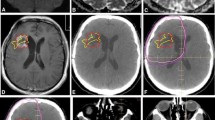
Abstract
Rationale
This systematic review aims to synthesise the outcomes of different strategies of incorporating functional biological markers in the radiation therapy plans of patients with glioblastoma to support clinicians and further research.
Methods
The systematic review protocol was registered on PROSPERO (CRD42021221021). A structured search for publications was performed following PRISMA guidelines. Quality assessment was performed using the Newcastle–Ottawa Scale. Study characteristics, intervention methodology and outcomes were extracted using Covidence. Data analysis focused on radiation therapy target volumes, toxicity, dose distributions, recurrence and survival mapped to functional image-guided radiotherapy interventions.
Results
There were 5733 citations screened, with 53 citations (n = 32 studies) meeting review criteria. Studies compared standard radiation therapy planning volumes with functional image-derived volumes (n = 20 studies), treated radiation therapy volumes with recurrences (n = 15 studies), the impact on current standard target delineations (n = 9 studies), treated functional volumes and survival (n = 8 studies), functionally guided dose escalation (n = 8 studies), radiomics (n = 4 studies) and optimal organ at risk sparing (n = 3 studies). The approaches to target outlining and dose escalation were heterogeneous. The analysis indicated an improvement in median overall survival of over two months compared with a historical control group. Simultaneous-integrated-boost dose escalation of 72–76 Gy in 30 fractions appeared to have an acceptable toxicity profile when delivered with inverse planning to a volume smaller than 100 cm\(^3\).
Conclusion
There was significant heterogeneity between the approaches taken by different study groups when implementing functional image-guided radiotherapy. It is recommended that functional imaging data be incorporated into the gross tumour volume with appropriate technology-specific margins used to create the clinical target volume when designing radiation therapy plans for patients with glioblastoma.
Similar content being viewed by others
Background
There is a clinical and economic need for improved outcomes for patients diagnosed with glioblastoma. Globally, over 300,000 primary brain and central nervous system cancers were diagnosed in 2020, with glioblastoma accounting for 48.6% of primary brain and central nervous system cancers in the United States (US) [1, 2]. The outcomes from care are poor; a patient’s relative survival post-diagnoses is under 7% at 5-years with current best practice [3]. In Australia, primary brain cancers account for more disability adjusted life-years lost per patient than any other adult cancer [4]. Further, brain cancer diagnoses have the largest financial cost of all cancers [5].
The World Health Organisation has classified four categories of diffuse gliomas: adult-type diffuse gliomas, pediatric-type diffuse high-grade gliomas, pediatric-type diffuse low-grade gliomas and circumscribed astrocytic gliomas with glioblastoma grouped with isocitrate dehydrogenase (IDH)-wildtype as adult-type diffuse gliomas [6]. The current standard treatment for glioblastoma consists of maximum safe surgical excision, followed by concurrent chemo-radiotherapy (typically 60 Gy/30 fractions or 40 Gy/15 fractions in elderly patients) with concurrent temozolomide (TMZ), followed by adjuvant TMZ [7]. Standard treatment has remained relatively unchanged in the last 17 years [8, 9]. Patient outcomes with standard treatment include a median overall survival of 14.6 months, a median progression-free survival of 6.9 months, a 12 months overall survival of 61.1% and a 12 months progression-free survival of 26.9% [9, 10]. Interestingly, the control arms of more recent randomised control trials which follow the Stupp et al. 2005 [11] guidelines report a median survival of approximately 20 months, most likely as a result of treatment refinement [11, 12].
Radiation therapy (RT) treatment quality has been shown to influence patient outcomes with target delineation identified as one of the largest variables in the treatment process [13, 14]. Currently, RT management of patients with glioblastoma is fragmented by the existence of variable target delineation guidelines which are based on magnetic resonance imaging (MRI) and computed tomography (CT) [15, 16]. This fragmentation in practice results in a significant difference in the outlined clinical target volume (CTV), for instance, in a study by Kumar et al. the CTV varied from 246 to 436 cm\(^3\) depending on the guidelines followed [17]. The guidelines documenting the acceptability of positron emission tomography (PET) scanning to support the RT planning of patients with gliomas have recently changed with PET scanning now more likely [18]. There is also increasing evidence to investigate dose-escalated RT in patients with glioblastoma, particularly in subgroups that do not receive TMZ or those who are known to have unmethylated DNA repair enzyme O-6-methylguanine-DNA methyltransferase (MGMT) [19]. Therefore, RT quality and patient outcomes are highly dependent on target voluming guidelines and the dose fractionation regimes used.
The main concern with dose escalation or increases in target volume size is an increased risk of side effects such as radiation necrosis [20]. This has prompted research to investigate biologically-derived target volumes and RT boosts to improve the specificity and sensitivity of the treated volumes and facilitate safe dose escalation [19, 21, 22]. Currently, 75% of patients receiving standard care for glioblastoma recur local to the high dose RT volume [23]. This highlights the need to improve both the local control and the sensitivity of target localisation, as nearly 100% of glioblastoma patients progress with current practice. Key to this goal, is capturing detailed recurrence information with geometric reference to the standard International Commission on Radiotherapy Units (ICRU) volumes and pre-treatment imaging, as this can be used to quantify the success of target contouring.
Functional imaging modalities have the advantage of being able to identify biochemical changes that often predate, or are distinct from, anatomical changes [24, 25]. A diverse range of functional imaging biological markers, including MRI, magnetic resonance spectroscopic imaging (MRSI) and nuclear medicine (NM), hold promise in this new era of functional image-guided radiotherapy (FIGR) [22, 25]. Additionally, the novel disciplines of radiomics and dosiomics, will have an increasingly synergistic role alongside functional imaging in the management of RT patients [26]. However, there is an evidence gap to support the development of guidelines to realise the benefits of FIGR. Therefore, the aim of this systematic review is to synthesise strategies and outcomes of functional imaging for RT planning in patients with glioblastoma. Outcomes related to RT target volumes, dose distributions, toxicity, recurrences and survival will be synthesised to support clinicians and research.
Method
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [27]. The review was prospectively registered on PROSPERO (CRD42021221021) [28]. The search strategy was conducted in consultation with a research librarian.
Search strategy
Literature searches were conducted in January 2021 in PubMed, CINAHL, Scopus, Cochrane Library, EMBASE, Web of Science and AMED. Three concepts were used to guide searches: functional imaging, glioblastoma multiforme and radiotherapy planning. Indexing thesaurus, keywords, MeSH terms, CINAHL headings, Indexed terms, Emtree terms, and synonyms were used, as relevant. Searches were restricted to texts available in English and published from January 2011 to January 2021, due to the rapid development in this field. A full overview of search terms, dates and boolean operations used for each database is available in Additional file 1.
Eligibility criteria
Eligibility criteria were defined according to participants, intervention, comparator, outcome and study type (PICOS framework). Participants and comparators were patients with primary glioblastoma undergoing external beam radiotherapy with a curative intent. Participants were 18 years of age or over. Mixed cohorts (i.e., glioblastoma and other high grade glioma) had to report separate outcomes for patients with glioblastoma to be eligible. Journal articles and published conference abstracts were included. Review articles and unpublished grey literature were excluded. Purely explorative articles (i.e., radiomics studies that were not trialled in patients with glioblastoma) were excluded.
Study selection
Duplicates were removed in Covidence [29]. Title and abstract screening against eligibility criteria was completed by two reviewers (from JR, MN, IG or LM). Conflicts were resolved by an independent reviewer (NH or JR). Eligibility of full text articles was determined by two reviewers (from JR, MN or IG) reading each paper in full. Conflicts were resolved by consultation. Data extraction was completed by JR. Figure 1 provides an overview of the screening, exclusion rationale and data extraction.
Data extraction and management
Citations were grouped according to study cohort. Study characteristics were extracted using a Covidence template and the Template for Intervention Description and Replication (TIDieR) checklist [30]. Data were extracted according to study ID, title, author, information source, location, funding, conflicts, study aim, study design, participant description, sample size, attrition, confounding variables, intervention and TIDieR components. Study quality including biases for journal articles was assessed using the Newcastle–Ottawa Scale (NOS) assessment tool (Table 1) [31].
Studies were grouped according to their aims, including improvement of standard target delineation, facilitation of dose escalation, improvement of organ at risk (OAR) sparing, mapping recurrence and treated volumes, mapping functional and planning volumes, mapping functional volumes and survival, and carrying out voxel/radiomics analyses (Fig. 1 and Table 1). Study outcomes and confounders are captured in Tables 2, 3 and 4. A study quality score of less than nine in Table 1 indicates an issue with data reliability in an individual study, such as reporting in a short time frame post-intervention or not reporting select patient data that may have biased the results.
Study synthesis
The tables present a snapshot of the included studies (Table 1) and examine clinically relevant RT planning and associated patient outcomes (Tables 2, 3 and 4). An analysis of outcomes relating to median overall survival, median progression-free survival, 12-month overall survival percentage, and 12-month progression-free survival was completed (Table 5). In Table 5 studies were weighted based on their population number and the summed averages compared to a historical control. Data were otherwise reported narratively.
Results
A total of 32 studies (53 citations) met inclusion criteria (Table 1 and Fig. 1) . TIDieR components were inconsistently reported [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. Information about who carried out the intervention and their expertise was lacking. Few studies used a prospective control group.
Included study overview
Included studies consisted of journal articles (n = 25) and conference presentations (n = 7). There were 20 retrospective cohort studies, eight prospective cohort studies, seven Phase I or Phase II trials and one Phase III randomised controlled trial.
Studies were grouped into seven categories comparing standard RT planning volumes with functional-derived volumes (n = 20), treated RT volumes with recurrences (n = 15), functional imaging into standard target delineations (n = 9), functional imaging volumes and survival (n = 8), functional imaging to guide dose escalation (n = 8), voxel or radiomics analyses (n = 4) and OAR sparing based on functional information (n = 3). There were 13 different technological interventions directly related to functional imaging, categorised according to NM, MRI, MRSI and FIGR boost. Dose escalation based on FIGR was implemented in eight studies using NM agents (n = 5), MRSI (n = 2) and MRI (n = 1).
Study outcomes
Twenty studies included outcomes on RT target volumes, 12 reported toxicity with a FIGR intervention, 10 reported survival with a FIGR intervention and 15 reported recurrence. Two of the 32 studies were not included in Tables 2, 3, 4 and 5 for analysis. Matsuo et al. [50, 51] conducted a volume comparison study and reported on the sensitivity and specificity of the CTV with different gross tumour volume (GTV) to CTV expansion margins. Lopez and colleagues [81] established a framework of co-dependencies between MRI, MRSI and radiotherapy planning volumes using radiomics.
Target volume size
Target volume size for RT planning volumes was recorded in Table 2. Target volume reporting between studies was varied, with 11 studies reporting median volumes and four studies reporting mean volumes. The GTV that incorporated functional and anatomical imaging was larger than the anatomical GTV in all studies. The intra-study variation in standard CTV size was greater than the inter-study variation in CTV size that incorporated functional and anatomical imaging. Target volume creation with functional imaging was heterogeneous. The most common approach was to incorporate the functional imaging data directly into the CTV or the planning target volume (PTV) without a specific uncertainty margin and by using standard GTV to CTV margin recipes. The CTV and PTV change with combined imaging was methodology dependent and varied between a 27% increase to a 50% decrease. The dose escalated boost volume was less than 100 cm\(^3\).
Dose distribution, toxicity and survival
Toxicity and survival metrics associated with the RT treatment process are presented (Table 3). Dose escalation was used in eight studies. The dose-escalated fractionation schedules had an equivalent 2 Gy doses (EQD\(_2\)) ranging between 74.4 and 104.8 Gy, with a given alpha/beta ratio of 10. Only one study used an EQD\(_2\) > 79.4 Gy [39]. Toxicity in the dose-escalated studies with an EQD\(_2\) of \(\leqslant\) 79.4 Gy were well tolerated, when delivered as a simultaneous integrated boost, with 60 Gy in 30 fractions prescribed to the standard PTV. A limited boost size \(\leqslant\) 65 cm\(^3\) was recommended in one study [85].
Recurrence patterns
Fifteen studies reported on recurrence outcomes (Table 4). These were broken down into three categories: studies that involved a retrospective comparison with a standard approach, studies that used functional imaging to derive the target volumes for treatment and studies that outlined extra target volumes with associated dose escalation. Table 4 indicates the relative location of a recurrence as a proportion of the total number of recurrences. Consistency was limited in the reporting of recurrence patterns and their location in relation to imaging and ICRU volumes. There were a higher proportion of recurrences central to the high therapeutic dose volume when functional and anatomical imaging were combined to outline the target.
Analysis of the effect of functional imaging on survival
Survival analysis (n = 10 studies, N = 686 participants) indicated improved survival outcomes with FIGR compared to the 2005 Stupp et al. trial (Table 5) [9]. Dose escalation, guided by functional imaging (n = 6 studies, N = 235 participants) further increased median overall survival and median progression-free survival, of these studies, only the Brinkmann study used a local current control group [59,60,61].
Discussion
To the authors knowledge this is the first systematic review of functional image-guided RT interventions in glioblastoma patients. Recent findings to support FIGR in glioblastoma patients have been shared and indicated FIGR can improve patient survival outcomes in certain patient cohorts [87, 88].
Standard target delineation
The classification of the target volumes for patients with glioblastoma tumours is not static, that is, it varies on the time-point, resources and local expertise available. Most studies using FIGR edited the CTV or PTV directly with historical margin recipes (Table 2). This is problematic as interventions that produce new functional imaging volumes have their own uncertainties that are inherent to the technologies and processes used. Study teams were typically cautious about incorporating functional imaging directly in the GTV, perhaps due to concerns about the final target volume size. However, an increased target size can be avoided with technology-specific GTV-CTV margin recipes (Table 3).
Dose escalation
Functional image-guided radiotherapy provides a mechanism to give a more personalised and possibly a more effective dose distribution. For example, where dose escalation was trialled based on a functionally-derived target volume there was a corresponding survival increase against the historical control (Table 5). This should be reviewed with the understanding that there has been substantial technological and practice refinement in glioblastoma treatment since 2005 and these changes may be due to other confounding factors like the categorisation of patients within individual studies, surgical practices, radiation therapy planning and delivery practices. There may be scope for further survival improvements with a more tailored approach to dose escalation, as boosting was done with a small number of discrete dose intervals.
Presently, research is concerned with ensuring the safety profile of dose-escalated RT for glioblastoma patients. Simultaneous-integrated boost dose prescriptions of 72–76 Gy delivered to volumes less than 100 cm\(^3\), with the standard PTV receiving 60 Gy, appeared relatively well tolerated (Table 3). The omission of a CTV-PTV boost volume margin in most studies may have limited the effectiveness of dose escalation. Further research is needed to compare the intrinsic differences in the boost volumes indicated by the different imaging agents and to derive ways to account for imaging agent variation via uncertainty margin recipes.
Organs at risk sparing
The specificity of target localisation in glioblastoma patients is directly linked to OAR sparing, particularly with inverse planning and intensity modulated radiation therapy (IMRT) delivery. Dose escalation with simultaneous-integrated boost techniques was common in the studies. Switching to IMRT delivery created OAR sparing capacity [41, 61]. However, current segmenting guidelines are based on anatomical imaging and thus inherently have to use relatively large isotropic expansions [89, 90]. Berberat and colleagues [69] demonstrated the feasibility of using functional imaging to map the white matter tracks in the brain and incorporated this information when deciding on target volumes. This mapping resulted in a 15% reduction in the PTV. This smaller PTV will most likely result in increased OAR sparing and increased potential for dose escalation via isotoxic planning. Both Wang [70] and Altabella [72] also used diffusion tensor imaging magnetic resonance imaging (DTI MRI) to map white matter tracks, but instead of using the information for target localisation, information was used as an IMRT OAR optimisation structure. A limitation with NM approaches to FIGR, is the lack of information regarding OAR functionality that can be incorporated into the RT plan.
Volume comparisons and radiomics
Volume comparison studies were the most prevalent study type (n = 21). Volumes were compared using absolute comparisons (mean/median), dice scores, Hausdorff distance metrics and radiomic voxel comparisons. The variation in data (Table 2), highlighted the need for volume comparison study guidelines. For example, two of the five studies that altered treated target volumes, based on FIGR, did not report the treated CTV size [43, 46].
A current gap in the literature is the unclear relationship between volumes outlined with different functional imaging agents for FIGR. None of the studies compared target volumes based on different functional scan information. The similarity between targets based on fluorine-18-fluoroethyltyrosine positron emission tomography (\(^{18}\)F-FET PET) and carbon-11-methionine positron emission tomography (\(^{11}\)C-MET PET) is well established but information about the crossover to other types of functional scan volumes is not present in the literature.
The value of recurrence analysis in volume comparison studies cannot be underestimated as they indicated where tumour volume was either missed or did not fully respond to treatment. However, the varied terminology used in the studies to describe recurrence location in relation to the outlined volumes makes interpretation and utilisation challenging. Despite this, there was a trend to suggest that a combined approach to target voluming would improve target sensitivity (Table 4).
Volume comparison studies that report survival are key to assessing the benefits of FIGR and critical to any practice reform. The methodologies used in the studies that implemented FIGR were heterogeneous, however, survival outcomes were reported in a uniform way. The improvements in survival with FIGR (n = 10) were modest, yet consistent (Tables 3 and 5). Further improvements may be possible with optimised dose escalation and outlining.
The radiomics studies presented a pathway to incorporate functional imaging data from diverse imaging techniques with a unified approach and could address limitations associated with each imaging technology.
Limitations and recommendations
Our study has several limitations. Variation in patient cohorts between studies should be acknowledged. Factors that influence outcomes, such as patient demographics, extent of surgery, MGMT status, chemotherapy protocol and RT treatment delivery were not clearly reported across all studies. It is not possible to verify the diagnosis and classification processes that led to patients being diagnosed with glioblastoma and thus meeting inclusion criteria for individual studies. The inclusion of published conference articles resulted in some studies having limited background detail to support data extraction.
Carrying out this review highlighted key recommendations for future FIGR studies. Implementation protocols should be published. Guideline development and implementation is needed for RT volume comparison and recurrence studies. Further, there is a need for prospective volume comparison studies between different FIGR intervention agents with radiomic analyses.
Conclusion
Functional image-guided radiotherapy is not currently standard practice for glioblastoma patients due to the lack of conclusive Phase III evidence. However, there are many variations possible when implementing FIGR and this makes assessing competing methodologies difficult. This review highlights the different approaches to FIGR for glioblastoma patients and relevant successes.
A three-pronged approach to FIGR for the RT treatment of patients with glioblastoma is recommended with optimised target voluming, dose prescription and OAR sparing. There is a need for a more structured approach to the testing and implementation of competing methodologies, with practical recommendations to account for the variations in available technology.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- \(^{11}\)C-AMT PET:
-
Carbon-11-alpha-methyltryptophan positron emission tomography
- \(^{11}\)C-MET PET:
-
Carbon-11-methionine positron emission tomography
- \(^{18}\)F-FAP:
-
Fluorine-18-fibroblast activating protein positron emission tomography
- \(^{18}\)F-FDG PET:
-
Fluorine-18-fluorodeoxyglucose positron emission tomography
- \(^{18}\)F-FDOPA PET:
-
Fluorine-18-fluorodihydroxyphenylalnine positron emission tomography
- \(^{18}\)F-FET PET:
-
Fluorine-18-fluoroethyltyrosine positron emission tomography
- BOLD MRI:
-
Blood oxygenation level dependent magnetic resonance imaging
- CT:
-
Computed tomography
- CTV:
-
Clinical target volume
- DCE MRI:
-
Dynamic contrast enhanced magnetic resonance imaging
- DTI MRI:
-
Diffusion tensor imaging magnetic resonance imaging
- DTT MRI:
-
Diffusion tensor tractography magnetic resonance imaging
- DW MRI:
-
Diffusion weighted magnetic resonance imaging
- EORTC:
-
European organization for research and treatment of cancer
- FIGR:
-
Functional image-guided radiotherapy
- GTV:
-
Gross tumour volume
- ICRU:
-
International commission on radiotherapy units
- IGRT:
-
Image-guided radiation therapy
- IMRT:
-
Intensity modulated radiation therapy
- IDH:
-
Isocitrate dehydrogenase
- MGMT:
-
O-6-methylguanine-DNA methyltransferase
- MRI:
-
Magnetic resonance imaging
- MRSI:
-
Magnetic resonance spectroscopic imaging
- NM:
-
Nuclear medicine
- NOS:
-
Newcastle–Ottawa scale
- OAR:
-
Organ at risk
- PET:
-
Positron emission tomography
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PTV:
-
Planning target volume
- PWI MRI:
-
Perfusion weighted imaging magnetic resonance imaging
- RT:
-
Radiation therapy
- RTOG:
-
Radiotherapy and oncology group
- TIDieR:
-
Template for intervention description and replication
- TMZ:
-
Temozolomide
- US:
-
United States
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–49.
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-Oncology. 2020;22(Supplement_1):1–96.
Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro-oncology. 2021;23(12 Suppl 2):III1–III105. Available from: https://pubmed.ncbi.nlm.nih.gov/34608945/.
Australian Institute of Health and Welfare (AIHW). Australian institute of health and welfare 2019. Cancer in Australia: In brief 2019. Canberra: Australian Government; 2019. Available from: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/summary.
Deloitte Access Economics. The economic cost of cancer in adolescents and young adults. Canteen; 2018. Available from: www.youthcancer.com.au.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncology. 2021;23(8):1231–51.
Weller M, Van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, et al. European association for neuro-oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–29.
Kazda T, Dziacky A, Burkon P, Pospisil P, Slavik M, Rehak Z, et al. Radiotherapy of glioblastoma 15 years after the landmark Stupp’s trial: More controversies than standards? Radiol Oncol. 2018;52(2):121–8.
Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–43.
Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–85.
Peters L, O’Sullivan B, Giralt J, Fitzgerald T, Trotti A, Bernier J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(18):2996–3001.
Segedin B, Petric P, Petrič P. Uncertainties in target volume delineation in radiotherapy-are they relevant and what can we do about them? Radiol Oncol. 2016;50(3):254–62.
Peeken JC, Molina-Romero M, Diehl C, Menze BH, Straube C, Meyer B, et al. Deep learning derived tumor infiltration maps for personalized target definition in Glioblastoma radiotherapy. Radiother Oncol. 2019;9(138):166–72.
Zhao F, Li M, Kong L, Zhang G, Yu J. Delineation of radiation therapy target volumes for patients with postoperative glioblastoma: a review. Onco Targets Ther. 2016;9:3197–204.
Kumar N, Kumar R, Sharma SC, Mukherjee KK, Khandelwal N, Kumar R, et al. To compare the treatment outcomes of two different target volume delineation guidelines (rtog vs md anderson) in glioblastoma multiforme patients: a prospective randomized study. Neuro-Oncology 2012;14(suppl_6):vi133–vi141. Available from: https://academic.oup.com/neuro-oncology/article/14/suppl_6/vi133/1058359.
Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and (18 F) FDG. Eur J Nucl Med Mol Imaging. 2019;33:540–57.
Singh R, Lehrer EJ, Wang M, Perlow HK, Zaorsky NG, Trifiletti DM, et al. Dose escalated radiation therapy for glioblastoma multiforme: an international systematic review and meta-analysis of 22 prospective trials. Int J Radiat Oncol Biol Phys. 2021;111(2):371–84.
Gondi V, Pugh S, Tsien C, Chenevert T, Gilbert M, Omuro A, et al. Radiotherapy (RT) dose-intensification (DI) using intensity-modulated RT (IMRT) versus standard-dose (SD) RT with temozolomide (TMZ) in newly diagnosed glioblastoma (GBM): preliminary results of NRG oncology BN001. Int J Radiat Oncol Biol Phys. 2020;108(3):S22–3.
Troost EGC, Thorwarth D, Oyen WJG. Imaging-based treatment adaptation in radiation oncology. J Nucl Med. 2015;56(12):1922–9.
Thorwarth D. Functional imaging for radiotherapy treatment planning: current status and future directions: a review. Br J Radiol. 2015;88(1051):20150056.
Jiang H, Yu K, Li M, Cui Y, Ren X, Yang C, et al. Classification of progression patterns in glioblastoma: analysis of predictive factors and clinical implications. Front Oncol. 2020;11(10):2408.
Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med Off Publ Soc Nucl Med. 2000;41(8):1369–79.
Gurney-Champion OJ, Mahmood F, van Schie M, Julian R, George B, Philippens MEP, et al. Quantitative imaging for radiotherapy purposes. Radiother Oncol. 2020;5(146):66–75.
Sun R, Lerousseau M, Henry T, Carré A, Leroy A, Estienne T, et al. Intelligence artificielle en radiothérapie : radiomique, pathomique, et prédiction de la survie et de la réponse aux traitements. Cancer /Radiothér. 2021;25(6–7):630–7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;3:372.
Ryan J, Gleeson I, Mannion L, Kelly J, Ng SP, Everitt S, et al. A systematic review of functional brain imaging in radiotherapy planning for glioblastoma multiforme patients using the TIDieR framework. PROSPERO: International prospective register of systematic reviews. PROSPERO; 2021. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021221021.
Covidence. Systematic review software. Melbourne,: Veritas Health Innovation; 2021. Available from: http://www.covidence.org/.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ (Online). 2014;3;348. Available from: https://pubmed.ncbi.nlm.nih.gov/24609605/.
Wells G, Shea B, O’Connell D, Peterson J, Welch V. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Newcastle & Ottawa Universities; 2000. Available from: http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf.
Geisler J, Niyazi M, Bartenstein P, Belka C, la Fougere C. FET-PET for radiotherapy planning in malignant glioma. J Nucl Med. 2011;52. Available from: \(<\)Go to ISI\(>\)://WOS:000443798902213.
Niyazi M, Geisler J, Siefert A, Schwarz SB, Ganswindt U, Garny S, et al. FET-PET for malignant glioma treatment planning. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2011;99(1):44–8.
Piroth MD, Holy R, Pinkawa M, Stoffels G, Kaiser HJ, Galldiks N, et al. Prognostic impact of postoperative, pre-irradiation (18)F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2011;99(2):218–24.
Piroth MD, Stoffels G, Kaiser HJ, Galldiks N, Herzog H, Coenen HH, et al. F-18-Fluoroethyltyrosine-PET imaging in glioblastoma patients for radiotherapy planning and response analysis - clinical and experimental evaluation. Strahlentherapie und Onkol. 2011;187(9):595.
Piroth MD, Pinkawa M, Holy R, Klotz J, Schaar S, Stoffels G, et al. Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Results of a prospective phase II study. Strahlenther Onkol. 2012;188(4):334–9.
Piroth MD, Galldiks N, Pinkawa M, Holy R, Stoffels G, Ermert J, et al. Relapse patterns after radiochemotherapy of glioblastoma with FET PET-guided boost irradiation and simulation to optimize radiation target volume. Radiation Oncol (London, England). 2016;6(11):87.
Laouiti M, Lauffer D, Garibotto V, Weber DC. Dose escalation using intensity modulated radiation therapy with a simultaneous integrated boost technique to FET PET avid regions and concomitant chemotherapy for newly diagnosed glioblastoma. Strahlentherapie und Onkol. 2013;189(12):1087.
Miwa K, Shinoda J, Yano H, Iwama T. Metabolically positive lesion before hypofractionated radiation therapy and its impact on outcome for patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2013;87(2):S247.
Munck af R, Costa J, Engelholm SA, Lundemann MJ, Law I, Ohlhues L, et al. Impact of F-18 -fluoro-ethyl-tyrosine PET imaging on target definition for radiation therapy of high-grade glioma. Neuro Oncol. 2015;17(5):757–63.
Munck Af Rosenschold P, Law I, Engelholm S, Engelholm SA, Muhic A, Lundemann MJ, et al. Influence of volumetric modulated arc therapy and FET-PET scanning on treatment outcomes for glioblastoma patients. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2019;1;130:149–55.
Lundemann J, Costa JC, Law I, Muhic A, Engelholm SA, af Rosenschold PM. Pattern of failure in glioblastoma patients after FET-PET and MRI-guided chemo-radiotherapy. Radiother Oncol. 2016;119:S304–S304.
Lundemann M, Costa JC, Law I, Engelholm SA, Muhic A, Poulsen HS, et al. Patterns of failure for patients with glioblastoma following O-(2-[(18)F]fluoroethyl)-L-tyrosine PET- and MRI-guided radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2017;122(3):380–6.
Lundemann M, Munck Af Rosenschöld P, Muhic A, Larsen VA, Poulsen HS, Engelholm SA, et al. Feasibility of multi-parametric PET and MRI for prediction of tumour recurrence in patients with glioblastoma. Eur J Nucl Med Mol Imaging. 2019;46(3):603–13.
Poulsen S, Urup T, Grunnet K, Christensen I, Larsen V, Per R, et al. The prognostic value of O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography (FET-PET) at radiotherapy planning in newly diagnosed glioblastoma. J Nucl Med. 2016;57(supplement 2):182.
Poulsen SH, Urup T, Grunnet K, Christensen IJ, Larsen VA, Jensen ML, et al. The prognostic value of FET PET at radiotherapy planning in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. 2017;44(3):373–81.
Harat M, Malkowski B, Wiatrowska I, Makarewicz R, Roszkowski K. Relationship between glioblastoma dose volume parameters measured by dual time point Fluoroethylthyrosine-PET and clinical outcomes. Front Neurol. 2018;8(JAN):756.
Hayes AR, Jayamanne D, Hsiao E, Schembri GP, Bailey DL, Roach PJ, et al. Utilizing 18F-fluoroethyltyrosine (FET) positron emission tomography (PET) to define suspected nonenhancing tumor for radiation therapy planning of glioblastoma. Pract Radiat Oncol. 2018;8(4):230–8.
Fleischmann DF, Unterrainer M, Schön R, Corradini S, Maihöfer C, Bartenstein P, et al. Margin reduction in radiotherapy for glioblastoma through (18)F-fluoroethyltyrosine PET? - A recurrence pattern analysis. Radiother Oncol. 2020;145:49–55.
Matsuo M, Miwa K, Tanaka O, Shinoda J, Nishibori H, Tsuge Y, et al. Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2012;82(1):83–9.
Matsuo M, Tanaka H, Hyodo F, Miwa K, Shinoda J. Methionine positron emission tomography for malignant brain tumors in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2019;105(1):E103.
Vigil C, Prieto E, Hernandez M, Caicedo C, Rodriguez-Ruiz M, Garcia-Granero M, et al. Radiotherapy planning of glioblastoma using C-11-methionine-PET/CT and MRI: prediction of recurrence and survival analysis. Eur J Nucl Med Mol Imaging. 2013;40:S238–9.
Vigil C, Prieto E, Ribelles M, Olarte A, Hernandez M, Valtuena G, et al. Prediction of recurrence and survival analysis after radiotherapy of glioblastoma using 11C-Methionine PET/CT and MR. J Nucl Med. 2014;55(supplement 1):12.
Hirata T, Kinoshita M, Tamari K, Seo Y, Suzuki O, Wakai N, et al. Impact of 11c-methionine/FDG dural tracer petbased, compared with MRI-based target delineation of malignant gliomas for radiation planning. Neuro Oncol. 2018;20:vi232–3.
Hirata T, Kinoshita M, Tamari K, Seo Y, Suzuki O, Wakai N, et al. 11C-methionine-18F-FDG dual-PET-tracer-based target delineation of malignant glioma: evaluation of its geometrical and clinical features for planning radiation therapy. J Neurosurg. 2019;131(3):676–86.
Christensen ME, Kamson D, Snyder M, Hallock A, Kim H, Mittal S, et al. Tumor volume for glioblastoma as defined by tryptophan PET offers superior coverage of recurrence site than standard MRI-based GTV. Int J Radiat Oncol Biol Phys. 2012;84(3):S271–S271.
Christensen M, Kamson DO, Snyder M, Kim H, Robinette NL, Mittal S, et al. Tryptophan PET-defined gross tumor volume offers better coverage of initial progression than standard MRI-based planning in glioblastoma patients. J Radiat Oncol. 2014;3(2):131–8.
Kosztyla R, Chan EK, Hsu F, Wilson D, Ma R, Cheung A, et al. High-grade glioma radiation therapy target volumes and patterns of failure obtained from magnetic resonance imaging and 18F-FDOPA positron emission tomography delineations from multiple observers. Int J Radiat Oncol Biol Phys. 2013;87(5):1100–6.
Brinkmann D, Pafundi D, Hunt C, Lowe V, Yan E, Brown PD, et al. Impact of 18f-dopa pet on radiotherapy target volumes for newly diagnosed mgmt unmethylated glioblastoma patients; preliminary results of a phase ii dose-escalation trial. Neuro Oncol. 2018;20:vi225.
Laack N, Pafundi D, Anderson S, Hunt C, Zakhary M, Kaufmann T, et al. Preliminary safety and efficacy of a phase ii trial of 18F-DOPA PET-guided, dose-escalated radiotherapy in the treatment of glioblastoma. Neuro Oncol. 2018;20:vi13.
Laack NN, Pafundi DH, Anderson SK, Kaufmann T, Lowe VJ, Hunt CH, et al. Initial results of a phase II trial of 18f-dopa pet-guided dose-escalated radiotherapy for glioblastoma. Int J Radiat Oncol Biol Phys. 2020;108(3):S23.
Windisch P, Röhrich M, Regnery S, Tonndorf-Martini E, Held T, Lang K, et al. Fibroblast activating protein specific PET for advanced target volume delineation in glioblastoma. Radiother Oncol. 2019;133:S672.
Windisch P, Rohrich M, Regnery S, Tonndorf-Martini E, Held T, Lang K, et al. Fibroblast activation protein (FAP) specific PET for advanced target volume delineation in Glioblastoma. Nuklearmedizin. 2020;59(2):114.
Munshi A, Ganesh T, Gupta RK, Vaishya S, Patir R, Sarkar B, et al. Perfusion magnetic resonance imaging in contouring of glioblastoma patients: preliminary experience from a single institution. J Cancer Res Ther. 2020;16(6):1488–94.
Wahl D, Kim M, Aryal M, Hartman H, Lawrence T, Schipper M, et al. Combining perfusion and high B-value diffusion MRI to inform prognosis and predict failure patterns in glioblastoma. Int J Radiat Oncol Biol Phys. 2018;102(4):757–64.
Kim M, Aryal M, Parmar H, Li P, Schipper M, Wahl D, et al. Response assessment using multiparametric MRI during chemoradiation predicts overall survival in patients with newly diagnosed glioblastoma. Neuro Oncol. 2019;21:vi179.
Kim MM, Parmar HA, Aryal MP, Mayo CS, Balter JM, Lawrence TS, et al. Developing a pipeline for multiparametric mri-guided radiation therapy: initial results from a phase II clinical trial in newly diagnosed glioblastoma. Tomogr (Ann Arbor, Mich). 2019;3;5(1):118–26.
Kim MM, Sun Y, Aryal MP, Parmar H, Piert M, Rosen BS, et al. A phase II study of dose-intensified chemoradiation using biologically-based target volume definition in patients with newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2020;108(3):S20.
Berberat J, McNamara J, Remonda L, Bodis S, Rogers S. Diffusion tensor imaging for target volume definition in glioblastoma multiforme. Strahlenther Onkol. 2014;190(10):939–43.
Wang M, Ma H, Wang X, Guo Y, Xia X, Xia H, et al. Integration of BOLD-fMRI and DTI into radiation treatment planning for high-grade gliomas located near the primary motor cortexes and corticospinal tracts. Radiat Oncol (London, England). 2015;3(10):64.
Zhang Y, Guo J, Zhou S, He D, Yu D, Liang J, et al. A new PTV strategy to improve the overall survival of glioblastoma patients treated with radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 2017;99(2):E119.
Altabella L, Broggi S, Mangili P, Conte GM, Pieri V, Iadanza A, et al. Integration of diffusion magnetic resonance tractography into tomotherapy radiation treatment planning for high-grade gliomas. Phys Med PM Int J Dev Appl Phys Med Biol Off J Ital Assoc Biomed Phys (AIFB). 2018;11(55):127–34.
Peeken JC, Molina-Romero M, Diehl C, Menze BH, Straube C, Meyer B, et al. Deep learning derived tumor infiltration maps for personalized target definition in glioblastoma radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2019;9(138):166–72.
Morin O, Held M, Wahl M, Braunstein S. Application of diffusion weighted MRI for target delineation in radiotherapy for glioblastoma. Med Phys. 2017;44(6):3096–7.
Anwar M, Molinaro AM, Morin O, Chang SM, Haas-Kogan DA, Nelson SJ, et al. Identifying voxels at risk for progression in glioblastoma based on dosimetry. Physiol Metab MRI Radiat Res. 2017;188(3):303–13.
Opposits G, Aranyi C, Glavák C, Cselik Z, Trón L, Sipos D, et al. OAR sparing 3D radiotherapy planning supported by fMRI brain mapping investigations. Med Dosim. 2020;45(4):e1–8.
Ken S, Vieillevigne L, Franceries X, Supper C, Lotterie JA, Filleron T, et al. MR spectroscopy imaging for glioblastoma dose painting with intensity modulated radiation therapy comprising simultaneous integrated boost on specific targets. Radiother Oncol. 2012;102:S103–5.
Ken S, Vieillevigne L, Franceries X, Simon L, Supper C, Lotterie JA, et al. Integration method of 3D MR spectroscopy into treatment planning system for glioblastoma IMRT dose painting with integrated simultaneous boost. Radiat Oncol (London, England). 2013;1(8):1.
Laprie A. Metabolic and functional MRI integration for glioblastoma dose-painting trial. Radiother Oncol. 2017;123:S160.
Laprie A, Ken S, Filleron T, Lubrano V, Vieillevigne L, Tensaouti F, et al. Dose-painting multicenter phase III trial in newly diagnosed glioblastoma: the SPECTRO-GLIO trial comparing arm A standard radiochemotherapy to arm B radiochemotherapy with simultaneous integrated boost guided by MR spectroscopic imaging. BMC Cancer. 2019;19(1):167.
Lopez CJ, Nagornaya N, Parra NA, Kwon D, Ishkanian F, Markoe AM, et al. Association of radiomics and metabolic tumor volumes in radiation treatment of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2017;97(3):586–95.
Mellon E, Gurbani S, Weinberg B, Kleinberg L, Schreibmann E, Barker P, et al. A feasibility study of radiation therapy dose escalation guided by spectroscopic MRI in patients with glioblastoma. Neuro Oncol. 2018;20:vi231.
Mellon E, Gurbani S, Ramesh K, Weinberg B, Kleinberg L, Schreibmann E, et al. A multisite clinical trial of spectroscopic MRI-guided radiation dose escalation in glioblastoma patients. Neuro Oncol. 2019;21:vi29.
Gurbani S, Weinberg B, Cooper L, Mellon E, Schreibmann E, Sheriff S, et al. The brain imaging collaboration suite (Br ICS): a cloud platform for integrating whole-brain spectroscopic MRI into the radiation therapy planning workflow. Tomography. 2019;5(1):184–91.
Gurbani S, Weinberg B, Cooper L, Mellon E, Schreibmann E, Sheriff S, et al. The brain imaging collaboration suite (Br ICS): a cloud platform for integrating whole-brain spectroscopic MRI into the radiation therapy planning workflow. Tomogr (Ann Arbor, Mich). 2019;5(1):184–91.
Kim MM, Parmar HA, Aryal MP, Mayo CS, Balter JM, Lawrence TS, et al. Developing a pipeline for multiparametric MRI-guided radiation therapy: initial results from a phase II clinical trial in newly diagnosed glioblastoma. Tomography. 2019;5(1):118–26.
Laack NN, Pafundi D, Anderson SK, Kaufmann T, Lowe V, Hunt C, et al. Initial results of a phase 2 trial of 18F-DOPA PET-guided dose-escalated radiation therapy for glioblastoma. Int J Radiat Oncol Biol Phys. 2021;110(5):1383–95.
Laprie Anne. Randomized phase III dose-painting trial SPECTRO GLIO high dose guided by MR spectroscopic imaging. Madrid: ESTRO; 2021. p. 2021.
Brouwer CL, Steenbakkers RJHM, Bourhis J, Budach W, Grau C, Grégoire V, et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG oncology and TROG consensus guidelines. Radiother Oncol. 2015;117(1):83–90.
Scoccianti S, Detti B, Gadda D, Greto D, Furfaro I, Meacci F, et al. Organs at risk in the brain and their dose-constraints in adults and in children: a radiation oncologist’s guide for delineation in everyday practice. Radiother Oncol J Eur Soc Therap Radiol Oncol. 2015;114(2):230–8.
Acknowledgements
Seonad Madden for all the proofreading and advice around systematic reviews.
Funding
John Ryan was supported by an Australian Government Research Training Program Fee-Offset Scholarship. Ian Gleeson is supported by Cancer Research UK RadNet Cambridge [C17918/A28870]. The 2020 Victorian Medical Radiation Practitioners Education Trust Award supported this research.
Author information
Authors and Affiliations
Contributions
JR developed the original concept for the review, drafted the review protocol, was the main coordinator for the review, carried out database searches, screening, data extraction and drafted and submitted this paper. MN carried out screening and paper submission review. IG carried out screening and paper submission review. LM carried out screening and paper submission review. MG is John Ryan’s primary PhD supervisor. He supported the review and added comments to submission drafts. JK is John Ryan’s associate PhD supervisor. She supported the review and added comments to submission drafts. SPN is John Ryan’s PhD clinical supervisor and a radiation oncologist. She provided pertinent clinical feedback to submission drafts. NH is John Ryan’s PhD clinical supervisor. He provided continuous input into systematic review design, carried out screening and pertinent clinical feedback on submission drafts. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Consent for publication
All the authors have approved the manuscript and agree with submission to your journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
A Database Search Strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ryan, J.T., Nakayama, M., Gleeson, I. et al. Functional brain imaging interventions for radiation therapy planning in patients with glioblastoma: a systematic review. Radiat Oncol 17, 178 (2022). https://doi.org/10.1186/s13014-022-02146-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02146-8