Abstract
Background
The acknowledgment that conservative oxygen therapy (COT) was related to better prognosis in the intensive care unit (ICU) was challenged recently. We conducted an updated meta-analysis aimed to determine whether liberal oxygen therapy (LOT) or COT is associated with better improve clinical outcomes.
Methods
We systematically searched the electronic databases (PubMed, Web of Science and Embase) up to May 2021 for randomized controlled trials (RCTs). The primary outcome was the mortality of the final follow-up time and secondary outcomes were ICU mortality, the ICU length of stay and the number of ventilator-free days.
Results
A total of 7 RCTs were included, with 2166 patients admitted to the ICU. There was no significant difference in the primary outcome between the LOT and COT. Additionally, LOT could not significantly increase ICU mortality and the ICU length of stay compared with COT.
Conclusions
The present study showed that COT was not significantly superior to LOT in clinical outcomes. Therefore, additional high-quality studies with novel designs are required to further elucidate this controversy.
Similar content being viewed by others
Background
In critically ill patients, the provision of supplemental oxygen is universal to patients who require invasive mechanical ventilation in the intensive care unit (ICU) [1]. The use of supplemental oxygen is aimed to prevent or reverse hypoxemia. The liberal oxygen therapy (LOT) may provide a baseline of safety against hypoxia [2, 3]. However, excess oxygen delivery could expose patients to hyperoxia that leads to potential iatrogenic harm, such as pulmonary injury, interstitial fibrosis, central nervous system toxicity, etc. [2, 4,5,6]. Conservative oxygen therapy (COT) could minimize the chance of exposure to high levels of oxygen and reduce the occurrence of hyperoxia [7]. In a previous meta-analysis of randomized trials about acutely ill adults, COT has been proved to be associated with lower in-hospital mortality compared with LOT [8].
Notably, several recent randomized controlled trials (RCTs) did not support the superiority of COT over LOT in ICU patients. Mackle et al. and Barrot et al. suggested that implementation of conservative-oxygenation strategy did not significantly affect the number of ventilator-free days and decrease the mortality rate when compared with LOT [7, 9]. Another RCT demonstrated that COT did not significantly improve the prognosis when compared to LOT in ICU patients with sepsis. The point estimate of treatment effect even preferred the LOT approach [10].
Therefore, giving the fact that several RCTs comparing LOT versus COT for ICU patients suggested conflicting results, we conducted an updated meta-analysis of RCTs involving ICU patients to compare LOT versus COT and synthesized the prognosis results.
Methods
Search strategies
The literature search was performed in Pubmed, Embase, and MEDLINE by combining the following keywords: (“conservative oxygen therapy” or “COT”), (“conventional oxygen therapy” or “liberal oxygen therapy” or “LOT” or “usual oxygen therapy”), (“ICU” or “intensive critical care” or “critical care”), and (“RCT” or “Randomized Controlled Trial” or “Controlled clinical trial” or “Random*”). In addition, the references of related articles were searched manually for studies if missed in the database searches.
Inclusion and exclusion criteria
The included studies in the meta-analysis met the following criteria: (1) randomized controlled trials; (2) subjects in studies were patients admitted in ICU who were expected to remain mechanically ventilated; (3) the patients were assigned to receive either COT or LOT; (4) the outcomes included deaths in final follow-up time, ICU mortality, length in ICU days, and mechanical ventilation-free days. The exclusion criteria were as follows: (1) meta-analyses, reviews, case reports, and protocols; (2) non-English articles. Two reviewers (LL, YT) independently screened the titles and abstracts following the criteria and review the full text of eligible studies to determine the final inclusion. Any difference was resolved with a third reviewer.
Data extraction
Data from the included studies were extracted by two reviewers (LL, YT) independently. A third reviewer was ready to adjudicate any unsolved disagreements. The following variables were extracted: the first author’s name, the country of study, the publication year, the study design, population, follow-up duration, in-hospital details, such as type of admission to ICU, median PaO2 and SpO2, and severity scores: the acute physiology and chronic health evaluation score-II (APACHE II); the simplified acute physiology score-II and III (SAPS II and SAPS III); sequential organ failure assessment (SOFA); intervention features, death in final follow-up time as the primary outcome, and secondary outcomes including ICU mortality, length in ICU days, and mechanical ventilation-free days.
Quality assessment
The risk of bias of each study was assessed by two independent reviewers using methods from The Cochrane Collaboration [11], which require response one of the “low risk”, “high risk”, or “unclear” to the following criteria as indicators of the quality of trials: (1) selection bias, including random sequence generation and allocation concealment; (2) performance bias or blinding of participants and personnel; (3) detection bias or blinding of outcome assessment; (4) attrition bias or incomplete outcome data; (5) detection attrition bias or incomplete outcome data; (6) reporting bias or selective reporting, and (7) other bias. Disagreements were adjudicated by the third reviewer.
Statistical analysis and assessment of heterogeneity
We performed all statistical analyses in R (version 4.0.3). Heterogeneity between studies was examined by using the I2 statistic. The magnitude of heterogeneity was considered as low, moderate, and high by I2 values of 25%, 50%, and 75%. When I2 > 50%, a random-effects model was applied in our meta-analyses. Otherwise, we used the fix-effects model. For dichotomized outcome data, such as mortality, we calculated the risk ratios (RRs) with the 95% confidence intervals (CIs), while we calculated mean differences (MDs) with the 95% CIs for continuous outcome data. Subgroup analyses were performed for the primary outcome according to the follow-up time and the publication year. In addition, we carried out the contour-enhanced funnel plots to examine the publication bias.
Results
Literature search
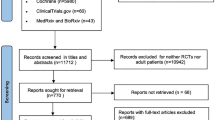
Sixty-four studied were identified by the literature search. After screening all the studies, 57 articles were excluded, including 22 duplications, 15 unrelated articles, 18 meta-analyses and reviews, and 2 non-English articles. Seven studies in total were included in this meta-analysis [7, 9, 10, 12,13,14,15] (Fig. 1).
Study characteristics
The included studies were published between 2014 and 2020 from 5 countries. There were 2166 patients from 7 studies included in our meta-analysis, 1077 of which received COT and 1089 patients received LOT. One study focused on children and other studies focused on adult patients. The details of each study were shown in Table 1.
Mortality in final follow-up and subgroup analysis
Mortality in the longest follow-up did not have significant difference between LOT and COT groups, with low heterogeneity (RR = 1.03; 95% CI [0.78; 1.36], I2 = 35%) (Fig. 2a). In the subgroup analysis, the results of mortality within 90-days (RR = 1.06; 95% CI [0.73; 1.55], I2 = 53%, Fig. 2b) and 180-days mortality (RR = 0.96; 95% CI [0.82; 1.12], I2 = 0%) were not significant (Fig. 2c). However, the result of 90-days mortality (RR = 0.88; 95% CI [0.76; 1.01], I2 = 0%, Fig. 2d) strongly favored the LOT, though not significant. When performing subgroup analysis according to the publication date, we found that COT significantly associated with reduced mortality based on the studies published before 2020 (RR = 1.34; 95% CI [1.06; 1.70], I2 = 0%, Fig. 2e), while the result was insignificant when it comes to the studies published after 2020 (RR = 0.91; 95% CI [0.79; 1.05], I2 = 29%) (Fig. 2f).
The forest plot of mortality between LOT and COT a The longest follow-up time. b Within 90-days. c In 90-days. d In 180-days. e According to the studies published before 2020. f According to the studies published after 2020 between LOT and COT. LOT liberal oxygen therapy, COT conservative oxygen therapy, RR relative risk
ICU mortality
A total of five studies provided available data with 1079 patients for ICU mortality (Fig. 3a). The meta-analysis showed that no significant correlation was found between ICU mortality and the two types of oxygen therapy, with moderate heterogeneity (RR = 0.97; 95% CI [0.57; 1.64], I2 = 63%).
ICU length of stay
A total of four studies showed relevant data of 1757 patients for ICU median days (Fig. 3b). We did not find significant difference between LOT and COT group, with low heterogeneity (SMD = 0.04; 95% CI [− 0.05; 0.13], I2 = 0%).
MV-free days
Six studies in total provided relevant data of 1951 patients for MV-free days (Fig. 3c). The pooled analysis showed that LOT was significantly related to reduced MV-free days compared with COT (SMD = − 0.02; 95% CI [− 0.24; − 0.20], I2 = 78%).
Publication bias
Funnel plots were performed to examine the publication bias of included studies (Fig. 4). We found no significant publication bias for the longest follow-up mortality, ICU mortality, ICU length of days, and MV-free days.
a The funnel plot for the death in final follow-up time. b The funnel plot for the ICU mortality. c The funnel plot for the length in ICU days. d The funnel plot for the mechanical ventilation-free days. LOT liberal oxygen therapy, COT conservative oxygen therapy, ICU intensive care unit, RR relative risk
The methodological quality of studies
The overall risk of bias of included studies is low (Fig. 5). The attrition bias of the two studies was “unclear”, while others were assessed as “low risk”. All studies had a “low risk” of reporting bias, except one with “unclear”. In the assessment of other biases, three studies had a quality indicator that was “unclear”, while others had “low risk”.
Discussion
The key finding of this meta-analysis was that the superiority of COT over LOT was challenged for patients admitted to ICU with the publishment of high-quality RCTs in 2020. In subgroup analysis, we also did not find significant differences in less than 90-day, 90-day, and 180-day mortality rates between the two therapies. We even observed the trend supporting a lower 90-day mortality rate in the LOT group.
Whereas in previous studies, meta-analyses of observational studies [16, 17] and RCTs [8] from various critical care settings and groups of patients have shown that LOT was associated with increased mortality risk in critically ill adults. Besides the reason mentioned in the previous section (LOT increases the probability of harm from hyperoxia), it might also be that excessive supplemental oxygen could lead to falsely reassuring SpO2 values in clinical practice [18, 19]. This might lessen clinician vigilance and delay the optimal treatment for patients. On the other hand, the fundamental diseases of patients also have major influences on the results. As illustrated previously, arterial hyperoxia was significantly related to the mortality in patients resuscitated from cardiac arrest but not in mechanically ventilated patients [16]. The reason could be hyperoxia-induced vasoconstriction and cardiac output reduction [16, 17].
To further illustrate the change of effect in LOT and COT to ICU patients, we conducted subgroup analysis according to follow-up time and publication year. Before 2020, the 4 articles we involved demonstrated that LOT could significantly result in more mortality than COT. These two papers [14, 15] contributed the most to the effect size. But the findings of 3 articles published after 2020 are at variance with the previous results. Young et al. found that the 90-day mortality of sepsis patients in ICU who received LOT was 7 percentage points lower than that of the COT group, although not significant [10]. For patients with acute respiratory distress syndrome (ARDS), Barrot et al. demonstrated that the 90-day mortality rate in LOT group was 14 percentage points lower than that in the COT group [9].
Several possible factors might explain the results. First, the patients’ characteristics should be an important concern. For patients with sepsis, oxygen delivery to the tissues could be impaired and excess oxygen delivery might help to reverse this situation and avoid cellular and organ dysfunction [10]. While for patients with hypoxic-ischemic encephalopathy, it is biologically plausible that COT reduces the incidence of secondary brain damage after resuscitation from cardiac arrest [7]. Moreover, based on the APACHE II score, the disease state of patients included in the Suzuki et al. [15] was severer than that in the Young et al. [10] and Mackle et al. [7]. Second, from the study design perspective, the follow-up duration of these two studies [14, 15] are relatively shorter than the studies published after 2020 and they are single-center studies. Third, targeting lower oxygenation might decrease oxygen content and transport and liberal use of oxygen may provide a baseline of safety against hypoxia in the long run.
This study also had some limitations. Firstly, the time of intervention, the duration of mechanical ventilation, and the definitions and implementation of LOT and COT are hard to be unified and impractical to be consistent in clinical work. It was also reported that clinicians might prone to switch from controlled ventilation to a mode allowing unassisted ventilation in the presence of a lower fraction of inspired oxygen (Fio2). Secondly, the methods of information gathering between publications could be diverse. Some papers are short of clinical parameters such as lactate and central venous oxygen saturation, which could be major influences during the treatment.
Conclusions
In conclusion, recent clinical trials targeting ICU patients showed that COT, as compared with LOT, did not significantly decrease the long-term and short-term mortality.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LOT:
-
Liberal oxygen therapy
- COT:
-
Conservative oxygen therapy
- RCTs:
-
Randomized controlled trials
- ICU:
-
Intensive care unit
- MV:
-
Mechanical ventilation
- CIs:
-
Confidence intervals
- MDs:
-
Mean differences
- RRs:
-
Risk ratios
- ARDS:
-
Respiratory distress syndrome
- APACHE II:
-
The acute physiology and chronic health evaluation score-II
- SAPA III:
-
The simplified acute physiology score-III
- SAPA II:
-
The simplified acute physiology score-II
- SOFA:
-
Sequential organ failure assessment
References
Suzuki S, Eastwood GM, Peck L, Glassford NJ, Bellomo R. Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. J Crit Care. 2013;28(5):647–54.
de Graaff AE, Dongelmans DA, Binnekade JM, de Jonge E. Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2. Intensive Care Med. 2011;37(1):46–51.
Panwar R, Capellier G, Schmutz N, Davies A, Cooper DJ, Bailey M, et al. Current oxygenation practice in ventilated patients-an observational cohort study. Anaesth Intensive Care. 2013;41(4):505–14.
Hadanny A, Zubari T, Tamir-Adler L, Bechor Y, Fishlev G, Lang E, et al. Hyperbaric oxygen therapy effects on pulmonary functions: a prospective cohort study. BMC Pulm Med. 2019;19(1):148.
Iscoe S, Beasley R, Fisher JA. Supplementary oxygen for nonhypoxemic patients: O2 much of a good thing? Crit Care. 2011;15(3):305.
de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12(6):R156.
Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382(11):989–98.
Chu DK, Kim LH, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693–705.
Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382(11):999–1008.
Young P, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, et al. Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX). Intensive Care Med. 2020;46(1):17–26.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Peters MJ, Jones GAL, Wiley D, Wulff J, Ramnarayan P, Ray S, et al. Conservative versus liberal oxygenation targets in critically ill children: the randomised multiple-centre pilot Oxy-PICU trial. Intensive Care Med. 2018;44(8):1240–8.
Panwar R, Hardie M, Bellomo R, Barrot L, Eastwood GM, Young PJ, et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016;193(1):43–51.
Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316(15):1583–9.
Suzuki S, Eastwood GM, Glassford NJ, Peck L, Young H, Garcia-Alvarez M, et al. Conservative oxygen therapy in mechanically ventilated patients: a pilot before-and-after trial. Crit Care Med. 2014;42(6):1414–22.
Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, de Jonge E. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43(7):1508–19.
Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18(6):711.
O’Driscoll BR, Howard LS, Earis J, Mak V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl 1):ii1–90.
Beasley R, Chien J, Douglas J, Eastlake L, Farah C, King G, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: “swimming between the flags.” Respirology. 2015;20(8):1182–91.
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
LL, YT collected, analyzed and interpreted the data. LL, YT contributed in writing the manuscript. LL, YT prepared the results. YT generated the idea, and revised the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, L., Tian, Y. Liberal or conservative oxygen therapy for ventilated patients in the ICU: a meta-analysis of randomized controlled trials. J Cardiothorac Surg 16, 261 (2021). https://doi.org/10.1186/s13019-021-01634-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-021-01634-4