8 million malaria cases reported in 2020 [1]. In sub-Saharan Africa, Plasmodium vivax and Plasmodium falciparum almost consistently co-occur and—in the epidemiological context—are equally important, although the latter is the most virulent of the two species [4]. In the last decades, most disease cases were attributed to P. falciparum, which is responsible for 90% of malaria-related deaths. In recent years, however, P. vivax has been reported to cause malaria complications among children in endemic regions [4,5,6].
In Ethiopia, there are more than 47 documented species of Anopheles mosquitoes [7, 8] of which Anopheles arabiensis, An. pharoensis, An. funestus and An. nili are recognized malaria vectors. The primary malaria vector is An. arabiensis while An. pharoensis, An. funestus and An. nili are secondary vectors that occur at varying densities, with limited distribution and vector competency [9, 10]. More recently, a new invasive Anopheles species, An. stephensi, has been documented in the country [11]. Populations of Anopheles mosquitoes, such as An. arabiensis, An. amharicus, An. pharoensis, An. coustani, An. nilli, An. funestus group and An. squamouse, usually co-occur in the East Wollega zone, western Ethiopia [12,13,14]. However, An. arabiensis, An. amharicus, An. coustani and An. pharoensis populations occur abundantly both in the rainy and dry season in western parts of Ethiopia [12].
Environmental modifications and water development projects may increase the risk of malaria transmission by contributing to the formation of additional breeding habitats with changes in micro-weather conditions and micro-ecological settings [15, 16]. Malaria distribution in different ecological settings is largely governed by the spatial and temporal distribution of malaria vectors [9]. The construction of irrigation schemes creates additional aquatic habitats, modifying the ecology of the area to conditions favoring mosquito breeding [17]. Such ecological change may also lead to shifts in mosquito fauna, distribution, abundance, vector diversity and proliferation [12, 13].
During the dry season, malaria transmission in irrigated areas and areas resulting from dam construction is supported by primary vectors, even though the abundance of secondary vectors is also relatively high [12]. The contribution of secondary and suspected vectors has not been well studied. However, the abundance and distribution of secondary and suspected vectors has increased considerably over the years [18], with modifications to environments creating new larval habitats [19]. However, the impact of this changed ecological setting due to the introduction of irrigation schemes and sugar plantations on the bionomics of Anopheles mosquitoes and their vector competence is not well studied in these areas.
In Ethiopia, studies on the role of secondary vectors are limited or missing entirely, possibly due to the extremely low infection rate (IR) or because no infections were reported from the field-collected adult mosquitoes that made up most of the collections [20, 33]. In addition, existing vector surveillance methods mainly focus on indoor resting and indoor biting species, with prioritization of the anthropophagic behavior and vectorial capacity of the primary vector [21, 22]. Data on secondary vectors is limited, and findings from the limited number of studies currently available indicate that most secondary vectors do have a short survival rate, with a natural mortality rate likely to be around 50–60% per gonotrophic cycle [23, 24]. However, experimental vector susceptibility studies that evaluate the vector capacity of suspected mosquito species are limited in Ethiopia [32]. Studies conducted in Ethiopia and the neighboring country of Kenya on wild-caught adult mosquitoes indicate that An. coustani is susceptible to Plasmodium infection [18, 33, 37]. Defining the receptiveness of Anopheles spp. to Plasmodium spp. in time and space is important in vector control programs, but few experimental infection studies on secondary and suspected vectors over both time and space have been conducted in Ethiopia. Therefore, the aim of the study reported here was to assess the susceptibility of Anopheles gambiae sensu lato (s.l.), the An. coustani complex and An. pharoensis to P. vivax and P. falciparum infection using indirect membrane feeding assay at the Arjo-Didessa irrigation scheme, south-western Ethiopia.
Methods
Study area
The study was conducted on the Arjo-Didessa sugarcane plantation irrigation scheme, southwest Ethiopia (8°41′35.5″N 36°25′54.9″E), located 575 km southwest of the capital city of Ethiopia, Addis Ababa. The altitude ranges from 1300 to 2280 m a.s.l., with a mean annual rainfall of 1477 mm that is distributed over a short rainy season between February and April and a long rainy season between June and September, corresponding to the low and high peak transmission seasons, respectively. The soil type at the Arjo-Didessa sugarcane plantation site is clay and clay loam with low porousness. Due to this slow rate of percolation, rainwater can accumulate in the top layers of the soil and form swamps in the area [25], creating a wide range of breeding sites for malaria vector mosquitoes. The study site is in a malaria-endemic area, and P. falciparum and P. vivax are the principal malaria parasite species responsible for the majority of the infections. Various Anopheles mosquitoes, including An. arabiensis, An. amharicus, An. pharoensis, An. coustani complex and An. squamous, occur in this area [12, 14].
Mosquito collection, rearing and identification
Anopheles larvae and pupae were collected from natural breeding habitats of the irrigation clusters and surrounding villages, and blood-fed adult and gravid mosquitoes for egg-laying were collected at dawn from human dwellings and animal indoor resting sites (sheds) using mouth aspirators. Field-collected adult mosquitoes were only used for oviposition purposes. Larvae and pupae were collected using a standard dipper (350 ml; BioQuip Products, Inc., Compton, CA, USA), immediately transported to the International Centre of Excellence for Malaria Research (ICEMR) field laboratory and reared to adults in enamel trays (27 × 16 × 6.5 cm). Immediately after collection, mosquito larvae were filtered remove competitors, predators and unwanted debris, transported to the insectary in water taken from the mosquito’s normal breading aquatic habitat and reared to adult stages on fish food (TetraMin; Tetra Werke, Melle, Germany). The three species under study were sorted and kept separately in different cages; all cages were provided daily with a 10% sucrose solution as a source of energy. Adult mosquitoes collected from the field were transferred for oviposition and rearing processes [26]. The eggs laid on filter papers were washed onto enamel trays and allowed to stand until emergence. Larvae were fed with fish food (TetraMin Baby; Tetra Werke). Pupae were transferred to cups containing water and allowed to develop to adults inside cages. The emerged adults were fed on cotton wool soaked in 10% sucrose solution. Rearing was done under laboratory conditions (25 ± 2 °C, 70% ± 10% relative humidity) [27].
Patient recruitment and sample collection
Persons suspected of having malaria, were febrile and had visited health facilities were eligible for enrollment in the study. As a first step, blood was obtained by finger prick from febrile patients who were screened for malaria infection at any of five health facilities (Arjo Diddessa Sugar Factory clinic, Abote Didessa Health Post, Kerka Health Post, Command Two Health Post and Command Five Heath Post), and examined as Giemsa-stained blood smear. Among these patients who tested positive for malaria, individuals diagnosed with malaria at the stage in which the parasite was circulating in the blood at the gametocyte stage were asked to consent to be enrolled in the study. Patients who refused to consent, had severe illness and/or were mentally sick, as well as those who had taken anti-malaria drugs within 2 weeks prior to screening were excluded from the study. All patients who agreed to participate in the study were transferred to the ICEMR Laboratory where venous blood was sampled and collected into heparin tubes [28].
Samples used for the membrane feeding assay were double screened (first for Plasmodium infection, then for the presence of gametocytes in the blood). Parasite and gametocyte densities were the blood smears were determined per leukocyte and assuming 8000 leukocytes/μl of blood. The sexual and asexual stages of the parasite were determined per leukocyte, against 500 and 200 leukocytes/μl, respectively. The time when the patient carrying gametocytes was available was not known; however, mosquitoes were always available for the membrane feeding assays to perform experimental infection on the same day. All persons who had fever during the physical examination and who tested positive for malaria parasites on the blood film examination were treated immediately as per the national malaria treatment guidelines [29].
Indirect membrane feeding assay
For the indirect membrane feeding assay (IDMFA), adult female mosquitoes aged 3–5 days were starved 8–12 h prior to membrane feeding as this was believed to increase the blood-feeding activity of the mosquitoes. The mosquitoes were provided with infected blood from patients who had tested positive for P. falciparum or P. vivax for a period of 30–60 min via an artificial membrane feeding apparatus; micro-glass feeders were used for experimental mosquito infection. Glass feeders were closed with membrane and placed on top of paper cups covered the meshes size (8.5/11 cm, diameter/depth) with starved mosquitoes. During the feeding process, the temperature was kept constant at 37 ± 0.1 °C using a high-speed water circulation system-connected to the glass feeders, which allowed the mosquitoes of the three species to feed in parallel and up to 450 mosquitoes to feed at one time [30].
An artificial membrane feeding apparatus with a water pump (model 8005; PolyScience, Niles, IL, USA) was connected to the nine glass feeders, and each feeder was filled with 200 μl of freshly drawn infected blood using a blunt needle. The starved mosquitoes in the paper cups enclosed within a mesh were then allowed to feed on infected blood from the same source for a period of 30–60 min under reduced light conditions. A total of nine paper cups were used in a single experiment; in each paper cup 50 starved mosquitoes, with three replicates for each species and three species, were allowed to feed in parallel on the same infected blood source [30]. Feeding success was measured after 30 min, at which time the glass feeders were detached and unfed, partially fed mosquitoes were killed and discarded. Fully fed mosquitoes were maintained at 25 ± 2 °C and 70 ± 10% relative humidity. Until day 8 post-feeding, infected mosquitoes were provided with cotton wool soaked in 10% sucrose solution.
Mosquito dissection
Eight days after feeding on infected blood, all experimentally infected and surviving mosquitoes were immobilized and dissected, and then examined for oocyst development on the midgut and enumerated. Midguts were removed, and oocyst counting was performed by examining the wet mount midgut stained with a 5% mercurochrome solution [30, 31]. Oocysts from the midgut were examined and enumerated under a microscope with a 40× objective. For each infection assay, the infection intensity (II) and IR were determined.
Data analysis
The R statistical software package version (4.2.0) was employed to analyze data. The P. vivax and P. falciparum IR for An. gambiae s.l., An. coustani complex and An. pharoensis was assessed by the presence of oocysts in the midguts. The IR was calculated as IR = \(\frac{{\text{oocyst positive mosquitoes}} }{{\text{total mosquitoes dissected in individual feeding}} } \times 100\). Similarly, the feeding rate was calculated as FR = \(\frac{{\text{Fed mosquitoes}}}{{\text{total exposed}}} \times 100.\)
The FR and infection frequency between Anopheles spp. were compared using the G-test; species pairwise comparisons were also computed. The overall II and IR were compared between populations of An. gambiae s.l., An. coustani complex and An. pharoensis using Pearson’s Chi-square test and the Kruskal–Wallis test, respectively. Gametocyte density with IR and mean oocyst number were correlated using Spearman’s rank correlation test.
Results
Anopheles mosquito exposure, feeding, survival and IR by the IDMFA
Overall, 586 febrile patients were screened for malaria during the study period. Of these, 94 individuals were unwilling to participate and 57 had taken anti-malarial drugs within the 48 h prior to sample collection and were excluded. Malaria screening confirmed that 125 patients had malaria, of whom 54 (43.2%) were infected with P. vivax and 71 (56.8%) with P. falciparum. Of these 54 and 71 patients, 40 (74%) and 19 (26.7%), respectively, were confirmed by microscopic examination of the blood smears to be gametocyte carriers.
Although all persons confirmed to be gametocyte carriers satisfied the inclusion criteria and were enrolled in the experimental infection study, only 32 parallel successful mosquito feeding assays were considered for analysis. Of these, 32 feeding experiments, 24 (68.6%) were infected with P. vivax and eight (31.4%) were infected with P. falciparum. The three mosquito species differed in feeding time, with An. gambiae s.l. feeding quickly (35% of individuals fully engorged in 30 min) and An. pharoensis and An. coustani complex feeding more slowly (< 35% fully engorged in approx. 40–60 min.)
Of the 5915 adult female mosquitoes allowed to feed on infected blood, 2106 (35.6%) fed successfully in the 32 independent parallel experimental infections. The feeding rate for An. gambiae s.l., An. pharoensis and An. coustani complex was 39.7% (1025 fed mosquitoes), 35.7% (594 fed mosquitoes) and 29.2% (487 fed mosquitoes), respectively. The proportion of fed mosquitoes after exposure to an infected blood meal varied significantly among species (G-test = 48.43, df = 2, P = 3.049e-11) (Table 1). The percentage of infected mosquitoes following feeding on an infected blood meal was significantly different among species (G-test = 6.4955, df = 2, P = 0.039) (Table 1).
Overall, pairwise comparison showed that only significantly different IR was between An. gambiae s.l. (61.1%) and An. coustani complex (56.8%) (P < 0.01) (Table 1). For all other comparisons, the differences were non-significant.
Measurement of II by the IDMFA
The mean II was significantly different among An. gambiae s.l., An. pharoensis and An. coustani complex (χ2 = 10.709, df = 2, P < 0.005) (Fig. 1). However, pairwise comparisons using the Wilcoxon test indicated that the differences were only significant between An. gambiae s.l. and An. coustani complex (P < 0.005). The median II for the An. coustani complex was 1.16 compared to 2.00 for An. gambiae s.l. and 1.25 for An. pharoensis.
Relationship between gametocyte abundance and oocyst density
There was a significant correlation between gametocyte and mean oocyst abundances (P < 0.001). Correlation comparison between gametocyte density and mean oocyst density showed a positive linear relationship although the association among species varied: An. gambiae s.l. (r = 0.65, P < 0.001), An. pharoensis (r = 0.66, P < 0.001) and An. coustani complex (r = 0.76, P < 0.001) (Fig. 2).
A positive correlation between the gametocytes/500 leukocytes and the IR of the mosquitoes was observed. However, the association varied among species: An. gambiae s.l. (r = 0.46, P < 0.01), An. pharoensis (r = 0.64, P < 0.001) and An. coustani complex (r = 0.44, P < 0.01) (Fig. 3).
Discussion
Mosquitoes belonging to the Anopheles coustani complex showed susceptibility for the development of P. falciparum and P. vivax parasites in experimental infection assays. However, An. gambiae s.l. and An. pharoensis had a significantly higher IR than the An. coustani complex, which is suspected to be a malaria vector in Ethiopia. In addition, all three species showed high mean oocyst density, with the highest oocyst density found in An. gambiae s.l., which is one of the main malaria vector species in sub-Saharan Africa. The results of some studies in a number of countries conducted under natural conditions indicate that An. coustani complex supports the development of both P. falciparum and P. vivax [33, 34]. In contrast to our results, results from similar experimental studies conducted in Jimma town, southwest Ethiopia, indicated that An. coustani complex is not susceptible to P. vivax development [32]. This discrepancy might be due to differences in larval breading sites and the presence of different member species of An. coustani complex: i.e. different sibling species may be present in different parts of the country.
Defining the susceptibility of anopheline species to malaria parasites in time and space is vital for designing efficient vector control and elimination programs [31,32,33]. In this study, we documented a difference in susceptibility among the three species tested. The rate of mosquito infection is affected by various factors, such as environmental, biological and behavioral factors [19, 35, 36]. Anopheles coustani complex is a suspected vector in Ethiopia [33, 34]. Immunological studies using wild catch mosquitoes of An. coustani in different countries in sub-Sharan Africa have shown its susceptibility to P. falciparum and P. vivax infection [33,34,35,36,37,38,39,40].
The susceptibility of An. gambiae s.l. and An. pharoensis to infection by the malaria parasites P. vivax and P. falciparum has long been established [41]. A growing body of evidence, including this study, confirms the susceptibility of An. coustani complex to both P. vivax and P. falciparum from Ethiopia, Kenya, Zambia, Democratic Republic of Congo and Cameroon [18, 33, 36, 38, 42] where field populations were susceptible [33, 34, 43].
Vector competence varies from species to species, and there is usually a high proportion of uninfected mosquitoes when the infection process is controlled. This has been confirmed in studies conducted in Ethiopia and elsewhere [32, 44, 45]. On the other hand, the IR of the three species examined in the present study are much higher in experimental studies than under condition of natural infection. As indicated by studies employing enzyme-linked immunosorbent assays, based on the use of species-specific anti-sporozoite monoclonal antibodies, anopheline populations have varied IRs in sub-Saharan countries. For example, it has been reported that the IR of An. gambiae s.l ranges from 0.3% to 9.3%, that of An. pharoensis from 0.4% to 5.2% and that of An. coustani complex from 0.3% to 1.81% [18, 33, 46].
Gametocyte density has been found to be significantly correlated with IR. In our study, irrespective of the differences in species, we found that Plasmodium gametocyte density was significantly correlated to IR and mean oocyst density. This is also true in other studies conducted in Manaus, in the western Brazilian Amazon and Bengbu, Anhui Province, central China [44, 45].
Infected blood with equivalent gametocyte density was provided to the three species of mosquitoes, but variable susceptibility was observed. This discrepancy in IR might be due to different factors, such as gametocyte maturity, gametocyte sex ratio, different Plasmodium genotypes, rhythms in the density and infectivity of transmission forms (gametocytes), immune factors in patient sera and/or mosquitoes’ innate immunity. All of these factors could alter gametocyte infectivity [47,48,49,50]. In addition, recent studies indicate that sub-microscopic gametocyte density is capable of infecting mosquitoes, which demonstrates that rather than gametocyte density, the above contributing factors play a role in the variability of the IR and mean oocyst density. Studies from different countries show that sub-microscopic infections might be the major contributor to malaria transmission [51,52,53,54], suggesting that gametocyte density is not the only factor that determines infection in mosquitoes.
Furthermore, secondary, and suspected vectors, such as An. pharoensis and An. coustani complex, have a relatively higher abundance during the dry season [12]. This relatively higher abundance in the dry season may be an important factor in terms of local malaria transmission it may help to increase or prolong the malaria transmission period [55]. Secondary vectors are most often outdoor biting and outdoor resting mosquitoes [46, 56]. The role of outdoor-resting Anopheles in malaria transmission is important as these secondary vectors contribute to malaria transmission in Africa, and their role in transmission is not negligible [56, 57]. Most secondary vectors have a short survival rate, with 50–60% natural mortality rates per gonotrophic cycle [23, 24]. This might be the reason that the population density of the secondary vector has been low in many settings.
Most Anopheles vectors found in nature have only a few oocysts, and oocysts are of little importance in malaria epidemiology. Rather, it is the outdoor biting and resting behavior of mosquitoes in nature that contribute to residual malaria transmission in different parts of sub-Saharan Africa and which pose new challenges as they cannot be consistently controlled using current tools. The proportion of outdoor biting mosquitoes has been reported to have increased by 10% [58,59,60]. More recent studies in sub-Saharan Africa have indicated that An. pharoensis and An. coustani complex have likely become primary role in malaria transmission, possibly because existing interventions target primary vectors only to achieve complete malaria control [61].
Conclusions
In the present study, we confirmed that primary, secondary and suspected vectors are receptive for the development of P. falciparum and P. vivax in this experimental infection model using IDMFA. For effective malaria elimination, there should be a focus on secondary and suspected vectors to avoid focal malaria transmission or limit residual malaria transmission. In addition, it is highly recommended that current vector surveillance and control tools be re-considered to target secondary and suspected vectors that may have an outdoor transmission role. In addition, further investigation of the ecology and dynamics of immature stages can improve our understanding of the bionomics of the receptiveness of these mosquitoes to Plasmodium development.
Availability of data and materials
The datasets used in this study are available upon reasonable request from the corresponding author.
Abbreviations
- ICEMR:
-
International Center Excellence for Malaria Research
- IDMFA:
-
Indirect membrane feeding assay
- II:
-
Infection intensity
- IR:
-
Infection rate
References
WHO. World Malaria Report 2021. 2021. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Accessed Feb 2021.
Deribew A, Dejene T, Kebede B, Tessema GA, Melaku YA, Misganaw A, et al. Incidence, prevalence and mortality rates of malaria in Ethiopia from 1990 to 2015: Analysis of the global burden of diseases 2015. Malar J. 2017;16:1–7.
Taffese HS, Hemming-Schroeder E, Koepfli C, Tesfaye G, Lee MC, Kazura J, et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty. 2018;7:1–9.
Saravu K, Rishikesh K, Kamath A, Shastry AB. Severity in Plasmodium vivax malaria claiming global vigilance and exploration—a tertiary care centre-based cohort study. Malar J. 2014;13:1–10.
Alexandre MA, Ferreira CO, Siqueira AM, Magalhães BL, Mourão MPG, Lacerda MV, et al. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis. 2010;16:1611–4.
Ketema T, Bacha K. Plasmodium vivax associated severe malaria complications among children in some malaria endemic areas of Ethiopia. BMC Public Health. 2013;13:1–7.
Gaffigan TV, Wilkerson RC, Pecor JE, Stoffer JA, Anderson T. Systematic catalog of Culicidae. 2018. Silver Spring: Walter Reed Army Institute of Research. http://www.mosquitocatalog.org. Accessed 17 Dec 2021.
Irish SR, Kyalo D, Snow RW, Coetzee M. Updated list of Anopheles species (Diptera: Culicidae) by country in the Afrotropical Region and associated islands. Zootaxa. 2020;4747:401.
Abeku TA, Van Oortmarssen GJ, Borsboom G, de Vlas SJHJ. Spatial and temporal variations of malaria epidemic risk in Ethiopia: factors involved and implications. Acta Trop. 2003;87:331–40.
White GB. Malaria vector ecology and genetics. Br Med Bull. 1982;38:207–12.
Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018;188:180–6.
Hawaria D, Demissew A, Kibret S, Lee MC, Yewhalaw D, Yan G. Effects of environmental modification on the diversity and positivity of Anopheline mosquito aquatic habitats at Arjo-Dedessa irrigation development site, Southwest Ethiopia. Infect Dis Poverty. 2020;9:1–11.
Jaleta KT, Hill SR, Seyoum E, Balkew M, Gebre-Michael T, Ignell R, et al. Agro-ecosystems impact malaria prevalence: large-scale irrigation drives vector population in western Ethiopia. Malar J. 2013;12:1–11.
Demissew A, Hawaria D, Kibret S, Animut A, Tsegaye A, Lee MC, et al. Impact of sugarcane irrigation on malaria vector Anopheles mosquito fauna, abundance and seasonality in Arjo-Didessa, Ethiopia. Malar J. 2020;19:1–8.
Baeza A, Bouma M, Dobson A, Dhiman R, Srivastava H, Pascual M. Climate forcing and desert malaria: the effect of irrigation. Malar J. 2011;10:1–10.
Keiser J, De Castro MC, Maltese MF, Bos R, Tanner M, Singer BH, et al. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am J Trop Med Hyg. 2005;72:392–406.
Ageep TB, Cox J, Hassan MM, Knols BG, Benedict MQ, Malcolm CA, et al. Spatial and temporal distribution of the malaria mosquito Anopheles arabiensis in northern Sudan: influence of environmental factors and implications for vector control. Malar J. 2009;8:1–14.
Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors. 2013;6:1–9.
Okech BA, Gouagna LC, Yan G, Githure JI, Beier JC. Larval habitats of Anopheles gambiae s.s. (Diptera: Culicidae) influences vector competence to Plasmodium falciparum parasites. Malar J. 2007;6:1–7.
Kibret S, Wilson GG, Ryder D, Tekie H, Petros B. Malaria impact of large dams at different eco-epidemiological settings in Ethiopia. Trop Med Health. 2017;45:1–4.
Seyoum A, Sikaala CH, Chanda J, Chinula D, Ntamatungiro AJ, Hawela M, et al. Human exposure to Anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, South-east Zambia. Parasit Vectors. 2012;5:1–10.
Killeen GF, Chaki PP, Reed TE, Moyes CL, Govella NJ. Entomological surveillance as a cornerstone of malaria elimination: a critical appraisal. In: Manguin S, Vas D, editors. Towards malaria elimination-a leap forward. London: IntechOpen; 2018. p. 403–29.
Gillies MT, Wilkes TJ. Observations on nulliparous and parous rates in a population of Anopheles funestus in East Africa. Ann Trop Med Parasitol. 1963;57:204–13.
Gillies MT. Observations on nulliparous and parous rates in some common East African mosquitoes. Ann Trop Med Parasitol. 1963;57:435–42.
Ethiopian Corporation. Arjo Dediessa Sugar Factory. 2020. https://www.ethiopiansugar.com/blog/arjo-dediessa-sugar-factory. Accessed 23 Aug 2021.
US Centers for Disease Control and Prevention (CDC). Methods in Anopheles research. MR4 methods in Anopheles research laboratory manual Atlanta: CDC.
Das S, Dimopoulos G. Molecular analysis of photic inhibition of blood-feeding in Anopheles gambiae. BMC Physiol. 2008;8:1–19.
Soumare HM, Guelbeogo WM, van de Vegte-Bolmer M, van Gemert GJ, Soumanaba Z, Ouedraogo A, et al. Maintaining Plasmodium falciparum gametocyte infectivity during blood collection and transport for mosquito feeding assays in the field. Malar J. 2021;20:1–9.
Federal Ministry of Health. National malaria guidelines, fourth edition. 2017. https://e-library.moh.gov.et/library/wp-content/uploads/2021/07/NATIONAL-MALARIA-GUIDELINES-UPDATED-JULY-3.pdf
Ouedraogo AL, Guelbéogo WM, Cohuet A, Morlais I, King JG, Gonçalves BP, et al. A protocol for membrane feeding assays to determine the infectiousness of P. falciparum naturally infected individuals to Anopheles gambiae. Malar World J. 2013;4:17–20.
Rios-Velasquez CM, Martins-Campos KM, Simoes RC, Izzo T, Dos Santos EV, Pessoa FA, et al. Experimental Plasmodium vivax infection of key Anopheles species from the Brazilian Amazon. Malar J. 2013;12:1–10.
Abduselam N, Zeynudin A, Berens-Riha N, Seyoum D, Pritsch M, Tibebu H, et al. Similar trends of susceptibility in Anopheles arabiensis and Anopheles pharoensis to Plasmodium vivax infection in Ethiopia. Parasit Vectors. 2016;9:1–9.
Haileselassie W, Zemene E, Lee MC, Zhong D, Zhou G, Taye B, et al. The effect of irrigation on malaria vector bionomics and transmission intensity in western Ethiopia. Parasit Vectors. 2021;14:1–11.
Yewhalaw D, Kelel M, Getu E, Temam S, Wessel G. Blood meal sources and sporozoite rates of Anophelines in Gilgel-Gibe dam area, Southwestern Ethiopia. https://www.researchgate.net/publication/292615632. Accessed 8 Mar 2022.
Fornadel CM, Norris LC, Glass GE, Norris DE. Analysis of Anopheles arabiensis blood feeding behavior in southern zambia during the two years after introduction of insecticide-treated bed nets. Am J Trop Med Hyg. 2010;83:848–53.
Fornadel CM, Norris LC, Franco V, Norris DE. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector-Borne Zoonotic Dis. 2011;11:1173–9.
Gillies MT. The role of secondary vectors of malaria in North-East Tanganyika. Trans R Soc Trop Med Hyg. 1964;58:154–8.
Antonio-Nkondjio C, Kerah CH, Simard F, Awono-Ambene P, Chouaibou M, Tchuinkam T, et al. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol. 2006;43:1215–21.
Ciubotariu II, Jones CM, Kobayashi T, Bobanga T, Muleba M, Pringle JC, et al. Genetic Diversity of Anopheles coustani (Diptera: Culicidae) in malaria transmission foci in Southern and Central Africa. J Med Entomol. 2020;57:1782–92.
Goupeyou-Youmsi J, Rakotondranaivo T, Puchot N, Peterson I, Girod R, Vigan-Womas I, et al. Differential contribution of Anopheles coustani and Anopheles arabiensis to the transmission of Plasmodium falciparum and Plasmodium vivax in two neighbouring villages of Madagascar. Parasit Vectors. 2020;13:1–6.
Nigatu WO, Abebe MA, Dejene AM. Plasmodium vivax and P. falciparum epidemiology in Gambella, south-west Ethiopia. Trop Med Parasitol. 1992;43:181–5.
Hendershot AL. Understanding the role of An. coustani complex members as malaria vector species in the Democratic Republic of Congo. PhD dissertation. Notre Dame: University Of Notre Dame; 2021.
Degefa T, Yewhalaw D, Zhou G, Lee MC, Atieli H, Githeko AK, et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malar J. 2017;16:1–13.
Martins-Campos KM, Kuehn A, Almeida A, Duarte APM, Sampaio VS, Rodriguez IC, et al. Infection of Anopheles aquasalis from symptomatic and asymptomatic Plasmodium vivax infections in Manaus, western Brazilian Amazon. Parasit Vectors. 2018;11:1–11.
Zhu G, Xia H, Zhou H, Li J, Lu F, Liu Y, et al. Susceptibility of Anopheles sinensis to Plasmodium vivax in malarial outbreak areas of central China. Parasit Vectors. 2013;6:1–9.
Nepomichene TNJJ, Tata E, Boyer S. Malaria case in Madagascar, probable implication of a new vector, Anopheles coustani. Malar J. 2015;14:1–8.
Robert V, Read AF, Essong J, Tchuinkam T, Mulder B, Verhave JP, et al. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans R Soc Trop Med Hyg. 1996;90:621–4.
Mitri C, Thiery I, Bourgouin C, Paul REL. Density-dependent impact of the human malaria parasite Plasmodium falciparum gametocyte sex ratio on mosquito infection rates. Proc R Soc B Biol Sci. 2009;276:3721–6.
Lambrechts L, Halbert J, Durand P, Gouagna LC, Koella JC. Host genotype by parasite genotype interactions underlying the resistance of Anopheline mosquitoes to Plasmodium falciparum. Malar J. 2005;4:1–8.
Schneider P, Rund SSC, Smith NL, Prior KF, O’Donnell AJ, Reece SE. Adaptive periodicity in the infectivity of malaria gametocytes to mosquitoes. Proc R Soc B Biol Sci. 2018;285:20181876.
Schneider P, Bousema JT, Gouagna LC, Otieno S, Van De Vegte-Bolmer M, Omar SA, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76:470–4.
Zemene E, Koepfli C, Tiruneh A, Yeshiwondim AK, Seyoum D, Lee MC, et al. Detection of foci of residual malaria transmission through reactive case detection in Ethiopia 11 medical and health sciences 1108 medical microbiology. Malar J. 2018;17:1–10.
Jiram AI, Ooi CH, Rubio JM, Hisam S, Karnan G, Sukor NM, et al. Evidence of asymptomatic submicroscopic malaria in low transmission areas in Belaga district, Kapit division, Sarawak, Malaysia. Malar J. 2019;18:1–12.
Ouedraogo AL, Gonçalves BP, Gnémé A, Wenger EA, Guelbeogo MW, Ouédraogo A, et al. Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis. 2016;213:90–9.
Wanji S, Tanke T, Atanga SN, Ajonina C, Nicholas T, Fontenille D. Anopheles species of the mount Cameroon region: biting habits, feeding behaviour and entomological inoculation rates. Trop Med Int Heal. 2003;8:643–9.
Burke A, Dahan-Moss Y, Duncan F, Qwabe B, Coetzee M, Koekemoer L, et al. Anopheles parensis contributes to residual malaria transmission in South Africa. Malar J. 2019;18:1–7.
Hoffman J. Investigating the biology and behavior of Anopheles Squamosus and its role in residual malaria. PhD dissertation. Baltimore: Johns Hopkins University; 2019.
Marrelli MT, Honório NA, Flores-Mendoza C, Lourenço-de-Oliveira R, Marinotti O, Kloetzel JK. Comparative susceptibility of two members of the Anopheles oswaldoi complex, An. oswaldoi and An. konderi, to infection by Plasmodium vivax. Trans R Soc Trop Med Hyg. 1999;93:381–4.
Adak T, Singh OP, Das MK, Wattal S, Nanda N. Comparative susceptibility of three important malaria vectors Anopheles stephensi, Anopheles fluviatilis, and Anopheles sundaicus to Plasmodium vivax. J Parasitol. 2005;91:79–82.
Bamou R, Rono M, Degefa T, Midega J, Mbogo C, Ingosi P, et al. Entomological and anthropological factors contributing to persistent malaria transmission in Kenya, Ethiopia, and Cameroon. J Infect Dis. 2021;223:155–70.
Mustapha AM, Musembi S, Nyamache AK, Machani MG, Kosgei J, Wamuyu L, et al. Secondary malaria vectors in western Kenya include novel species with unexpectedly high densities and parasite infection rates. Parasit Vectors. 2021;14:1–11.
Acknowledgements
We would like to acknowledge the Arjo-Didessa sugar factory and the surrounding community for their cooperation during this study. We are very grateful to the ICEMR field Entomology data collectors for their help in collecting and rearing of the mosquitoes. We are also very grateful to Jimma University Tropical and Infectious Diseases Research Center (TIDRC) Laboratory staff.
Funding
The study was supported financially by the National Institutes of Health (Grant No.: U19AI129326, D43TW001505 and R01A1050243). The funders had no role in the study design, analysis or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
AT, DY and GY conceived the study. AT drafted the manuscript, analyzed and interpreted the data. AT, AD, DH, KH and HG were involved in field data collection and patient sample collection. AT, HG and KH performed mosquito dissection and oocyst detection and quantification. DY, GY and AA critically reviewed the manuscript for significant intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical consideration and consent to participate
The protocol was reviewed and approved by National Research Ethics Review committee (NRERC) of the Ethiopia (Ref. no.: 10/31/2018). Permission was also obtained from the Buno Bedele and East Wollega Zonal Health Offices, and from the Arjo-Didessa Sugar factory, Oromia Regional State, Ethiopia. Gametocyte carriers were recruited among febrile patients visiting selected health facilities to seek treatment for malaria. Gametocytemic study participants with P. vivax and P. falciparum infection were informed of the risks and the benefits of the study. Consent was obtained from participants or legal guardians/parents in the case of children. Throughout the study, all malaria-positive patients, as determined by microscopic examination of blood film, were treated as per the national malaria treatment guideline for free.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tsegaye, A., Demissew, A., Hawaria, D. et al. Susceptibility of primary, secondary and suspected vectors to Plasmodium vivax and Plasmodium falciparum infection in Ethiopia. Parasites Vectors 15, 384 (2022). https://doi.org/10.1186/s13071-022-05467-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05467-5