Abstract
With the increasing prevalence and mortality, chronic kidney disease (CKD) has become a world public health problem. As the primary pathological manifestation in CKD, renal fibrosis is often used as a critical target for the treatment of CKD and inhibits the progression of CKD to end-stage renal disease (ESRD). As a potential drug, natural products have been confirmed to have the potential as a routine or supplementary therapy for chronic kidney disease, which may target renal fibrosis and act through various pharmacological activities such as anti-inflammatory and anti-oxidation of natural products. This article briefly introduces the pathological mechanism of renal fibrosis and systematically summarizes the latest research on the treatment of renal fibrosis with natural products of Chinese herbal medicines.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) has become a world public health problem with the increasing prevalence and mortality. In 2017, the number of patients with CKD reached 697.5 million, and the global prevalence of CKD was 9.1% [1]. At present, the treatment of CKD is mainly based on the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. However, this does not better prevent the progression of CKD [2]. Continuously progressive CKD will eventually develop into end-stage renal disease. At this time, patients can only rely on renal replacement therapy, seriously affecting the quality of life, so the search for better CKD treatment strategies has become a current research hotspot.
The pathological manifestations of CKD due to different causes may vary slightly. However, the main pathological feature is renal fibrosis driven by renal injury stimuli such as inflammation and oxidative stress, so anti-renal fibrosis is widely studied as a potential CKD therapeutic target. Traditional Chinese Medicine (TCM), as an alternative therapy in modern medicine, has attracted much attention in recent years. A large number of studies have demonstrated that natural products in TCM play a role in anti-renal fibrosis through their anti-oxidation and anti-inflammation pharmacological activities.
In this paper, we introduce the pathological mechanism involved in renal fibrosis, summarize the latest research on the treatment of renal fibrosis with natural products in recent years, and discuss the future direction and challenges of natural products of Chinese herbal medicines and renal fibrosis.
Pathological mechanisms of renal fibrosis
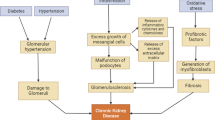
Renal fibrosis is the main pathological feature of CKD and plays a vital role in CKD progression to ESRD. The essence of renal fibrosis is that various injury reactions stimulate renal resident cells, causing excessive extracellular matrix (ECM) deposition, tubulointerstitial fibrosis, and glomerulosclerosis, ultimately leading to the destruction of renal parenchyma and loss of renal function [3]. Renal fibrosis involves a series of complex cellular and molecular mechanisms. Almost all renal resident cells are involved in the process of fibrosis. Generally, renal fibrosis can be divided into four overlapping processes: priming, activation, execution, and progression. It is worth noting that these four stages are not strictly chronological. Since fibrosis is a dynamic pathological process, many events may occur simultaneously [4]. This paper will briefly introduce the cellular and molecular pathways involved in these four stages (Fig. 1).
Priming: formation of the fibrotic microenvironment
In the initiation phase, various renal injurious stimuli such as infection, trauma, inflammation and autoimmunity act on renal resident cells to induce the initiation of fibrosis, the most important of which is the inflammatory response [5]. Inflammation is the most important initiator of renal fibrosis. Under various injury stimuli, inflammatory cells such as lymphocytes, macrophages and dendritic cells are recruited into the glomeruli and renal interstitium. At the same time, these injury stimuli will also activate the resident immune cells of the kidney, produce inflammatory mediators and form an inflammatory microenvironment [6]. Normally, inflammation is conducive to the repair of body injury. However, persistent inflammation is the key cause of initiating fibrosis. Renal resident cells and recruited inflammatory cells stimulated by persistent inflammation release pro-fibrotic cytokines such as inflammatory and growth factors [7] and form a fibrotic microenvironment. The formation of a fibrotic microenvironment promotes the activation and proliferation of myofibroblasts and the imbalance between ECM production and degradation. And then, the process of fibrosis also enters the activation stage.
Activation: activation of myofibroblasts
Under the stimulation of pro-fibrotic cytokines, matrix-producing cells in the kidney are activated, and fibroblasts, tubular epithelial cells, endothelial cells, podocytes, cells, and macrophages can produce ECM, but usually myofibroblasts are the main effector cells leading to excessive ECM deposition [3]. Myofibroblasts are considered to be a type of cell with both smooth muscle cell and fibroblast characteristics, which are rarely seen in the normal kidney, but are abundant in the fibrotic environment, so the source of myofibroblasts has been a research hotspot and is still controversial. The possible sources are renal resident fibroblasts, pericytes, epithelial cells, endothelial cells and circulating bone marrow-derived fibrocytes, which transform and proliferate into myofibroblasts under the action of pro-fibrotic cytokines. These cytokines also act on myofibroblasts to produce a large amount of ECM and αSMA, which leads to renal fibrosis [8,9,10].
At this stage, numerous molecular pathways activate myofibroblasts, and the most studied ones are mainly focused on signaling pathways such as TGF-β, Wnt, and Hedgehog, which also play an important role in the next stage. TGF-β is now recognized as the most critical pro-fibrotic factor that can activate myofibroblasts through standard Smad and non-standard MAPK signaling pathways. Wnt/β-catenin can activate myofibroblasts by regulating the expression of downstream genes and can also act by regulating the renin–angiotensin system (RAS). The Hedgehog pathway acts primarily through its ligand Sonic hedgehog (Shh) to regulate the transcription factor Gli. Some reviews have comprehensively summarized the relationship between these pathways and renal fibrosis, so it will not be introduced in detail here [11,12,13,14].
Execution: excessive deposition of ECM
In this stage, matrix-producing cells (especially myofibroblasts) activated by the above pathways begin to synthesize and secrete a large amount of ECM. At the same time, due to the influence of the fibrotic microenvironment, the balance between ECM production and degradation is out of balance, so that they are excessively deposited in glomeruli and renal tubules. This abnormal ECM accumulation will lead to glomerulosclerosis and tubulointerstitial fibrosis [4, 15]. Renal ECM is a non-cellular three-dimensional macromolecular network composed of various glycoproteins such as collagen, elastin, proteoglycan and fibronectin, of which type I and type III collagen and fibronectin play a major role in renal fibrosis, and these proteins play an important role in the process of renal fibrosis under the regulation of integrins and their downstream signals [16, 17].
The abnormal deposition of ECM is mainly because of the imbalance between production and degradation. Among them, the molecular pathways leading to increased ECM production mainly involve two aspects, on the one hand, TGF-β and other signaling pathways activate a large number of myofibroblasts to synthesize and secrete ECM during the activation stage, and on the other hand, these pro-fibrotic signals can directly promote the synthesis and secretion of ECM transcriptionally, in which anti-fibrotic factors (e.g., BMP-7, HGF) can inhibit the production of ECM by antagonizing the TGF-β signaling pathway [3]. The molecular pathways leading to reduced ECM degradation are mainly associated with changes in the expression of metalloproteinases (e.g., MMPs, ADAMs and ADAMTSs, etc.) and metalloproteinase inhibitors (TIMPs) in fibrotic environments [17].
Progression: progressive renal failure
A large amount of ECM has been deposited in the glomeruli and tubulointerstitium, resulting in the destruction of the original structure and the loss of renal function. At this time, renal fibrosis has entered a vicious cycle, which means that ECM is not only the result after injury, but also can act as a new stimulus to promote fibrosis. This pro-fibrotic effect may be related to the synergistic regulation of the YAP/TAZ and TGF-β signaling pathway [18]. In addition to the effects of ECM on renal function, since numerous pro-fibrotic factors similarly involve inflammation [19], oxidative stress [20], autophagy [21], and other signaling pathways, these cellular and molecular events also damage renal resident cells while promoting fibrosis, which can also lead to further loss of renal function. The progressive stage is the final stage of renal fibrosis, during which renal function continues to decline until ESRD is entered.
Therapeutic effects of natural products on renal fibrosis
Natural products have been considered as one of the essential sources for drug research and development, and in fact, 441 natural products and their derivatives were approved by the FDA for clinical use as drugs in the course of 1981 to 2019 [22]. Of the 371 medicinal substances included in the Ninth Edition of International Pharmacopoeia, more than 80 are natural products and their derivatives [23]. In the related research of renal fibrosis, a large number of natural products (especially the natural products in Traditional Chinese Medicine) have been confirmed to alleviate the process of renal fibrosis, protect the renal structure and improve renal function by regulating a variety of cytokines. This paper is divided into the following categories according to their different chemical structures and systematically summarizes the mechanism of action of natural products in Traditional Chinese Herbal Medicine in protecting renal fibrosis [24] (Table 1).
Flavonoids
Flavonoids are widely present in a variety of Chinese herbal medicines and are common natural products, which have various biological activities such as regulating oxidative stress, participating in cell cycle arrest, inducing apoptosis, autophagy, and so on [25]. In recent years, the anti-fibrotic effects of some flavonoids have become a research hotspot.
Quercetin is a natural flavonoid, which exists in many kinds of Chinese herbs and has many pharmacological effects, such as anti-inflammatory and anti-oxidation. Quercetin has been found to inhibit the expression of NF-κB p65 and IRF5 signaling pathways in the kidneys of UUO mice, which in turn inhibit M1 macrophage polarization and the expression of inflammatory factors and treat kidney injury. At the same time, it can reduce the expression of NF-κB p50 and IRF4 signaling pathways, inhibit M2 macrophage polarization, which reduces the deposition of ECM and alleviate renal interstitial fibrosis [26]. Liu et al. found that quercetin can also inhibit the expression of SHH signaling in the kidneys of UUO rats, prevent EMT in tubular epithelial cells, reduce excessive accumulation of ECM, and treat renal fibrosis [27]. In addition, it has also been found that quercetin can inhibit tubular epithelial cell senescence and reduce renal fibrosis by activating SIRT1/PINK1/Parkin-mediated mitosis [28].
Puerarin, a natural product extracted from Radix Puerariae, has been found to have an anti-fibrotic effect in recent years, and Zhou et al. found that puerarin can inhibit oxidative stress-induced tubular epithelial cell apoptosis and improve renal fibrosis by decreasing ROS production and the expression of MAPK signaling pathways in the kidneys of UUO mice [29]. Others have found that puerarin can reduce fibrosis by inhibiting the NF-κB p65/STAT3 and TGF-β/Smad signaling pathways and inhibiting the inflammation and excessive deposition of ECM in the kidney [30].
Dihydromyricetin is mainly derived from Chinese herbal medicines such as Ampelopsis Japonica and has a wide range of pharmacological activities. In the UUO mice model, dihydromyricetin inhibited TGF-β1-mediated miR-34a expression in the kidney, which up-regulated Klotho expression in tubular epithelial cells and alleviated renal fibrosis [31]. In high glucose-induced glomerular cells, dihydromyricetin can also improve renal fibrosis by regulating the Nrf2/HO-1 signaling pathway and inhibiting the deposition of ECM and the expression of fibronectin [32].
Calycosin is the main component of Astragalus membranaceus, and recent studies have shown that calycosin can improve the inflammatory response and fibrosis in diabetic nephropathy and protect the renal structure by inhibiting the expression of inflammatory mediators IL-33/ST2 signaling pathway and its downstream inflammatory factors [33].
Isoliquiritigenin is a natural flavonoid from Glycyrrhiza uralensis and has anti-fibrotic effects. Studies have shown that isoliquiritigenin directly inhibits the Mincle/Syk/NF-κB signaling pathway in UUO mice while inhibiting the polarization of M1 macrophages and reducing renal inflammation and fibrosis [34]. In addition, isoliquiritigenin also has a good therapeutic effect on kidney injury in diabetic nephropathy and treats renal fibrosis by regulating oxidative stress and inflammation mediated by the SIRT1 pathway [35]. Isoliquiritigenin can also inhibit the expression of ITGB3, ameliorate tubular cell senescence, and reduce renal fibrosis caused by senescence in the kidney [36].
5,7,3ʹ,4ʹ,5ʹ-pentahydroxy flavanone, Barleriside A, and Rhoifolin are natural flavonoids derived from Semen Plantaginis. 5,6,7,8,3ʹ,4ʹ-hexamethoxyflavone is a natural flavonoid derived from Poria cocos. Although they have different structures, they are all aryl hydrocarbon receptor (AHR) antagonists. In 5/6 nephrectomy rat models, they significantly reduced the secretion of ECM by regulating the aromatic hydrocarbon receptor signaling pathway, while inhibiting EMT of epithelial cells and alleviating renal fibrosis [37, 38].
Polyphenols
Polyphenols, also known as polyhydroxyphenols, have anti-inflammatory and anti-oxidation pharmacological effects, but also can regulate immunity and cell proliferation, and have a good therapeutic effect on various chronic inflammatory diseases [39,40,41]. Polyphenolic compounds have therefore also attracted much attention in the field of anti-fibrosis.
Curcumin is the main active component in Curcumaelongae Rhizoma, which has been demonstrated to have an excellent anti-fibrotic effect. It was found that mitochondrial dysfunction was significantly improved in renal interstitial cells of UUO rats after curcumin treatment, which in turn inhibited the activation of NLRP3 inflammasome and the expression of PI3K/AKT/mTOR signaling pathway, alleviating the progression of renal fibrosis by reducing the inflammatory response and regulating autophagy [42]. Curcumin can also attenuate EndMT and fibrosis occurring after kidney transplantation, which is similarly accomplished by activating cellular autophagy [43]. In addition, curcumin can act as an anti-oxidant that can alleviate renal fibrosis induced by scavenging excess ROS, inhibiting the activity of NADPH oxidase, improving mitochondrial redox balance [44].
Resveratrol is mainly derived from plants such as Cassiae Semen and Polygoni Cuspidati Rhizoma Et Radix and can also be obtained in plants such as grapes and peanuts, which are widely used in traditional medicines and dietary supplements. Resveratrol has been found to inhibit tubular epithelial cell EMT and fibroblast proliferation and differentiation, prevent myofibroblasts’ activation and improve renal fibrosis by inhibiting the activity of proliferation-related signaling pathways of tubular epithelial cells and interstitial cells [45]. In addition, resveratrol has been found to reduce renal oxidative stress and delay glomerulosclerosis and renal interstitial fibrosis in the aging kidney by regulating the renin–angiotensin system [46]. Chen et al. found that resveratrol could up-regulate SIRT1-mediated Klotho expression and the expression of anti-oxidant factors such as SOD and GSH and ameliorate progressive glomerulosclerosis in aging kidneys [47].
Epigallocatechin gallate (EGCG) is the most important polyphenolic compound in green tea and has an excellent protective effect on kidney injury caused by various causes. For chronic kidney injury due to cadmium intoxication, EGCG can ameliorate renal fibrosis by regulating the expression of TGF-β1 and its mediated microRNAs, restoring anti-oxidation enzymes activity in renal cells, inhibiting EMT and reducing the excessive deposition of ECM in renal cells [48]. In renal injury caused by salt-sensitive hypertension, EGCG reduces renal cellular inflammatory infiltration and oxidative stress, improves renal injury through anti-inflammatory and anti-oxidation effects, and improves renal fibrosis by inducing fibroblast apoptosis [49]. In diabetic nephropathy, EGCG can inhibit the expression of the TGF-β/Smad3 signaling pathway by binding with Notch1, attenuating fibrosis [50].
Salvianolic acid A is a natural product derived from Radix Salviae. In 5/6 nephrectomy rats model, salvianolic acid A significantly reduced the expression of p38 MAPK and its downstream signal inflammatory factors such as NF-κB, while inhibiting the expression of TGF-β1 and α-SMA in renal cells, reducing renal inflammation and renal interstitial fibrosis, and exerting a protective effect on the kidney [51].
Schisandrin B is mainly derived from the traditional Chinese medicine Schisandrae Chinensis Fructus, which has been found to inhibit the expression of Snail, Slug and Zeb2, preventing EMT in tubular epithelial cells, and reduce TGF-β1-mediated renal interstitial fibrosis by up-regulating the expression of miR-30e in renal cells [52].
Terpenoids
Terpenoids are important natural products in Chinese herbal medicines, which have many potential pharmacological activities such as anti-cancer, anti-fibrosis, anti-inflammatory, etc. [53,54,55]. As a potential drug, many studies have reported on the anti-fibrosis effects of terpenoids.
Poricoic acid A is one of the main active ingredients in Poria cocos and has an excellent anti-renal fibrosis effect. In the UUO mice model, poricoic acid A reduced the activity of the Wnt/β-catenin signaling pathway by enhancing the expression of tryptophan hydroxylase-1 (TPH-1), and also inhibited renal cell injury and fibroblast activation, exerting an anti-renal fibrosis effect [56]. In addition, poricoic acid A can also inhibit renal fibrosis by activating the AMPK signaling pathway to inhibit TGF-β1/Smad3 pathway-mediated deposition of ECM and activation of myofibroblasts [57].
Poricoic acid ZC, ZD, ZE, ZG, ZH, ZI, ZM, and ZP are novel tetracyclic triterpenoid compounds newly discovered in recent years, which are the main components of Poria cocos, and have renoprotective effects. Among them, Poricoic acid ZC, ZD, ZE, ZG, and ZH significantly ameliorate renal tubular interstitial fibrosis by inhibiting TGF-β/Smad and Wnt/β-catenin signaling pathways [58, 59]. Poricoic acid ZI reduces the secretion of ECM and attenuates epithelial cells EMT by inhibiting the activity of MMP-13 [60]. Poricoic acid ZM, ZP inhibits the expression of NF-κB and its downstream genes, promotes the expression of the Nrf2 signaling pathway, regulates AHR signaling pathway, attenuates oxidative stress and inflammatory response in the kidney, and treats renal fibrosis [61].
Alisol B23-acetate is a triterpenoid derived from Alisma Orientale. Chen et al. found that alisol B23-acetate could reduce renal fibrosis in UUO rats and 5/6 nephrectomy rats, which may be associated with improving gut microbiota and then reducing blood pressure and regulating the RAS. In addition, alisol B23-acetate can also inhibit the activation of Smad3 and the activation of the Wnt/β-catenin signaling pathway, induce fibroblast apoptosis and inhibit their activation and proliferation, reduce renal interstitial fibrosis [62].
Triptolide is mainly derived from Tripterygii Radix and has good efficacy in various kidney diseases. Studies have found that triptolide can specifically bind to MEX3C protein in the kidney and inhibit MEX3C-mediated K27-linked polyubiquitin chain modification of phosphatase and tensin homolog, thereby inhibiting EMT in tubular epithelial cells and protecting renal function [63]. In particular, in diabetic nephropathy models, triptolide can also restore autophagy in glomerular fibrotic cells by regulating the miR-141-3p/PTEN/Akt/mTOR signaling pathway to reduce fibrosis [64].
Alkaloids
Alkaloids have many pharmacological activities, such as anti-inflammatory, anti-oxidation, and anti-cancer. They are one of the natural sources of drugs and are the active ingredients of many kinds of traditional Chinese medicines [55, 65, 66]. At present, the research on the anti-fibrosis of alkaloids has become a current research hotspot.
Ligustrazine is a natural product of Chuanxiong Rhizoma and is mainly used to treat various kidney injuries. Yuan et al. showed that ligustrazine decreased the expression of TGF-β1 and CTGF, up-regulated the expression of HGF and BMP-7 in tubular epithelial cells, and inhibited EMT in tubular epithelial cells to alleviate renal interstitial fibrosis [67].
Oxymatrine can be mainly found in Sophorae Flavescentis Radix and has been demonstrated to have anti-organ fibrosis effects. Liu et al. found in vitro that oxymatrine could inhibit TGF-β1/Smad-mediated EMT in epithelial cells by up-regulating the expression of nuclear transcription co-repressor Ski-related novel protein N [68]. In addition, it has been found that in diabetic nephropathy mice treated with oxymatrine, the expression of inhibitor of differentiation 2 (Id2) was significantly increased in the kidney, which suggests that oxymatrine may play a role in anti-renal fibrosis by restoring the expression of Id2 and promoting the binding of Id2 and Twist in the damaged kidney thereby regulating the expression of genes downstream of Twist and inhibiting EMT in tubular epithelial cells [69].
Leonurine is an active component in Leonuri Herba and has pharmacological activities of anti-inflammatory and anti-oxidation. In UUO mice, leonurine ameliorates inflammation and renal interstitial fibrosis in the kidney by inhibiting the ROS-mediated NF-κB signaling pathway and TGF-β/Smad3 signaling pathway [70].
Berberine is a natural product of Chinese herbal medicine such as Coptidis Rhizoma and Phellodendri Chinrnsis Cortex, widely used in clinical practice. Berberine has been found to inhibit the expression of the TGF-β/Smad pathway while promoting the Nrf2/HO-1 pathway, preventing EMT and excessive accumulation of ECM in tubular epithelial cells and alleviating renal fibrosis [71]. In addition, berberine also inhibits Notch/snail expression in tubular epithelial cells and prevents EMT progression and renal interstitial fibrosis [72].
Glycosides
Glycosides are the active ingredients of many kinds of traditional Chinese medicines, which have many potential pharmacological activities and also have good efficacy in anti-inflammatory and anti-fibrosis [55, 73].
Astragaloside IV (AS-IV) is a natural product in Astragalus membranaceus, which has a good renoprotective effect and can improve renal fibrosis mainly through anti-inflammatory and anti-oxidative stress. Zhang et al.’s study found that AS-IV could significantly up-regulate the expression of TRX1, decrease the expression of cytokines such as TXNIP, PANX1, NOD2, and JUN in the kidneys of DN rats, inhibit inflammation-related NLR signaling pathway expression by enhancing the TRX anti-oxidant system, and attenuates renal injury, fibrosis, and microstructural changes induced by diabetic nephropathy [74]. Zhou et al. found that AS-IV can attenuate inflammation and inhibit renal fibrosis by inhibiting TLR4/NF-κB signaling pathway [75]. In vitro experiments have shown that AS-IV can also inhibit EMT in tubular epithelial cells and ameliorate renal fibrosis by inhibiting the mTORC1/p70S6K signaling pathway [76]. In addition, AS-IV also has a good therapeutic effect in kidney injury induced by some nephrotoxic drugs. In a study on tacrolimus-induced chronic nephrotoxicity, AS-IV was found to reduce ROS accumulation and renal interstitial fibrosis by regulating the p62-Keap1-Nrf2 signaling pathway [77].
Salidroside is the main component of Rhodiola Rosea, which has the function of protecting the kidney. Studies have confirmed that salidroside reduces excessive deposition of ECM, prevents epithelial cell EMT, and ameliorates renal fibrosis by inhibiting the expression of TLR4/MAPK/NF-κB signaling pathway and its downstream pro-inflammatory and pro-fibrotic factors [78]. Salidroside can also regulate the SIRT1/PGC-1α signaling pathway to improve mitochondrial dysfunction, reduce renal fibrosis in diabetic nephropathy, and protect renal function [79]. Salidroside can also modulate Wnt/β-catenin signaling in a model of adriamycin-induced nephropathy that alleviates podocyte injury and renal fibrosis [80].
Dioscin, a natural product in Rhizoma Dioscoreae, has been found to up-regulate the expression of the SIRT3 gene, inhibit renal fibrosis mediated by TGF-β1/Smad3 signaling pathway, and ameliorate fructose-induced kidney injury [81].
Quinones
Quinones are natural products widely distributed in a variety of traditional Chinese medicines and have been reported to have various pharmacological activities such as antimalarial and anti-tumor activities, and quinones also play an important role in anti-fibrosis [82, 83].
Tanshinone IIA is mainly derived from Radix Salviae and has a significant therapeutic effect on various acute and chronic kidney injuries. Tanshinone IIA significantly reduced excessive deposition of ECM and inflammatory cell infiltration, inhibited renal fibrosis and renal inflammation, and protected renal function by regulating the expression of TGF-β/Smad and NF-κB signaling pathways in 5/6 nephrectomized rats [84]. In folic acid-induced acute kidney injury, tanshinone IIA attenuates tubular inflammatory infiltration and improves renal interstitial fibrosis by inhibiting the excessive activation of GSK3β and subsequent excessive activation of the MAPK pathway [85]. In addition, Xu et al. found that tanshinone IIA can also alleviate oxidative stress status by increasing SOD activity, which inhibits ER stress mediated by the PERK pathway and reduces the expression of TGF-β1, and ameliorates renal fibrosis caused by diabetic nephropathy [86].
Emodin is a natural product in Chinese herbal medicine such as Rheum Offcinale and Polygoni Cuspidati Rhizoma Et Radix, which has anti-fibrotic pharmacological effects. Emodin has been found to reduce renal fibrosis in DN rats by regulating the AMPK/mTOR signaling pathway in the kidney, promoting podocyte autophagy, and inhibiting apoptosis [87]. Emodin can also improve renal interstitial fibrosis by up-regulating the expression of BMP7 and promoting autophagy in tubular epithelial cells and inhibiting their EMT [88]. In addition, in the UUO rats model, emodin inhibited the expression of enhancer of zeste homolog 2, which in turn inhibited trimethylation on Lysine 27 of histone H3 and alleviated the process of tubulointerstitial fibrosis [89].
Chrysophanol is a natural anthraquinone compound in Rheum Officinale with a variety of pharmacological activities. It was found that Chrysophanol alleviated renal fibrosis in UUO mice by modulating the TGF-β/Smad signaling pathway, especially inhibiting phosphorylation of Smad3 [90].
Conclusion
The incidence and mortality of CKD are increasing yearly worldwide, and renal fibrosis, as the primary pathological manifestation of CKD, has been a targeted therapeutic target. Natural products in Chinese herbal medicine perform well in the process of anti-renal fibrosis due to their anti-oxidation and anti-inflammation pharmacological effects. This review comprehensively summarizes the therapeutic effects and the molecular mechanisms of natural products in Chinese herbal medicine on renal fibrosis in recent years. These studies have shown that natural products have great potential in anti-fibrosis and are promising as novel therapeutic drugs for CKD.
However, some issues deserve our consideration: First, these studies are based on animal experiments and cell experiments, they are not enough to support the clinical application of these natural products, and we should identify natural products with a precise mechanism of action based on high-quality studies further to confirm the safety and effectiveness of clinical efficacy. Second, existing studies mainly focus on inflammation and oxidative stress, TGF-β/Smad, and Wnt/β-catenin signaling pathways, which have limitations and lack the diversity of therapeutic targets, so more studies are needed to explore other cellular and molecular pathways that may be involved. Third, the effects of the kinetics and pharmacodynamics of natural products on the treatment of renal fibrosis should also be considered. For example, emodin has been shown to have an anti-fibrotic effect in animal experiments and in vitro experiments. However, its poor oral availability may affect clinical efficacy [91]. Finally, there is also a relatively interesting question, whether it needs to rely on the guidance of TCM theory in the search for natural products in Chinese herbal medicines to treat renal fibrosis, because some natural products may not be commonly used drugs in TCM to treat kidney disease, but they have been shown to have an anti-fibrosis effect on other organs, and whether this anti-fibrosis effect is also applicable in the kidney is also a question worth pondering.
In conclusion, this review introduces the pathological processes involved in renal fibrosis, systematically summarizes the latest research on the treatment of renal fibrosis with natural products of Chinese herbal medicines, and points out the problems that need attention in future research, hoping that this paper can provide help for further research in the future.
Availability of data and materials
All the data used to support the findings of this study are available from the corresponding author upon reasonable request.
Change history
24 October 2022
A Correction to this paper has been published: https://doi.org/10.1186/s13020-022-00668-7
Abbreviations
- AS-IV:
-
Astragaloside IV
- CKD:
-
Chronic kidney disease
- ECM:
-
Extracellular matrix
- EGCG:
-
Epigallocatechin gallate
- EMT:
-
Epithelial–mesenchymal transition
- ESRD:
-
End-stage renal disease
- RAS:
-
Renin–angiotensin system
- TCM:
-
Traditional Chinese medicine
References
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (Lond, Engl). 2020;395(10225):709–33. https://doi.org/10.1016/S0140-6736(20)30045-3.
Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16(5):269–88. https://doi.org/10.1038/s41581-019-0248-y.
Nastase MV, Zeng-Brouwers J, Wygrecka M, Schaefer L. Targeting renal fibrosis: mechanisms and drug delivery systems. Adv Drug Deliv Rev. 2018;129:295–307. https://doi.org/10.1016/j.addr.2017.12.019.
Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7(12):684–96. https://doi.org/10.1038/nrneph.2011.149.
Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16–36. https://doi.org/10.1016/j.mam.2018.06.002.
Andrade-Oliveira V, Foresto-Neto O, Watanabe I, Zatz R, Câmara N. Inflammation in renal diseases: new and old players. Front Pharmacol. 2019;10:1192. https://doi.org/10.3389/fphar.2019.01192.
Lv W, Booz GW, Wang Y, Fan F, Roman RJ. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018;820:65–76. https://doi.org/10.1016/j.ejphar.2017.12.016.
LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19(8):1047–53. https://doi.org/10.1038/nm.3218.
Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015;87(2):297–307. https://doi.org/10.1038/ki.2014.287.
Yuan Q, Tan RJ, Liu Y. Myofibroblast in kidney fibrosis: origin, activation, and regulation. Adv Exp Med Biol. 2019;1165:253–83. https://doi.org/10.1007/978-981-13-8871-2_12.
Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–38. https://doi.org/10.1038/nrneph.2016.48.
Edeling M, Ragi G, Huang S, Pavenstädt H, Susztak K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol. 2016;12(7):426–39. https://doi.org/10.1038/nrneph.2016.54.
Hu HH, Cao G, Wu XQ, Vaziri ND, Zhao YY. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev. 2020;60:101063. https://doi.org/10.1016/j.arr.2020.101063.
Zhou D, Tan RJ, Liu Y. Sonic hedgehog signaling in kidney fibrosis: a master communicator. Sci China Life Sci. 2016;59(9):920–9. https://doi.org/10.1007/s11427-016-0020-y.
Sun YB, Qu X, Caruana G, Li J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 2016;92(3):102–7. https://doi.org/10.1016/j.diff.2016.05.008.
Bülow RD, Boor P. Extracellular matrix in kidney fibrosis: more than just a scaffold. J Histochem Cytochem. 2019;67(9):643–61. https://doi.org/10.1369/0022155419849388.
Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. https://doi.org/10.1016/j.addr.2015.11.001.
Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, et al. YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J Am Soc Nephrol. 2016;27(10):3117–28. https://doi.org/10.1681/ASN.2015050499.
Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15(3):144–58. https://doi.org/10.1038/s41581-019-0110-2.
Duni A, Liakopoulos V, Roumeliotis S, Peschos D, Dounousi E. Oxidative stress in the pathogenesis and evolution of Chronic Kidney Disease: untangling Ariadne’s thread. Int J Mol Sci. 2019;20(15):3711. https://doi.org/10.3390/ijms20153711.
Tang C, Livingston MJ, Liu Z, Dong Z. Autophagy in kidney homeostasis and disease. Nat Rev Nephrol. 2020;16(9):489–508. https://doi.org/10.1038/s41581-020-0309-2.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. https://doi.org/10.1021/acs.jnatprod.9b01285.
Zhang L, Song J, Kong L, Yuan T, Li W, Zhang W, et al. The strategies and techniques of drug discovery from natural products. Pharmacol Ther. 2020;216:107686. https://doi.org/10.1016/j.pharmthera.2020.107686.
Chen DQ, Hu HH, Wang YN, Feng YL, Cao G, Zhao YY. Natural products for the prevention and treatment of kidney disease. Phytomedicine. 2018;50:50–60. https://doi.org/10.1016/j.phymed.2018.09.182.
Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12(2):457. https://doi.org/10.3390/nu12020457.
Lu H, Wu L, Liu L, Ruan Q, Zhang X, Hong W, et al. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem Pharmacol. 2018;154:203–12. https://doi.org/10.1016/j.bcp.2018.05.007.
Liu X, Sun N, Mo N, Lu S, Song E, Ren C, et al. Quercetin inhibits kidney fibrosis and the epithelial to mesenchymal transition of the renal tubular system involving suppression of the Sonic Hedgehog signaling pathway. Food Funct. 2019;10(6):3782–97. https://doi.org/10.1039/c9fo00373h.
Liu T, Yang Q, Zhang X, Qin R, Shan W, Zhang H, et al. Quercetin alleviates kidney fibrosis by reducing renal tubular epithelial cell senescence through the SIRT1/PINK1/mitophagy axis. Life Sci. 2020;257:118116. https://doi.org/10.1016/j.lfs.2020.118116.
Zhou X, Bai C, Sun X, Gong X, Yang Y, Chen C, et al. Puerarin attenuates renal fibrosis by reducing oxidative stress induced-epithelial cell apoptosis via MAPK signal pathways in vivo and in vitro. Ren Fail. 2017;39(1):423–31. https://doi.org/10.1080/0886022X.2017.1305409.
Wang J, Ge S, Wang Y, Liu Y, Qiu L, Li J, et al. Puerarin alleviates UUO-induced inflammation and fibrosis by regulating the NF-κB P65/STAT3 and TGFβ1/Smads signaling pathways. Drug Des Dev Ther. 2021;15:3697–708. https://doi.org/10.2147/DDDT.S321879.
Liu Y, Bi X, Xiong J, Han W, Xiao T, Xu X, et al. MicroRNA-34a promotes renal fibrosis by downregulation of klotho in tubular epithelial cells. Mol Ther. 2019;27(5):1051–65. https://doi.org/10.1016/j.ymthe.2019.02.009.
Dong C, Wu G, Li H, Qiao Y, Gao S. Ampelopsin inhibits high glucose-induced extracellular matrix accumulation and oxidative stress in mesangial cells through activating the Nrf2/HO-1 pathway. Phytother Res. 2020;34(8):2044–52. https://doi.org/10.1002/ptr.6668.
Elsherbiny NM, Said E, Atef H, Zaitone SA. Renoprotective effect of calycosin in high fat diet-fed/STZ injected rats: effect on IL-33/ST2 signaling, oxidative stress and fibrosis suppression. Chem Biol Interact. 2020;315:108897. https://doi.org/10.1016/j.cbi.2019.108897.
Liao Y, Tan RZ, Li JC, Liu TT, Zhong X, Yan Y, et al. Isoliquiritigenin Attenuates UUO-induced renal inflammation and fibrosis by inhibiting Mincle/Syk/NF-Kappa B signaling pathway. Drug Des Dev Ther. 2020;14:1455–68. https://doi.org/10.2147/DDDT.S243420.
Huang X, Shi Y, Chen H, Le R, Gong X, Xu K, et al. Isoliquiritigenin prevents hyperglycemia-induced renal injuries by inhibiting inflammation and oxidative stress via SIRT1-dependent mechanism. Cell Death Dis. 2020;11(12):1040. https://doi.org/10.1038/s41419-020-03260-9.
Li S, Jiang S, Zhang Q, Jin B, Lv D, Li W, et al. Integrin β3 induction promotes tubular cell senescence and kidney fibrosis. Front Cell Dev Biol. 2021;9:733831. https://doi.org/10.3389/fcell.2021.733831.
Miao H, Cao G, Wu XQ, Chen YY, Chen DQ, Chen L, et al. Identification of endogenous 1-aminopyrene as a novel mediator of progressive chronic kidney disease via aryl hydrocarbon receptor activation. Br J Pharmacol. 2020;177(15):3415–35. https://doi.org/10.1111/bph.15062.
Miao H, Wu XQ, Wang YN, Chen DQ, Chen L, Vaziri ND, et al. 1-Hydroxypyrene mediates renal fibrosis through aryl hydrocarbon receptor signalling pathway. Br J Pharmacol. 2022;179(1):103–24. https://doi.org/10.1111/bph.15705.
Silva AS, Reboredo-Rodríguez P, Süntar I, Sureda A, Belwal T, Loizzo MR, et al. Evaluation of the status quo of polyphenols analysis: part I-phytochemistry, bioactivity, interactions, and industrial uses. Compr Rev Food Sci Food Saf. 2020;19(6):3191–218. https://doi.org/10.1111/1541-4337.12629.
Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. https://doi.org/10.3390/nu10111618.
Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Woźniak K, Aprotosoaie AC, et al. Bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr. 2020;60(4):626–59. https://doi.org/10.1080/10408398.2018.1546669.
Lu M, Li H, Liu W, Zhang X, Li L, Zhou H. Curcumin attenuates renal interstitial fibrosis by regulating autophagy and retaining mitochondrial function in unilateral ureteral obstruction rats. Basic Clin Pharmacol Toxicol. 2021;128(4):594–604. https://doi.org/10.1111/bcpt.13550.
Zhou J, Yao M, Zhu M, Li M, Ke Q, Wu B, et al. Curcumin blunts IL-6 dependent endothelial-to-mesenchymal transition to alleviate renal allograft fibrosis through autophagy activation. Front Immunol. 2021;12:656242. https://doi.org/10.3389/fimmu.2021.656242.
Trujillo J, Molina-Jijón E, Medina-Campos ON, Rodríguez-Muñoz R, Reyes JL, Loredo ML, et al. Curcumin prevents cisplatin-induced decrease in the tight and adherens junctions: relation to oxidative stress. Food Funct. 2016;7(1):279–93. https://doi.org/10.1039/c5fo00624d.
Zhang X, Lu H, Xie S, Wu C, Guo Y, Xiao Y, et al. Resveratrol suppresses the myofibroblastic phenotype and fibrosis formation in kidneys via proliferation-related signalling pathways. Br J Pharmacol. 2019;176(24):4745–59. https://doi.org/10.1111/bph.14842.
Jang IA, Kim EN, Lim JH, Kim MY, Ban TH, Yoon HE, et al. Effects of resveratrol on the renin-angiotensin system in the aging kidney. Nutrients. 2018;10(11):1741. https://doi.org/10.3390/nu10111741.
Chen CC, Chang ZY, Tsai FJ, Chen SY. Resveratrol pretreatment ameliorates concanavalin A-induced advanced renal glomerulosclerosis in aged mice through upregulation of Sirtuin 1-mediated Klotho expression. Int J Mol Sci. 2020;21(18):6766. https://doi.org/10.3390/ijms21186766.
Chen J, Du L, Li J, Song H. Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem Toxicol. 2016;96:70–8. https://doi.org/10.1016/j.fct.2016.07.030.
Luo D, Xu J, Chen X, Zhu X, Liu S, Li J, et al. (-)-Epigallocatechin-3-gallate (EGCG) attenuates salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Sci Rep. 2020;10(1):4783. https://doi.org/10.1038/s41598-020-61794-6.
Zhu QQ, Yang XY, Zhang XJ, Yu CJ, Pang QQ, Huang YW, et al. EGCG targeting Notch to attenuate renal fibrosis via inhibition of TGFβ/Smad3 signaling pathway activation in streptozotocin-induced diabetic mice. Food Funct. 2020;11(11):9686–95. https://doi.org/10.1039/d0fo01542c.
Zhang HF, Wang YL, Gao C, Gu YT, Huang J, Wang JH, et al. Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-κB and p38 MAPK signaling pathways in 5/6 nephrectomized rats. Acta Pharmacol Sin. 2018;39(12):1855–64. https://doi.org/10.1038/s41401-018-0026-6.
Cao G, Li S, Shi H, Yin P, Chen J, Li H, et al. Schisandrin B attenuates renal fibrosis via miR-30e-mediated inhibition of EMT. Toxicol Appl Pharmacol. 2019;385:114769. https://doi.org/10.1016/j.taap.2019.114769.
Hortelano S, González-Cofrade L, Cuadrado I, de Las Heras B. Current status of terpenoids as inflammasome inhibitors. Biochem Pharmacol. 2020;172:113739. https://doi.org/10.1016/j.bcp.2019.113739.
Masood N, Dubey V, Luqman S. Activation of Caspase-3 by terpenoids and flavonoids in different types of cancer cells. Curr Top Med Chem. 2020;20(21):1876–87. https://doi.org/10.2174/1568026620666200710101859.
Ma X, Jiang Y, Wen J, Zhao Y, Zeng J, Guo Y. A comprehensive review of natural products to fight liver fibrosis: alkaloids, terpenoids, glycosides, coumarins and other compounds. Eur J Pharmacol. 2020;888:173578. https://doi.org/10.1016/j.ejphar.2020.173578.
Chen DQ, Wu XQ, Chen L, Hu HH, Wang YN, Zhao YY. Poricoic acid A as a modulator of TPH-1 expression inhibits renal fibrosis via modulating protein stability of β-catenin and β-catenin-mediated transcription. Ther Adv Chronic Dis. 2020;11:2040622320962648. https://doi.org/10.1177/2040622320962648.
Chen DQ, Wang YN, Vaziri ND, Chen L, Hu HH, Zhao YY. Poricoic acid A activates AMPK to attenuate fibroblast activation and abnormal extracellular matrix remodelling in renal fibrosis. Phytomedicine. 2020;72:153232. https://doi.org/10.1016/j.phymed.2020.153232.
Wang M, Chen DQ, Chen L, Cao G, Zhao H, Liu D, et al. Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β-catenin pathway against renal fibrosis. Br J Pharmacol. 2018;175(13):2689–708. https://doi.org/10.1111/bph.14333.
Wang M, Chen DQ, Chen L, Liu D, Zhao H, Zhang ZH, et al. Novel RAS inhibitors poricoic acid ZG and poricoic acid ZH attenuate renal fibrosis via a Wnt/β-catenin pathway and targeted phosphorylation of smad3 signaling. J Agric Food Chem. 2018;66(8):1828–42. https://doi.org/10.1021/acs.jafc.8b00099.
Chen L, Cao G, Wang M, Feng YL, Chen DQ, Vaziri ND, et al. The matrix metalloproteinase-13 inhibitor poricoic acid ZI ameliorates renal fibrosis by mitigating epithelial-mesenchymal transition. Mol Nutr Food Res. 2019. https://doi.org/10.1002/mnfr.201900132.
Wang M, Hu HH, Chen YY, Chen L, Wu XQ, Zhao YY. Novel poricoic acids attenuate renal fibrosis through regulating redox signalling and aryl hydrocarbon receptor activation. Phytomed Int J Phytother Phytopharmacol. 2020;79:153323. https://doi.org/10.1016/j.phymed.2020.153323.
Chen H, Wang MC, Chen YY, Chen L, Wang YN, Vaziri ND, et al. Alisol B 23-acetate attenuates CKD progression by regulating the renin-angiotensin system and gut-kidney axis. Ther Adv Chronic Dis. 2020;11:2040622320920025. https://doi.org/10.1177/2040622320920025.
Li Y, Hu Q, Li C, Liang K, Xiang Y, Hsiao H, et al. PTEN-induced partial epithelial-mesenchymal transition drives diabetic kidney disease. J Clin Invest. 2019;129(3):1129–51. https://doi.org/10.1172/JCI121987.
Li XY, Wang SS, Han Z, Han F, Chang YP, Yang Y, et al. Triptolide restores autophagy to alleviate diabetic renal fibrosis through the miR-141-3p/PTEN/Akt/mTOR pathway. Mol Ther Nucleic Acids. 2017;9:48–56. https://doi.org/10.1016/j.omtn.2017.08.011.
Liu C, Yang S, Wang K, Bao X, Liu Y, Zhou S, et al. Alkaloids from Traditional Chinese Medicine against hepatocellular carcinoma. Biomed Pharmacother. 2019;120:109543. https://doi.org/10.1016/j.biopha.2019.109543.
Alasvand M, Assadollahi V, Ambra R, Hedayati E, Kooti W, Peluso I. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. https://doi.org/10.1155/2019/9475908.
Yuan XP, Liu LS, Fu Q, Wang CX. Effects of ligustrazine on ureteral obstruction-induced renal tubulointerstitial fibrosis. Phytother Res. 2012;26(5):697–703. https://doi.org/10.1002/ptr.3630.
Liu L, Wang Y, Yan R, Li S, Shi M, Xiao Y, et al. Oxymatrine inhibits renal tubular EMT induced by high glucose via upregulation of SnoN and inhibition of TGF-β1/Smad signaling pathway. PLoS ONE. 2016;11(3):e0151986. https://doi.org/10.1371/journal.pone.0151986.
Xiao Y, Peng C, Xiao Y, Liang D, Yuan Z, Li Z, et al. Oxymatrine inhibits twist-mediated renal tubulointerstitial fibrosis by upregulating Id2 expression. Front Physiol. 2020;11:599. https://doi.org/10.3389/fphys.2020.00599.
Cheng H, Bo Y, Shen W, Tan J, Jia Z, Xu C, et al. Leonurine ameliorates kidney fibrosis via suppressing TGF-β and NF-κB signaling pathway in UUO mice. Int Immunopharmacol. 2015;25(2):406–15. https://doi.org/10.1016/j.intimp.2015.02.023.
Zhang X, He H, Liang D, Jiang Y, Liang W, Chi ZH, et al. Protective effects of berberine on renal injury in Streptozotocin (STZ)-induced diabetic mice. Int J Mol Sci. 2016;17(8):1327. https://doi.org/10.3390/ijms17081327.
Yang G, Zhao Z, Zhang X, Wu A, Huang Y, Miao Y, et al. Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the Notch/snail pathway in diabetic nephropathy model KKAy mice. Drug Des Dev Ther. 2017;11:1065–79. https://doi.org/10.2147/DDDT.S124971.
Wu S, Pang Y, He Y, Zhang X, Peng L, Guo J, et al. A comprehensive review of natural products against atopic dermatitis: flavonoids, alkaloids, terpenes, glycosides and other compounds. Biomed Pharmacother. 2021;140:111741. https://doi.org/10.1016/j.biopha.2021.111741.
Zhang Y, Tao C, Xuan C, Jiang J, Cao W. Transcriptomic analysis reveals the protection of Astragaloside IV against diabetic nephropathy by modulating inflammation. Oxid Med Cell Longev. 2020;2020:9542165. https://doi.org/10.1155/2020/9542165.
Zhou X, Sun X, Gong X, Yang Y, Chen C, Shan G, et al. Astragaloside IV from Astragalus membranaceus ameliorates renal interstitial fibrosis by inhibiting inflammation via TLR4/NF-кB in vivo and in vitro. Int Immunopharmacol. 2017;42:18–24. https://doi.org/10.1016/j.intimp.2016.11.006.
Chen X, Yang Y, Liu C, Chen Z, Wang D. Astragaloside IV ameliorates high glucose-induced renal tubular epithelial-mesenchymal transition by blocking mTORC1/p70S6K signaling in HK-2 cells. Int J Mol Med. 2019;43(2):709–16. https://doi.org/10.3892/ijmm.2018.3999.
Gao P, Du X, Liu L, Xu H, Liu M, Guan X, et al. Astragaloside IV alleviates tacrolimus-induced chronic nephrotoxicity via p62-Keap1-Nrf2 pathway. Front Pharmacol. 2021;11:610102. https://doi.org/10.3389/fphar.2020.610102.
Li R, Guo Y, Zhang Y, Zhang X, Zhu L, Yan T. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-κB and MAPK signaling pathways. Int J Mol Sci. 2019;20(5):1103. https://doi.org/10.3390/ijms20051103.
Xue H, Li P, Luo Y, Wu C, Liu Y, Qin X, et al. Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine. 2019;54:240–7. https://doi.org/10.1016/j.phymed.2018.10.031.
Huang X, Xue H, Ma J, Zhang Y, Zhang J, Liu Y, et al. Salidroside ameliorates Adriamycin nephropathy in mice by inhibiting β-catenin activity. J Cell Mol Med. 2019;23(6):4443–53. https://doi.org/10.1111/jcmm.14340.
Qiao Y, Xu L, Tao X, Yin L, Qi Y, Xu Y, et al. Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicol Lett. 2018;284:37–45. https://doi.org/10.1016/j.toxlet.2017.11.031.
Patel O, Beteck RM, Legoabe LJ. Antimalarial application of quinones: a recent update. Eur J Med Chem. 2021;210:113084. https://doi.org/10.1016/j.ejmech.2020.113084.
Siegel D, Yan C, Ross D. NAD(P)H:quinone oxidoreductase 1 (NQO1) in the sensitivity and resistance to antitumor quinones. Biochem Pharmacol. 2012;83(8):1033–40. https://doi.org/10.1016/j.bcp.2011.12.017.
Wang DT, Huang RH, Cheng X, Zhang ZH, Yang YJ, Lin X. Tanshinone IIA attenuates renal fibrosis and inflammation via altering expression of TGF-β/Smad and NF-κB signaling pathway in 5/6 nephrectomized rats. Int Immunopharmacol. 2015;26(1):4–12. https://doi.org/10.1016/j.intimp.2015.02.027.
Jiang C, Zhu W, Yan X, Shao Q, Xu B, Zhang M, et al. Rescue therapy with Tanshinone IIA hinders transition of acute kidney injury to chronic kidney disease via targeting GSK3β. Sci Rep. 2016;6:36698. https://doi.org/10.1038/srep36698.
Xu S, He L, Ding K, Zhang L, Xu X, Wang S, et al. Tanshinone IIA ameliorates streptozotocin-induced diabetic nephropathy, partly by attenuating PERK pathway-induced fibrosis. Drug Des Dev Ther. 2020;14:5773–82. https://doi.org/10.2147/DDDT.S257734.
Liu H, Wang Q, Shi G, Yang W, Zhang Y, Chen W, et al. Emodin ameliorates renal damage and podocyte injury in a rat model of diabetic nephropathy via regulating AMPK/mTOR-mediated autophagy signaling pathway. Diabetes Metab Syndr Obes. 2021;14:1253–66. https://doi.org/10.2147/DMSO.S299375.
Liu W, Gu R, Lou Y, He C, Zhang Q, Li D. Emodin-induced autophagic cell death hinders epithelial-mesenchymal transition via regulation of BMP-7/TGF-β1 in renal fibrosis. J Pharmacol Sci. 2021;146(4):216–25. https://doi.org/10.1016/j.jphs.2021.03.009.
Xu L, Gao J, Huang D, Lin P, Yao D, Yang F, et al. Emodin ameliorates tubulointerstitial fibrosis in obstructed kidneys by inhibiting EZH2. Biochem Biophys Res Commun. 2021;534:279–85. https://doi.org/10.1016/j.bbrc.2020.11.094.
Dou F, Ding Y, Wang C, Duan J, Wang W, Xu H, Zhao X, Wang J, Wen A. Chrysophanol ameliorates renal interstitial fibrosis by inhibiting the TGF-β/Smad signaling pathway. Biochem Pharmacol. 2020;180:114079. https://doi.org/10.1016/j.bcp.2020.114079.
Huang J, Gong W, Chen Z, Huang J, Chen Q, Huang H, et al. Emodin self-emulsifying platform ameliorates the expression of FN, ICAM-1 and TGF-β1 in AGEs-induced glomerular mesangial cells by promoting absorption. Eur J Pharm Sci. 2017;99:128–36. https://doi.org/10.1016/j.ejps.2016.12.012.
Acknowledgements
We are grateful the support from First Teaching Hospital of Tianjin University of Traditional Chinese Medicine.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ZJZ, YHQ and BY contributed to the project design and paper writing. YRZ, XC and JL contributed to search literature. HQZ and QML contributed to drawing the figure and table. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The co-first authors have been updated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Z., Qiao, Y., Zhao, Y. et al. Natural products: potential drugs for the treatment of renal fibrosis. Chin Med 17, 98 (2022). https://doi.org/10.1186/s13020-022-00646-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-022-00646-z