Abstract
Background
Lyme borreliosis and other tick-borne diseases emerge from increased interactions between humans, other animals, and infected ticks. The risk of acquiring a tick-borne infection varies across space and time, so knowledge of the occurrence and prevalence of pathogens in ticks can facilitate disease diagnosis in a specific area and the implementation of mitigation measures and awareness campaigns. Here we identify the occurrence and prevalence of several pathogens in Ixodes ricinus ticks in Wester Ross, Northwest Scotland, a region of high tourism and tick exposure, yet data-poor in terms of tick-borne pathogens.
Methods
Questing I. ricinus nymphs (n = 2828) were collected from 26 sites in 2018 and 2019 and tested for the presence of tick-borne pathogens using PCR-based methods. Prevalence was compared with other regions of Scotland, England, Wales, and the Netherlands.
Results
Anaplasma phagocytophilum (4.7% prevalence), Borrelia burgdorferi sensu lato (s.l.) (2.2%), Babesia from clade X (0.2%), Rickettsia helvetica (0.04%), and Spiroplasma ixodetis (0.4%) were detected, but no Neoehrlichia mikurensis, Borrelia miyamotoi, or Babesia microti. Typing of A. phagocytophilum using a fragment of the GroEL gene identified the presence of both ecotype I and ecotype II. Genospecies identification of Borrelia burgdorferi s.l. revealed B. afzelii (53% of infected nymphs), B. garinii (9%), B. burgdorferi sensu stricto (7%), and B. valaisiana (31%). We found similar prevalence of A. phagocytophilum in Wester Ross as in the Netherlands, but higher than in other parts of Great Britain. We found lower B. burgdorferi s.l. prevalence than in England or the Netherlands, and similar to some other Scottish studies. We found higher prevalence of B. valaisiana and lower prevalence of B. garinii than in other Scottish studies. We found S. ixodetis at much lower prevalence than in the Netherlands, and R. helvetica at much lower prevalence than in England and the Netherlands.
Conclusions
As far as we know, this is the first description of S. ixodetis in Great Britain. The results are relevant for disease surveillance and management for public and veterinary health. The findings can also aid in designing targeted public health campaigns and in raising awareness among outdoor recreationists and professionals.
Graphical abstract

Similar content being viewed by others
Background
Ixodes ricinus is the most abundant and widespread tick species in Europe [1] and, as a generalist feeder, transmits multiple pathogens of medical and veterinary importance [2]. The primary public health concerns are Lyme borreliosis, caused by Borrelia burgdorferi sensu lato (s.l.) infection, and tick-borne encephalitis (TBE), both of which have increased in incidence in several European countries in recent decades [3,4,5,6,7]. TBE virus was detected in the UK (England) for the first time in 2018 [8].
Ixodes ricinus also transmits other pathogens, including Anaplasma phagocytophilum, Borrelia miyamotoi, Neoehrlichia mikurensis, Rickettsia helvetica, Spiroplasma ixodetis, and several Babesia species. These are currently the key known pathogens that can cause disease in livestock, wild animals, and/or humans [9, 10]. Although the number of studies describing human infections involving these other tick-borne pathogens is increasing, their incidence is largely unknown, awareness is low, and adequate diagnostic modalities are often lacking in routine settings [10, 11].
Information on the geographical distribution and prevalence of pathogens in ticks helps to evaluate the risk of exposure through tick bites and, consequently, the risk of disease. This raises awareness of the appropriate tick-borne pathogens in areas of risk and can contribute to prevention, identification, diagnosis, and treatment of these diseases in humans and animals. This is particularly relevant to regions where the pathogens, and hence the accompanying diseases, are currently considered absent due to the lack of data on the presence and prevalence of these pathogens in ticks. For example, soon after becoming aware of the presence of B. miyamotoi and TBE virus in questing ticks in the Netherlands, local health professionals identified the first cases of hard tick-borne relapsing fever and TBE in human patients and could thus administer appropriate treatment [12, 13]. Awareness, accurate diagnosis, and hence treatment of a disease can therefore depend on the knowledge of the local presence of the pathogen in the environment.
One region in Europe that is data-deficient in terms of these emerging pathogens is Northwest Scotland. It is an area of potential high relevance to tick-borne pathogens, as it has high tick host populations, both wild, such as deer and small mammals, and livestock, especially sheep [14]. Northwest Scotland, including the Wester Ross area, is a sparsely populated region that has long been visited by tourists and is still growing in popularity. In 2019, 12 million visitors visited the Scottish Highlands [15], and Wester Ross is one of the most popular destinations in the Highlands, thanks to its dramatic mountain scenery and beaches. Many visitors engage in recreational outdoor activities that have potentially high exposure to ticks. Moreover, woodland expansion is being actively encouraged by government policies and supporting subsidies, and this is resulting in land cover changes [16] with potential consequences for the populations of ticks, their hosts, and hence the prevalence of tick-borne pathogens. We therefore consider Northwest Scotland to be of interest in view of the potential health concerns arising from disease caused by exposure to tick-borne pathogens.
The aim of this study is to provide a first assessment of the presence of a range of pathogens in questing I. ricinus ticks from Wester Ross, including a preliminary estimate of their prevalence. Specifically, we aim to test I. ricinus for the presence of A. phagocytophilum, B. burgdorferi s.l., B. miyamotoi, N. mikurensis, R. helvetica, S. ixodetis, Ba. microti, and Babesia species from clade X, formerly known as Babesia sensu stricto (s.s.) [17,18,19]. Samples positive for A. phagocytophilum, B. burgdorferi s.l., and Babesia species from clade X are further specified to the ecotype, genospecies, and species level, respectively. Importantly, we place these new presence and prevalence data in context by comparing them with previously published and other unpublished data from other regions of Scotland, England and Wales, and the Netherlands, as an example from continental Europe.
Methods
Tick collection
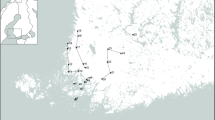
Ticks were collected from 26 sites in Wester Ross in Northwest Scotland, United Kingdom (57°24′–57°42′ N, 5°–5°54′ W), in 2018 and 2019 (Fig. 1). The four habitats of the sites were young Scots pine (Pinus sylvestris) woodlands (15–20 years old), mature Scots pine woodlands (minimum 50 years old), mature birch (Betula spp.) woodlands, and open moorland sites dominated by heather (Calluna and Erica spp.). A total of 2828 questing I. ricinus ticks (69 adult females, 72 adult males, and 2687 nymphs) were collected using the blanket drag/cloth lure method and kept for analysis in Eppendorf vials containing 70% ethanol. Previous studies have confirmed that 100% of the ticks identified from blanket drags in Scotland, including the Northwest region, are I. ricinus: in woodland sites across Scotland including Northwest Scotland, 2000 questing nymphs identified to species level were I. ricinus [20], in two studies carried out in the Northeast region of Scotland, 2500 and 1000 nymphs, respectively, identified to species level were I. ricinus [21], and in the North of Scotland, 4383 questing ticks (2700 larvae, 1550 nymphs, and 133 adults) identified were I. ricinus [22]. Therefore, we assumed that all ticks counted were I. ricinus. All the 2828 questing ticks were used for molecular analysis. For detailed information on the geographical location, land cover type, and times of data collection on the sites, see Fig. 1 and Additional file 1.
Geographical locations of the sites used for tick collection in Wester Ross, Northwest Scotland. The map was created using the free and open source QGIS 3.8. The location of the sites was recorded with a handheld GPS, the shapefile of the United Kingdom was obtained from Runfola D, Anderson A, Baier H, Crittenden M, Dowker E, Fuhrig S, et al. (2020) geoBoundaires: A global database of political administrative boundariies. PLoS ONE 15(4)
Pathogen identification
Ticks were kept in Eppendorf vials filled with 70% ethanol until return from the field. All ticks (n = 2828) were then washed in distilled water, briefly dried, separated individually into polymerase chain reaction (PCR) plates, and kept at –20 °C until DNA extraction. All ticks were extracted with ammonium hydroxide as described previously [23]. The ticks were analysed individually for the presence of tick-borne pathogens with different (multiplex) real-time PCR (qPCR) protocols, based on various target genes, as described: B. burgdorferi s.l. [24], B. miyamotoi [13], N. mikurensis [25], A. phagocytophilum [26, 27], Babesia microti [28], S. ixodetis [28], R. helvetica [29], and Babesia spp. from clade X, which has been designed to detect Ba. divergens, Ba. venatorum (formerly called EU1-3), Ba. capreoli and Ba. odocoilei [30]. Samples positive for B. burgdorferi s.l. were subjected to conventional PCR and Sanger sequencing of the intergenic spacer region for genospecies identification [31]. Samples positive for A. phagocytophilum were subjected to conventional PCR and Sanger sequencing of a fragment of the GroEL region for ecotyping [32]. All qPCRs were carried out on a LightCycler 480 (Roche Diagnostics Nederland B.V., Almere, the Netherlands) in a final volume of 20 μl with iQ Multiplex Powermix, 3 μl of sample, and 0.2 μM for all primers and different concentrations for probes [33]. Positive controls and negative water controls were used on every plate tested. To minimise contamination and false-positive samples, the DNA extraction, PCR mix preparation, sample addition, and qPCR analyses were performed in separate air-locked dedicated labs.
We could not test for the presence of tick-borne viruses such as TBE virus and Louping ill virus, as this would have required a more expensive and labour-intensive sampling strategy and testing procedure.
Comparison with other regions
We sourced data from different studies to compare the presence and prevalence of the tested pathogens in questing I. ricinus from Wester Ross, Northwest Scotland, with those in other regions of Scotland, England and Wales, and the Netherlands [28] as an example from mainland Europe. A previously unpublished dataset from 26 sites in Scotland (Grampian and Inverness-shire) was also used for prevalence of B. burgdorferi s.l., A. phagocytophilum, and Babesia species from clade X (Gilbert and Rocchi, unpublished data). For additional B. burgdorferi prevalence in Scotland and for the prevalence of all the pathogens in England and Wales, data from published studies were used [21, 25, 34,35,36,37,38,39,40,41,42,43]. The ticks from the Netherlands and the ticks from Wester Ross were analysed with the same molecular methods in the same laboratory, following the methods described above, allowing direct comparison of the prevalence. Note that the prevalence from the published literature [21, 25, 34,35,36,37, 40,41,42,43] and unpublished data from Gilbert and Rocchi were derived using slightly different DNA extraction and PCR protocols, which may have slightly different specificities and sensitivities, so direct comparisons are undertaken with that caveat in mind. Gilbert and Rocchi (unpublished) used the following techniques: DNA from nymphs (pools of three) was extracted using a KingFisher Flex magnetic particle processor (Thermo Scientific) and a MagMAX CORE nucleic acid purification kit (Thermo Fisher). Anaplasma phagocytophilum and B. burgdorferi s.l. nucleic acids were subjected to specific TaqMan RT-PCR [26], which is the same method used for A. phagocytophilum [26] but different from B. burgdorferi s.l. in our Wester Ross ticks [24]. Babesia spp. DNA was amplified according to Hilpershauser et al. (2020) [44]. Positive samples were Sanger-sequenced (Eurofins MWG) and analysed using DNAStar Lasergene (version 15), and sequences were compared against the BLAST repository (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to obtain parasite speciation. Prevalence estimates of nymphs assumed that a positive pool resulted from one positive nymph, i.e. the number of positive pools divided by the number of nymphs tested for each site. The overall prevalence for each pathogen cited in the results from the Gilbert and Rocchi data was the mean of all the site-level prevalence values.
Results
We detected the following pathogens tested in the 2828 questing I. ricinus ticks from Wester Ross, from highest to lowest prevalence: A. phagocytophilum (4.7% prevalence; 132/2828), B. burgdorferi s.l. (2.2%; 63/2828), S. ixodetis (0.4%; 12/2828), Babesia spp. from clade X (0.2%; 5/2828), and R. helvetica (0.04%; 1/2828). See Additional file 1 for more details. Of the Anaplasma-positive ticks, 86% (80/93) were identified as the zoonotic ecotype I and 14% (13/93) as the non-zoonotic ecotype II. Four genospecies of B. burgdorferi s.l. were detected in the following proportions of positive nymphs: B. afzelii (53%, 24/63), B. valaisiana (31%, 14/63), B. garinii (9%, 4/63), and B. burgdorferi s.s. (7%, 3/63). Two genospecies of Babesia from clade X were detected, in the following proportions of positive nymphs: Ba. venatorum (40%, 2/5) and Ba. divergens (60%, 3/5).
Comparison with other regions
The presence and prevalence of the tick-borne pathogens from other regions of Scotland, England and Wales, and the Netherlands are summarised in Table 1.
Anaplasma phagocytophilum was present at a higher prevalence than in studies from England, and at a similar prevalence as the Netherlands. We found B. burgdorferi s.l. prevalence to be lower than in England or the Netherlands, and similar to some other Scottish studies. We found a higher prevalence of B. valaisiana and lower prevalence of B. garinii than in other studies from Scotland (not shown in table). We found S. ixodetis at much lower prevalence than in the Netherlands, and R. helvetica at much lower prevalence than in England and the Netherlands.
Discussion
We revealed the presence of the following tick-borne pathogens in ticks in Wester Ross, Northwest Scotland: A. phagocytophilum, B. burgdorferi s.l., Babesia species from clade X (Ba. venatorum and Ba. divergens), R. helvetica, and S. ixodetis, the last being the first record described for Great Britain. This study assessed only the presence of the DNA from the chosen pathogens, and not their viability or infectivity. However, numerous studies already implicate I. ricinus as their vector. We cannot assume infectiousness from PCR results, and therefore we cannot translate prevalence estimates of the pathogens to infection risk in humans.
Anaplasma phagocytophilum
The most frequently detected pathogen group was A. phagocytophilum, with a prevalence higher than in England and Wales, but comparable to locations in the Netherlands and elsewhere in Europe [45]. The two ecotypes, I and II, are known to circulate in I. ricinus [27], and we found both in Wester Ross. The ecotype II is probably non-zoonotic, and most likely maintained by roe deer (Capreolus capreolus), which are present at our survey sites in Wester Ross [46]. Anaplasma phagocytophilum ecotype I is probably zoonotic and can also cause disease in livestock. It is probably maintained in enzootic cycles by deer species other than roe deer [32]. In Northwest Scotland, red deer (Cervus elaphus) are the most common deer species, and likely to be the main natural reservoir host.
Borrelia burgdorferi s.l.
The second most abundant group of tick-borne pathogens was B. burgdorferi s.l., with a prevalence of 2.2%. This prevalence is comparable to that reported in two of the four other large cross-sectional surveys from further south and east in Scotland, in the Grampian region (2.2%) [21], and across Scotland but focussing mainly in West and Central Scotland (1.7%) [34]. The other two large-scale Scottish surveys found higher prevalence: 5.6% from across Scotland, but mainly focussed on the Grampian region [35], and 5.9% from Grampian, Moray, and Inverness-shire (Gilbert and Rocchi, unpubl.). Studies from England and Wales showed similar prevalence to studies in Scotland, except in a study from Salisbury (South England), where the prevalence was 18.1% [37]. This higher figure is more comparable to the prevalence from the Netherlands (13.3%) and other parts of continental Western Europe (10.2%) [47].
Similarly to other studies in Scotland [21, 34, 43], the rodent-associated B. afzelii was the genospecies that constituted most (53%) of the B. burgdorferi s.l. complex of spirochetes in Wester Ross.
We found that in Wester Ross, 7% of the B. burgdorferi s.l. complex was comprised of B. burgdorferi s.s., which is a similar proportion as that reported in all the large Scottish surveys [21, 34, 43]. The reservoir host of B. burgdorferi s.s. zoonotic genospecies in Europe has not been firmly established, but recent studies, including from Scotland, suggest a dominant role for squirrels [48,49,50].
Interestingly, the two bird-associated genospecies, B. garinii and B. valaisiana, comprised markedly different proportions in Wester Ross from those in previous Scottish surveys, which showed far greater proportions of B. garinii (18.2% [21], 28.8% [34], and 36% [43]) and smaller proportions of B. valaisiana (7.6% [34], 8% [43], and 10.7% [21]). We found the opposite trends, with only 9% of B. burgdorferi s.l. comprising B. garinii and 31% comprising B. valaisiana. The habitats surveyed in Wester Ross (pine and birch woodlands and open moorland) are similar to those in the other Scottish surveys, and are expected to comprise similar bird species, so we do not know why B. valaisiana dominates in Wester Ross as compared to the apparent dominance of B. garinii in the rest of Scotland. One possible explanation is competition between the pathogens within the host or the vector, and/or that there is a certain amount of host specificity of each of the bird genospecies between bird species. There is some evidence that B. garinii can associate with squirrels [49], so perhaps there are higher squirrel densities in some of the more southern Scottish survey sites than in Wester Ross [51].
The genospecies composition is relevant from a public health perspective, as different genospecies lead to different clinical symptoms in human Lyme disease patients. Borrelia afzelii is associated with a skin condition, acrodermatitis chronica atrophicans (ACA), B. garinii with neuroborreliosis, and B. burgdorferi s.s. with Lyme arthritis [2, 52, 53], whereas B. valaisiana may not be pathogenic [54]. As we found a high percentage of B. afzelii, general practitioners need to be aware of ACA and its manifestations.
The genospecies composition is also interesting from an ecological perspective. Habitat is an important determinant of vertebrate community composition. All the B. burgdorferi s.l.-positive ticks in this study were from mature birch and mature Scots pine woodlands, and both these habitat types are likely to support diverse vertebrate communities, but may differ in their species composition. The low bird-associated B. garinii prevalence suggests that rodents play a more important role in Lyme disease hazard than do birds in the study area. Further research on bird and rodent densities at the sites could elucidate the mechanisms driving the relative composition of Borrelia burgdorferi s.l. genospecies in Wester Ross.
Babesia species
Babesia species from clade X were detected in Wester Ross at a prevalence of 0.2%, compared to 0.6% in other regions of Scotland (Gilbert and Rocchi, unpubl.). Two of the four common Babesia species from I. ricinus were detected. The first, Ba. venatorum, has been associated with some disease cases in humans in Europe [55, 56] and has recently been found in sheep [57] and dogs [58] in the UK, although the main natural vertebrate host for Ba. venatorum is probably roe deer in Europe [59, 60]. Babesia divergens, the second detected genospecies, is of zoonotic importance: it has several zoonotic reservoirs, including deer and cattle, both of which have high cultural and economic value in Wester Ross. It has been found all across Europe affecting cattle [61, 62] as well as humans [61, 63,64,65], Recently, in July 2020, the first human case of Ba. divergens in the UK was reported in Southwest England [66]. Babesia capreoli and Ba. odocoilei were not detected, but they are present in the Netherlands. No human cases have been associated with either of the two latter pathogens in Europe [67].
Rickettsia helvetica
We found very low prevalence (0.04%) of R. helvetica in Wester Ross, Northwest Scotland. This pathogen has previously been detected in ticks in England at a 3% prevalence [42] and in several European countries at prevalence between 4.2% and 16.7%, including the Netherlands (this study), Poland [69], Germany [70], Austria [71], Slovenia [72], and Spain [73], and has been the subject of several cases in humans with febrile illness and perimyocarditis [74,75,76]. It is therefore considered an emerging human pathogen [77]. As R. helvetica is efficiently transmitted vertically in ticks (i.e. transovarial and trans-stadial transmission), ticks themselves can be considered as reservoirs hosts [68]. Which vertebrate hosts contribute most to the life cycle of R. helvetica has not be fully investigated yet, but the pathogen has been detected in bank vole, wood mouse, shrew, wild boar, and roe deer [68, 78, 79]. The prevalence of R. helvetica is very low, and one may wonder whether or how a tick-borne pathogen can be maintained in an enzootic cycle with such a low prevalence. One possible explanation is that ticks with R. helvetica are not maintained in a regional enzootic cycle but (continuously) introduced by migratory birds [80].
Spiroplasma ixodetis
We detected the relatively unknown tick-borne pathogen S. ixodetis (0.4% prevalence), and this is the first record for Great Britain. The bacterial parasite Spiroplasma ixodetis was first isolated from Ixodes pacificus ticks in the USA in 1995 [81], and it has previously been found in Slovakia in all active life stages of I. ricinus including larvae [82,83,84], which suggests that transmission can be transovarial. The role of hosts in the S. ixodetis transmission cycle is unknown [28]. Several European studies have described human cases of cataract and uveitis in newborn babies that showed evidence of transplacental infection with S. ixodetis [85, 86].
Neoehrlichia mikurensis, Borrelia miyamotoi, and Babesia microti
Although we did not observe N. mikurensis, B. miyamotoi, or Ba. microti in questing nymphs from Wester Ross, this cannot necessarily be interpreted as a complete absence of enzootic circulation of these pathogens, because the prevalence of some of these pathogens can be low, for example, 0.1% for N. mikurensis in regions of Denmark [87], 0.6% for Ba. microti in Poland [88] and 1.5% in the UK (ticks pulled from dogs) [89], and 0.8% for B. miyamotoi in regions of Germany [90] and 0.5% in England (Table 1). In our sample of 2828 ticks, a prevalence of 0.1% would mean that there would be an expectation of around three ticks that test positive in our sample size. Therefore, it could be that the pathogens are present but were undetected, especially if the circulation of the pathogens is very localised or occurs in habitats insufficiently monitored in this study. In a separate study, also in Wester Ross, we did find Ba. microti in one engorged tick pulled from a wood mouse (Apodemus sylvaticus) (Olsthoorn et al. unpubl.). Neoehrlichia mikurensis has so far not been found in any study that tested for it in Great Britain [36, 37], so there is a realistic possibility that this pathogen is not circulating in Scotland.
We tested 8282 questing nymph and adult ticks, and it is possible that a tiny proportion of the ticks analysed may have been non-ricinus, although this is highly unlikely, as previous studies from Scotland identified 100% of questing ticks (9883 in total) to be I. ricinus [20,21,22].
Conclusions
In the first surveys of tick-borne pathogens in Northwest Scotland, in a region much frequented by tourists, we identified and quantified the prevalence of A. phagocytophilum, B. burgdorferi s.l., Babesia species from clade X (Ba. venatorum and Ba. divergens), R. helvetica, and S. ixodetis, the latter being the first record described for Great Britain. Anaplasma phagocytophilum occurred at higher prevalence than elsewhere in Great Britain, while B. burgdorferi s.l. was less prevalent than in England or the Netherlands, though similar to other Scottish studies. This new knowledge has implications for public and veterinary health in terms of improving diagnoses and treatment and better targeting of awareness and mitigation strategies.
Further research is needed to investigate the life cycles and mechanisms of transmission of these pathogens. Anaplasma phagocytophilum and Ba. divergens, both of zoonotic importance, are transmitted by deer, an important cultural and economic asset in the Wester Ross area as well as other parts of Europe. Rickettsia helvetica has been found in several vertebrates, but its life cycle is unclear and needs further investigation. More studies in different habitats are needed to shed light on the ecological determinants of genospecies composition of B. burgdorferi s.l.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
ECDC. European Centre for Disease Prevention and Control and European Food Safety Authority. Ixodes ricinus - current known distribution: May 2020. Stockholm. 2020. https://www.ecdc.europa.eu/en/publications-data/ixodes-ricinus-current-known-distribution-may-2020.
Sprong H, Azagi T, Hoornstra D, Nijhof AM, Knorr S, Baarsma ME, et al. Control of Lyme borreliosis and other Ixodes ricinus-borne diseases. Parasit Vectors. 2018;11:1.
Smith R, Takkinen J. Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Euro Surveill. 2006;11:2977.
Hofhuis A, Harms M, van den Wijngaard C, Sprong H, van Pelt W. Continuing increase of tick bites and Lyme disease between 1994 and 2009. Ticks Tick Borne Dis. 2015;6:69–74.
Dubrey SW, Bhatia A, Woodham S, Rakowicz W. Lyme disease in the United Kingdom. Postgrad Med J. 2014;90:33–42.
Kunze U. The International Scientific Working Group on Tick-Borne Encephalitis (ISW TBE): review of 17 years of activity and commitment. Ticks Tick Borne Dis. 2016;7:399.
Vandekerckhove O, De Buck E, Van Wijngaerden E. Lyme disease in Western Europe an emerging problem? A systematic review. Acta Clin Belgica. 2019. https://doi.org/10.1080/17843286.2019.1694293.
Holding M, Dowall SD, Medlock JM, Carter DP, McGinley L, Curran-French M, et al. Detection of new endemic focus of tick-borne encephalitis virus (TBEV), Hampshire/Dorset border, England, september 2019. Euro Surveill. 2019;24:1900658.
Koenen F, Pascucci I, Jaenson TGT, Madder M, de Sousa R, Estrada-Peña A, et al. Tick-borne infections including zoonoses in europe and the mediterranean basin. In: Salman M, Tarrescall J, editors., et al., Ticks and tick-borne diseases geographical distribution and control strategies in the Euro-Asian region. Wallingford: CABI Publishing; 2012.
Azagi T, Hoornstra D, Kremer K, Hovius JWR, Sprong H. Evaluation of disease causality of rare ixodes ricinus-borne infections in Europe. Pathogens. 2020. https://doi.org/10.3390/pathogens9020150.
Davidson MM, Williams H, Macleod JAJ. Louping ill in man: A forgotten disease. J Infect. 1991;23:241–9.
Weststrate AC, Knapen D, Laverman GD, Schot B, Prick JJ, Spit SA, et al. Increasing evidence of tick-borne encephalitis (TBE) virus transmission, the Netherlands, June 2016. Euro Surveill. 2017;22:30482.
Hovius JWR, De Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658.
Scottish Natural Heritage. Updating the estimates of national trends and regional differences in red deer densities on open-hill ground in Scotland. 2019.
VisitScotland. Insight Department: Highland Factsheet 2019. 2020. https://www.visitscotland.org/binaries/content/assets/dotorg/pdf/research-papers-2/regional-factsheets/highland-factsheet-2019.pdf.
Scottish Government. Draft climate change plan, The draft third report on policies and proposals 2017–2032. 2017.
Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect Genet Evol. 2012;12:1788–809.
Jalovecka M, Sojka D, Ascencio M, Schnittger L. Babesia life cycle—when phylogeny meets biology. Trends Parasitol. 2019;35:356–68.
Schreeg ME, Marr HS, Tarigo JL, Cohn LA, Bird DM, Scholl EH, et al. Mitochondrial genome sequences and structures aid in the resolution of Piroplasmida phylogeny. PLoS ONE. 2016;11:165702.
James MC. The ecology, genetic diversity and epidemiology of Lyme borreliosis in Scotland (PhD thesis). University of Aberdeen, 2010.
Gandy SL. The impacts of host community composition on Lyme disease risk in Scotland (PhD thesis). University of Glasgow, 2020.
Ruiz-Fons F, Gilbert L. The role of deer as vehicles to move ticks, Ixodes ricinus, between contrasting habitats. Int J Parasitol. 2010;40:1013–20.
Wielinga PR, Gaasenbeek C, Fonville M, De Boer A, De Vries A, Dimmers W, et al. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in the Netherlands. Appl Environ Microbiol. 2006;72:7594–601.
Heylen D, Tijsse E, Fonville M, Matthysen E, Sprong H. Transmission dynamics of Borrelia burgdorferi s.l. in a bird tick community. Environ Microbiol. 2013;15:663–73.
Jahfari S, Fonville M, Hengeveld P, Reusken C, Scholte EJ, Takken W, et al. Prevalence of Neoehrlichia mikurensis in ticks and rodents from North–west Europe. Parasit Vectors. 2012. https://doi.org/10.1186/1756-3305-5-74.
Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol. 2004;42:3164–8.
Jahfari S, Coipan EC, Fonville M, Van Leeuwen AD, Hengeveld P, Heylen D, et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. 2014;7:365.
Krawczyk AI, Van Duijvendijk GLA, Swart A, Heylen D, Jaarsma RI, Jacobs FHH, et al. Effect of rodent density on tick and tick-borne pathogen populations: consequences for infectious disease risk. Parasit Vectors. 2020. https://doi.org/10.1186/s13071-020-3902-0.
Heylen D, Fonville M, van Leeuwen AD, Sprong H. Co-infections and transmission dynamics in a tick-borne bacterium community exposed to songbirds. Environ Microbiol. 2016;18:988–96.
Øines Ø, Radzijevskaja J, Paulauskas A, Rosef O. Prevalence and diversity of Babesia spp. in questing Ixodes ricinus ticks from Norway. Parasit Vectors. 2012;5:156.
Coipan EC, Fonville M, Tijsse-Klasen E, van der Giessen JWB, Takken W, Sprong H, et al. Geodemographic analysis of Borrelia burgdorferi sensu lato using the 5S–23S rDNA spacer region. Infect Genet Evol. 2013;17:216–22.
Jaarsma RI, Sprong H, Takumi K, Kazimirova M, Silaghi C, Mysterud A, et al. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasit Vectors. 2019. https://doi.org/10.1186/s13071-019-3583-8.
Kazimírová M, Hamšíková Z, Špitalská E, Minichová L, Mahríková L, Caban R, et al. Diverse tick-borne microorganisms identified in free-living ungulates in Slovakia. Parasit Vectors. 2018. https://doi.org/10.1186/s13071-018-3068-1.
Millins C, Gilbert L, Johnson P, James M, Kilbride E, Birtles R, et al. Heterogeneity in the abundance and distribution of Ixodes ricinus and Borrelia burgdorferi (sensu lato) in Scotland: implications for risk prediction. Parasit Vectors. 2016;9:595.
James MC, Bowman AS, Forbes KJ, Lewis F, McLeod JE, Gilbert L. Environmental determinants of Ixodes ricinus ticks and the incidence of Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in Scotland. Parasitology. 2013;140:237–46.
Hansford KM, Fonville M, Jahfari S, Sprong H, Medlock JM. Borrelia miyamotoi in host-seeking Ixodes ricinus ticks in England. Epidemiol Infect. 2015;143:1079–87.
Hansford KM, Fonville M, Gillingham EL, Coipan EC, Pietzsch ME, Krawczyk AI, et al. Ticks and Borrelia in urban and peri-urban green space habitats in a city in southern England. Ticks Tick Borne Dis. 2017;8:353–61.
Bettridge J, Renard M, Zhao F, Bown KJ, Birtles RJ. Distribution of Borrelia burgdorferi sensu lato in Ixodes ricinus populations across central Britain. Vector Borne Zoonotic Dis. 2013;13:139–46.
Hall J. Ecology of Borrelia burgdorferi sensu lato and epidemiology of borrelial infections in Cumbria. University of Salford, 2018.
Cull B, Hansford K, McGinley L, Gillingham E, Vaux A, Smith R, et al. A nationwide study on Borrelia burgdorferi s.l. infection rates in questing Ixodes ricinus: a six year snapshot study in protected recreational areas in England and Wales. Med Vet Entomol. 2021. https://doi.org/10.1111/mve.12503.
Bown KJ, Lambin X, Telford GR, Ogden NH, Telfer S, Woldehiwet Z, et al. Relative importance of Ixodes ricinus and Ixodes trianguliceps as vectors for Anaplasma phagocytophilum and Babesia microti in field vole (Microtus agrestis) populations. Appl Environ Microbiol. 2008;74:7118–25.
Tijsse-Klasen E, Jameson LJ, Fonville M, Leach S, Sprong H, Medlock JM. First detection of spotted fever group rickettsiae in Ixodes ricinus and Dermacentor reticulatus ticks in the UK. Epidemiol Infect. 2011;139:524–9.
James MC, Gilbert L, Bowman AS, Forbes KJ. The heterogeneity, distribution, and environmental associations of Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in Scotland. Front Public Heal. 2014;2:1–10.
Hilpertshauser H, Deplazes P, Schnyder M, Gern L, Mathis A. Babesia spp. identified by PCR in ticks collected from domestic and wild ruminants in Southern Switzerland. Appl Environ Microbiol. 2006;72:6503–7.
Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum-a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013. https://doi.org/10.3389/fcimb.2013.00031.
The Scottish Government. Scotland’s Wild Deer: a national approach, including 2015–2020 priorities. 2016. https://www.nature.scot/scotlands-wild-deer-national-approach-2015-2020-priorities.
Strnad M, Hönig V, Růžek D, Grubhoffer L, Rego ROM. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl Environ Microbiol. 2017. https://doi.org/10.1128/AEM.00609-17.
Mysterud A, Stigum VM, Jaarsma RI, Sprong H. Genospecies of Borrelia burgdorferi sensu lato detected in 16 mammal species and questing ticks from northern Europe. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-41686-0.
Millins C, Magierecka A, Gilbert L, Edoff A, Brereton A, Kilbride E, et al. An invasive mammal (the gray squirrel, Sciurus carolinensis) commonly hosts diverse and atypical genotypes of the zoonotic pathogen Borrelia burgdorferi sensu lato. Appl Environ Microbiol. 2015;81:4236–45.
Majerová K, Hönig V, Houda M, Papežík P, Fonville M, Sprong H, et al. Hedgehogs, squirrels, and blackbirds as sentinel hosts for active surveillance of Borrelia miyamotoi and Borrelia burgdorferi complex in urban and rural environments. Microorganisms. 2020;8:1–16.
Gurnell J, Lurz P, Bertoldi W. The changing patterns in the distribution of red and grey squirrels in the North of England and Scotland between 1991 and 2010 based on volunteer surveys. Hystrix. 2014;25:83–9.
Coipan EC, Jahfari S, Fonville M, Oei GA, Spanjaard L, Takumi K, et al. Imbalanced presence of Borrelia burgdorferi s.l. multilocus sequence types in clinical manifestations of Lyme borreliosis. Infect Genet Evol. 2016;42:66–76.
Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–73.
Margos G, Sing A, Fingerle V. Published data do not support the notion that Borrelia valaisiana is human pathogenic. Infection. 2017;45:567–9.
Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis. 2015;15:196–203.
Zhao L, Jiang R, Jia N, Ning N, Zheng Y, Huo Q, et al. Human case infected with Babesia venatorum: a 5-year follow-up study. Open Forum Infect Dis. 2020. https://doi.org/10.1093/ofid/ofaa062.
Gray A, Capewell P, Loney C, Katzer F, Shiels BR, Weir W. Sheep as host species for zoonotic Babesia venatorum, United Kingdom. Emerg Infect Dis. 2019;25:2257–60.
Smith FD, Wall LER. Prevalence of Babesia and Anaplasma in ticks infesting dogs in Great Britain. Vet Parasitol. 2013;198:18–23.
Michel AO, Mathis A, Ryser-Degiorgis MP. Babesia spp. in European wild ruminant species: parasite diversity and risk factors for infection. Vet Res. 2014;45:65.
Zanet S, Trisciuoglio A, Bottero E, De Mera IGF, Gortazar C, Carpignano MG, et al. Piroplasmosis in wildlife: Babesia and Theileria affecting free-ranging ungulates and carnivores in the Italian Alps. Parasit Vectors. 2014. https://doi.org/10.1186/1756-3305-7-70.
Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16:622–36.
Bock R, Jackson L, De Vos A, Jorgensen W. Babesiosis of cattle. Parasitology. 2004;129:S247.
Herwaldt BL, Cacciò S, Gherlinzoni F, Aspöck H, Slemenda SB, Piccaluga PP, et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9:942–8.
Qi C, Zhou D, Liu J, Cheng Z, Zhang L, Wang L, et al. Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol Res. 2011;109:241–5.
Haapasalo K, Suomalainen P, Sukura A, Siikamäki H, Sakari JT. Fatal babesiosis in man, Finland, 2004. Emerg Infect Dis. 2010;16:1116–8.
Johnson N, Phipps P, Godbole G, Hansford K, Johnston C, White M, et al. Preventing tick exposure in vets and farmers. Vet Rec. 2020. https://doi.org/10.1136/vr.m3334.
Young KM, Corrin T, Wilhelm B, Uhland C, Greig J, Mascarenhas M, et al. Zoonotic Babesia: a scoping review of the global evidence. PLoS ONE. 2019;14:e0226781.
Sprong H, Wielinga PR, Fonville M, Reusken C, Brandenburg AH, Borgsteede F, et al. Ixodes ricinus ticks are reservoir hosts for Rickettsia helvetica and potentially carry flea-borne Rickettsia species. Parasit Vectors. 2009. https://doi.org/10.1186/1756-3305-2-41.
Stańczak J, Racewicz M, Michalik J, Buczek A. Distribution of Rickettsia helvetica in Ixodes ricinus tick populations in Poland. Int J Med Microbiol. 2008;298:231–4.
Wölfel R, Terzioglu R, Kiessling J, Wilhelm S, Essbauer S, Pfeffer M, et al. Rickettsia spp. in Ixodes ricinus ticks in Bavaria, Germany. Ann NY Acad Sci. 2006;1078:509–11.
Rehacek J, Kocianova E, Lukacova M, Stanek G, Khanakah G, Vyrostekova V, et al. Detection of spotted fever group (SFG) rickettsia in Ixodes ricinus ticks in Austria. Acta Virol. 1997;41:355–6.
Prosenc K, Petrovec M, Trilar T, Duh D, Avšič-Županc T. Detection of rickettsiae in Ixodes ricinus ticks in Slovenia. Ann NY Acad Sci. 2003;990:201–4.
Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A, Sanz RÁ. Detection and identification of Rickettsia helvetica and Rickettsia sp. IRS3/IRS4 in Ixodes ricinus ticks found on humans in Spain. Eur J Clin Microbiol Infect Dis. 2004;23:648–9. https://doi.org/10.1007/s10096-004-1184-7.
Fournier PE, Grunnenberger F, Jaulhac B, Gastinger G, Raoult D. Evidence of Rickettsia helvetica infection in humans, Eastern France. Emerg Infect Dis. 2000;6:389–92.
Nilsson K, Lindquist O, Påhlson C. Association of Rickettsia helvetica with chronic perimyocarditis in sudden cardiac death. Lancet. 1999;354:1169–73.
Nilsson K. Septicaemia with Rickettsia helvetica in a patient with acute febrile illness, rash and myasthenia. J Infect. 2009;58:79–82.
Scarpulla M, Barlozzari G, Salvato L, De Liberato C, Lorenzetti R, Macrì G. Rickettsia helvetica in human-parasitizing and free-living Ixodes ricinus from urban and wild green areas in the metropolitan city of Rome, Italy. Vector Borne Zoonotic Dis. 2018;18:404–7.
Mardosaitė-Busaitienė D, Radzijevskaja J, Balčiauskas L, Paulauskas A. First detection of Rickettsia helvetica in small mammals in Lithuania. New Microbes New Infect. 2018;22:19–23.
Fischer S, Spierling NG, Heuser E, Kling C, Schmidt S, Rosenfeld UM, et al. High prevalence of Rickettsia helvetica in wild small mammal populations in Germany. Ticks Tick Borne Dis. 2018;9:500–5.
Pedersen BN, Jenkins A, Kjelland V. Tick-borne pathogens in Ixodes ricinus ticks collected from migratory birds in southern Norway. PLoS ONE. 2020. https://doi.org/10.1371/journal.pone.0230579.
Tully JG, Rose DL, Yunker CE, Carle P, Bove JM, Williamson DL, et al. Spiroplasma ixodetis sp. nov., a new species from Ixodes pacificus ticks collected in Oregon. Int J Syst Bacteriol. 1995;45:23–8.
Bell-Sakyi L, Palomar AM, Kazimirova M. Isolation and propagation of a Spiroplasma sp. from Slovakian Ixodes ricinus ticks in Ixodes spp. cell lines. Ticks Tick Borne Dis. 2015;6:601–6.
Subramanian G, Sekeyova Z, Raoult D, Mediannikov O. Multiple tick-associated bacteria in Ixodes ricinus from Slovakia. Ticks Tick Borne Dis. 2012;3:406–10.
Binetruy F, Bailly X, Chevillon C, Martin OY, Bernasconi MV, Duron O. Phylogenetics of the Spiroplasma ixodetis endosymbiont reveals past transfers between ticks and other arthropods. Ticks Tick Borne Dis. 2019;10:575–84.
Lorenz B, Schroeder J, Reischl U. First evidence of an endogenous Spiroplasma sp. infection in humans manifesting as unilateral cataract associated with anterior uveitis in a premature baby. Graefes Arch Clin Exp Ophthalmol. 2002;240:348–53.
Matet A, Le Flèche-Matéos A, Doz F, Dureau P, Cassoux N. Ocular spiroplasma ixodetis in Newborns, France. Emerg Infect Dis. 2020;26:340–4.
Portillo A, Santibáñez P, Palomar AM, Santibáñez S, Oteo JA. Candidatus Neoehrlichia mikurensis in Europe. New Microbes New Infect. 2018;22:30–6.
Siński E, Bajer A, Welc R, Pawełczyk A, Ogrzewalska M, Behnke JM. Babesia microti: Prevalence in wild rodents and Ixodes ricinus ticks from the Mazury Lakes District of north-eastern Poland. Int J Med Microbiol. 2006;296:137–43.
Abdullah S, Helps C, Tasker S, Newbury H, Wall R. Prevalence and distribution of Borrelia and Babesia species in ticks feeding on dogs in the U.K. Med Vet Entomol. 2018;32:14–22.
Rǎileanu C, Tauchmann O, Vasić A, Wöhnke E, Silaghi C. Borrelia miyamotoi and Borrelia burgdorferi (sensu lato) identification and survey of tick-borne encephalitis virus in ticks from north-eastern Germany. Parasit Vectors. 2020;13:106.
Acknowledgements
We thank the student team who participated in data collection in Wester Ross, Northwest Scotland, namely Kirsten van der Hulst, Livia May, Flavia Mondini, Ronny Rotbarth, and Corinne Schlierenzauer. We thank Doug Bartholomew and all staff members at Nature Scotland Beinn Eighe National Nature Reserve for accommodating and supporting the fieldwork team, as well as Andrew Cope and Beverly and Charlie Hill, for the accommodation at Glen Mhor estate. We also thank Linda and Soeren Hoejlund, and Kay and Wang Liston for support and accommodation. We thank involved land owners, managers, and inhabitants in the Wester Ross area for their warm welcome. We thank the laboratory technician at ETH Zürich, Kirsti Määttänen, for coordinative, organisational, and preparatory work in the laboratory, Lukas Schwyter and Eliane Steiner for their help in preparatory laboratory work, and the Genetic Diversity Centre (GDC) for their support in acquiring laboratory materials. We thank Glauco Camenish for support in cross-border transport arrangements of the scientific samples.
Funding
F.O. is supported by the Chair of Ecosystem Management at ETH Zurich. H.S. and M.F. were supported by a grant the European Interreg North Sea Region program, as part of the NorthTick project. The pathogen detection was financially supported by the Dutch Ministry of Health, Welfare, and Sports. L.G. and M.R. were supported by the Scottish Government’s Rural and Environmental Science and Analytical Services Division (RESAS).
Author information
Authors and Affiliations
Contributions
Conceptualisation–HS; methodology–MF, HS, FO, LG; software–FO; validation–MF; formal analysis–FO; investigation–MF; resources–MF, LG, MR; field data collection–FO, LG; lab analysis–MR, MF; data curation–FO, LG; writing—original draft–FO, HS; writing—review and editing–HS, MF, LG, JM, MR, JG, FO; visualisation–FO; supervision–HS, JG, LG; project administration–HS, JG; funding acquisition–HS, JG. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Text S1. Field methods explained. Table S1. Sum of ticks collected at each visit on each plot is summarised, as well as the sum of ticks positive for each of the eight pathogens tested for in the study, namely Borrelia miyamotoi, Borrelia burgdorferi s.l., Anaplasma phagocytophilum, Neoehrlichia mikurensis, Babesia spp. from clade X, Spiroplasma ixodetis, Babesia microti, and Rickettsia helvetica. Table S2. Molecular analysis results of the 2828 adult and nymph ticks.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Olsthoorn, F., Sprong, H., Fonville, M. et al. Occurrence of tick-borne pathogens in questing Ixodes ricinus ticks from Wester Ross, Northwest Scotland. Parasites Vectors 14, 430 (2021). https://doi.org/10.1186/s13071-021-04946-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-021-04946-5