Abstract
Introduction
The aim of the narrative review was to analyse the applications of nuclear medicine (NM) techniques such as PET/CT with different tracers in combination with radiotherapy for the clinical management of glioblastoma patients.
Materials and methods
Key references were derived from a PubMed query. Hand searching and clinicaltrials.gov were also used.
Results
This paper contains a narrative report and a critical discussion of NM approaches in combination with radiotherapy in glioma patients.
Conclusions
NM can provide the Radiation Oncologist several aids that can be useful in the clinical management of glioblastoma patients. At the same, these results need to be validated in prospective and multicenter trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) incidence is about 2–3 cases per 100,000 people [1], and its prognosis is extremely poor with a median survival time of only 14.5 months from diagnosis in clinical trials [2]. Despite that the 3-year survival rates rarely reach 5% [3], in clinical practice a great variability in terms of prognosis exists in unselected patients.
Extensive characterisation by multiple omic platforms is improving our knowledge of the molecular bases underlying the nature of GBM aggressiveness [4,5,6,7,8,9,10,11].
Radiotherapy (RT) represents one of the most effective anticancer agents, that can be used either alone or in combination with other strategies (surgery, chemotherapy, immunotherapy). In the field of glioblastoma it represents one of the three modalities that are used together with surgery and temozolomide [2].
In the past decade, several techniques of Nuclear Medicine (NM) have been developed in the field of primary central nervous system cancer, with the aim of increasing the early detection of the pathophysiological changes in oncological patients, including those with brain tumours [12, 13]. Nowadays, the most used NM imaging technique in this field is Positron emission tomography (PET) [12, 13].
Herein, we will discuss the impact of PET in the clinical management of glioblastoma patients.
We will focus on the application of PET/CT with different tracers for the treatment planning of radiotherapy and for response assessment and to distinguish tumour progression from pseudoprogression or radiation necrosis after radiotherapy.
Following a literature search, we will provide a narrative overview of these topics.
Materials and methods
Methods
Evidence acquisition
Electronic literature search was conducted in the PubMed database for English articles published up November 30, 2021. Boolean operators (OR, AND) were used to combine the following search terms: “glioblastoma”, “radiotherapy”, “nuclear medicine”, “PET”. Two independent reviewers (VN, ID) screened titles and abstracts and performed final article selection. Any discrepancy was resolved by discussion with a third reviewer (AR). Meeting proceedings (European Society of Medical Oncology—ESMO—, European SocieTy for Radiotherapy & Oncology—ESTRO—, American Society of Clinical Oncology—ASCO—and American Society for Radiation Oncology—ASTRO—), trial registries (clinicaltrials.gov), reference lists of published studies, review articles and relevant books were also considered.
Nuclear medicine: applications in glioblastoma
Positron emission tomography (PET) is a nuclear medicine imaging technique that, using different radiotracers evaluating different metabolic patterns, is able to detect in advance pathophysiological changes in oncological patients, including those with brain tumours. These functional changes usually occur before the development of morphological changes detected by conventional radiological imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) [14]. Even though contrast-enhanced conventional MRI is the diagnostic method of choice for patients with primary and secondary (metastatic) brain tumours, its specificity for neoplastic tissue is low, resulting in challenges regarding the distinction between cancer and non-neoplastic lesions, the delineation of tumour extent, especially of non-enhancing tumour portions, and the differentiation of treatment-related changes from tumour relapse [15, 16]. Over the past decades, PET with numerous radiolabeled molecules has been evaluated to overcome these limitations of conventional MRI and its clinical use has been also emphasized by the PET task force of the Response Assessment in Neuro-Oncology (RANO) working group. Different PET radiotracers have been used to evaluate brain tumours, in particular for the delineation of tumour extent, diagnosis of treatment-related changesand the assessment of treatment response.
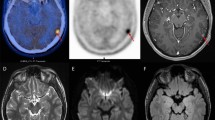
18F-2-deoxy-2-fluoro-D-glucose (18F-FDG) is the most used PET radiotracer in oncology; it is a radiolabelled glucose analogue taken up by neoplastic cells via cell membrane glucose transporters (GLUT) and subsequently phosphorylated through the activity of intracellular hexokinase. 18F-FDG allows the detection of neoplastic cells due to their frequently increased glucose metabolism [14]. In the central nervous system, the uptake of 18F-FDG is physiologically high and varying in healthy brain parenchyma hampering the delineation of brain tumours (see Fig. 1). Furthermore the cerebral inflammatory processes may also exhibit high FDG uptake, thereby diminishing its diagnostic accuracy for the correct identification of treatment-related changes and assessment of treatment response in gliomas and brain metastases [13, 17]. However, 18F-FDG PET seems to be of value for the delineation of tumour extent and assessment of treatment response in patients with primary central nervous system lymphoma [18, 19]. In recent decades other PET tracers, such as radiolabeled amino acids, have been developed. Radiolabeled amino acids are of particular interest for brain tumour imaging using PET because of their increased uptake in neoplastic tissue but low uptake in normal brain parenchyma, resulting in an improved tumour-to-brain. An important feature of these tracers is their ability to cross the intact blood–brain barrier via the transport system L for large neutral amino acids, allowing for visualization of tumour extent beyond contrast enhancement on MRI [12]. Compared to 18F-FDG, radiolabelled amino acid PET showed higher sensitivity and specificity in differentiating between high-grade and low-grade gliomas [20] and can provide valuable information for planning stereotactic biopsies, resection, and radiotherapy [21,22,23].
11C-methionine (11C-MET) is a radiolabelled amino acid; methionine is used by the cells in the following two main metabolic functions: protein synthesis and conversion to S-adenosylmethionine. In many neoplastic cells, there is an increase in protein synthesis, transmethylation and transsulfuration, leading to an increased uptake of 11C-MET [14, 24]. Unfortunately, the use of this PET tracer is restricted to PET centers with a cyclotron facility because of the shorter half-life of 11C compared to 18F (20 min versus 110 min, respectively) [24].
18F-fluoro-ethyl-tyrosine (18F-FET) is a fluorinated amino acid used to detect brain tumours. 18F-FET is taken up into neoplastic cells due to their increased amino acid uptake through an L-type amino acid transport system, and it is not incorporated into proteins [25] (see Fig. 1).
6-Fluoro-(18F)-l-3,4-dihydroxyphenylalanine (18F-FDOPA) has been proposed as a useful PET tracer for imaging brain tumours (see Fig. 1). 18F-FDOPA is transported across the blood–brain barrier by a number of amino acid transporters, which have been shown to be overexpressed in brain tumours. After intracellular uptake through the large amino acid transporter, 18F-FDOPA is decarboxylated by DOPA decarboxylase to 18F-dopamine, which is transported into storage granules by vesicular monoamine transporters and trapped intracellularly [14, 26].
Another biomarker used in brain tumour imaging is the 18F-fluoro-3-deoxy-3-L-fluorothymidine (18F-FLT), a biomarker of cell proliferation, which is increased in neoplastic cells; during the S phase of the cell cycle, 18F-FLT is phosphorylated by thymidine-kinase-1 and trapped inside the cell but not incorporated into the DNA. The cellular thymidine-kinase-1 activity has been reported to be proportional to the proliferation activity of the tumour [27]. However, its diagnostic use is limited by the requirement of a disrupted blood–brain barrier [28]. In terms of tumour detection and delineation, 18F-FLT PET was less sensitive than 11C-methionine PET to detect WHO grade II gliomas, which usually show no contrast enhancement [29]. On the other hand, 18F-FLT PET seems to be useful for the assessment of response to antiangiogenic therapy with bevacizumab in patients with recurrent malignant glioma.
As tumour cells present a high turnover of cellular membranes, radiolabelled choline (using 11C or 18F) may be used to detect brain tumours. The uptake of radiolabelled choline increases in tumour tissue to keep up with the demands of phospholipids synthesis in cellular membranes [14, 30]. In brain tumours 11C or 18F-fluorocholine as markers of cell membrane phospholipids can only detect tumour in disrupted blood–brain barrier areas and are, therefore, less suitable for the delineation of tumour extent [31].
Another approach to study brain tumour is the evaluation of hypoxia, a phenomenon associated with tumour persistence and resistance to cancer treatment. Using PET tracers such as 18F-fluoromisonidazole (FMISO), which is trapped in hypoxic but viable cells, it is possible to identify the hypoxic areas of brain tumour and make an accurate target volume delineation for radiotherapy planning in glioblastoma patients.
Last, PET ligands targeting the 18 kDa mitochondrial translocator protein (TSPO), located at the outer mitochondrial membrane, which is strongly expressed in gliomas, are also of interest in neuro-oncology. TSPO is associated with neuroinflammation due to its expression in activated microglia, endothelial cells, and infiltrating macrophages [32]. The TSPO ligand GE-180 labelled with [18F], recently introduced, offers an increased binding specificity and was tested in patients with gliomas [33]. However, the importance of this radiotracer for radiotherapy planning has not yet been established.
Radiation therapy: applications in glioblastoma
The role of radiation treatment in the management of glioma, both low- and high-grade (LGG and HGG, respectively), is a mainstay.
In Laperriere review [34] post-operative external beam radiotherapy (EBRT) is recommended as standard therapy for patients with malignant glioma.
According to EORTC guidelines, for LGG in absence of favorable prognostic factors (age < 40, no median shift, absence of neurological symptoms, oligodendritic features and maximum diameter < 6 cm), radiotherapy is indicated after surgery with a FTD of 50.4/54 Gy in fractions of 1.8/2 Gy [35,36,37]. In this case, GTV is represented by high intensity area on postoperative T2w MRI and CTV is GTV plus a 1-cm margin [38, 39].
In the management of anaplastic oligodendroglioma, RT leads to a risk reduction in association with surgery and chemotherapy with lomustine, vincristine and procarbazine (PCV), especially in 1p-19q codeleted ones [40]. Conventional fractionation is recommended (60 Gy in 30 fractions or 59.4 Gy in 33 fractions). Also in anaplastic astrocytoma adjuvant radiation treatment is the standard of care at the same FTD in association to sequential temozolomide [41, 42].
Glioblastoma Multiforme (GBM) is the most common type of malignant glioma—and brain tumors as well—among adults.
Radiation treatment in the setting of GBM has a crucial role, as demonstrated by several studies in which radiotherapy following surgery led to an improved survival, if compared to surgical treatment alone [43,44,45].
The current clinical management of GBM is still based on the STUPP trial [2] in which the addition of concomitant temozolomide 75 mg/mq daily plus sequential temozolomide 150–200 mg/mq to radiotherapy treatment significantly improved median OS (14.6 mo vs 12.1 mo with only post-operative RT). Radiation treatment was delivered for a Fractionated Total Dose of 60 Gy in 30 fractions of 2 Gy each (in patients aged 18–72 and with PS < = 2) and it was delivered to the Gross Tumour Volume (GTV) with a 2-to 3-cm margin for the Clinical Target Volume (CTV) 60 Gy in 30 fractions.
As a consequence, the current standard of care in the management of GBM is maximal safe neurosurgical resection followed by radiation treatment to the involved brain with concurrent and adjuvant temozolomide.
For what concerns contouring and planning, according to ESTRO-ACROP guidelines [46], to ensure accurate re-positioning, the patient’s head should be immobilized using an individually adapted mask system (e.g. thermoplastic systems). A CT scan should be obtained using 1–3 mm slice thickness from the vertex to the lower border of C3. Contrast enhanced MRI scan should be fused with the planning CT to aid target delineation. If a recent MRI is not available, for example if MRI is contraindicated, then intravenous contrast should be administered during the planning CT scan to help identification of residual disease. Target delineation should be performed using contrast-enhanced T1 + T2/FLAIR sequences.
GTV is usually identified with surgical resection cavity plus any residual enhancing tumour (post-contrast T1 weighted MRI scans) without inclusion of peri-tumoural oedema (see Fig. 2).
CTV is defined as the GTV plus a margin to account for microscopic spread. Based on studies of recurrence pattern and tumour infiltration, 20 mm is the recommended margin applied in all directions, but CTV margin may be reduced to 0.5 cm around natural barriers to tumour growth (see Fig. 2).
More specifically, CTV reduced at such as the skull (0 mm, using bone window), ventricles (5 mm), falx (5 mm), tentorium cerebelli (5 mm), visual pathway/optic chiasm and (each 0 mm), provided the tumour is distant from the white matter tracts extending to these regions (e.g. midbrain).
Planning Target Volume (PTV) should take into account uncertainties of planning, including those arising from CT-MRI fusion and patient setup. Restricting the CTV to PTV margin to a maximum value around 3–5 mm is recommended (see Fig. 2).
In Europe, single-phase treatment is advocated, with a dose of 60 Gy delivered in 30 fractions of 2 Gy each.
The American Radiation Therapy Oncology Group (RTOG), on the other hand, recommends two phases starting with a larger volume that receives 46 Gy before “coning down” for the additional 14 Gy. In phase 1, a total dose of 46 Gy is delivered in 23 fractions of 2 Gy each. GTV1 is defined as the surgical resection cavity plus any residual enhancing tumour (postcontrast T1-weighted MRI scans) plus surrounding oedema (hyperintensity on T2 or FLAIR MRI scans). CTV1 is defined as the GTV1 plus a margin of 2 cm (if no surrounding oedema is present, the CTV is the contrast enhancing tumour plus 2.5 cm). PTV1 is defined as CTV1 plus a margin of 3–5 mm).
Phase 2 is represented by boost dose of 14 Gy in 7 fractions. GTV2 can be identified with surgical resection cavity plus any residual enhancing tumour (postcontrast T1-weighted MRI scans); CTV2 is GTV2 plus a margin of 2 cm and PTV2 is a margin of 3–5 mm around CTV2.
For what concerns organs at risk (OARs), they include the optic nerves, eyes, lenses, brain and brainstem which all should be contoured. Some also contour the hippocampus when the tumour is in a location that will allow sparing without compromising dose to the target; there is currently insufficient evidence to support recommendations on hippocampal sparing.
Expansion of OARs to create a planning risk volume (PRV) for each OAR is frequently applied; the margin should reflect the accuracy of daily set-up. Overlaps between PRVs and PTV should be considered and may necessitate reducing PTV dose adjacent to OARs. In elderly patients (> 70 years) or those with poor performance status (KPS < 70) hypofractionated schedules are appropriate, such as 40 Gy delivered in 15 fractions of 2.67 Gy [47] or 34 Gy in 10 fractions of 3.4 Gy [48].
Results
The role of NM before glioblastoma radiotherapy: treatment planning, dose escalation
Radiotherapy plays important role in the complex oncological treatment of glioblastoma multiforme (GBM). The current clinical standard consists of surgery followed by radiotherapy plus concomitant and adjuvant temozolomide, providing a median overall survival of 12–16 months [2]. Concomitant temozolomide appears to be most effective in young and fit patients with GBM who have had debulking surgery [49]. Several technological advances have changed the radiotherapy in GBM patients, such as the use of intensity modulated radiotherapy (IMRT) [50], proton therapy and so on [51, 52].
At the same time, there is still an unmet need for the development of additional imaging techniques to complement the standard planning imaging with computed tomography (CT) and magnetic resonance imaging (MRI), in order to increase the therapeutic ratio of radiotherapy. In this context, numerous studies have indicated that the use of MET/FET PET, MRS in conjunction with MRI was superior to MRI alone in determining the extent of malignant involvement [53, 54]. With these techniques it is possible to define the so-called biological target volume (BTV) [55] and although the modern radiotherapy treatments are based on cross-sectional CT and MRI information, more attention is being paid to functional hybrid imaging describing the biological and functional morphology of tumour lesions and newer radiopharmaceuticals for imaging [56].
Conventional PET-CT uses the most commonly tracer 18F-FDG but in this context it may show limited utility because of the high metabolic rate of normal brain tissue [17, 57]. In 2002 a preliminary analysis of 27 GBM patients showed that there was a mean difference of 25% between the 18F-FDG PET and the MRI delineation volume [58]. However, when PET is analysed approximately 3.5 h following FDG injection, it allows the washout of FDG from normal brain cells while abnormal tissue retains FDG. This delayed-phase PET has been shown to be beneficial in detecting both primary and metastatic brain lesions as well as differentiating between residual or recurrent tumour and radiation necrosis following treatment [59, 60].
Within the past decade, several other radiotracers have been investigated in GBM, such as 11C-MET and 18F-FET, that are able to cross the blood–brain barrier [17].
Comparing the 11C-MET PET and MRI, Grosu et al. [61] found that in operated patients with brain gliomas, the size and location of residual 11C-MET uptake differs considerably from what found on postoperative MRI. This consideration has led to new investigations in the radiotherapy treatments planning, initially in the context of re-irradiation at the disease progression, and subsequently in the definition of first treatment target for dose escalation of a limited area (boost).
Douglas et al., explored the use of a 18F-FDG PET based boost (up to a total dose of 79.4 Gy), but unfortunately they found no significant differences in OS or PFS in comparison with the historical data [62].
Miwa et al. investigated a simultaneous integrated boost (SIB) with helical tomotherapy system (HT) planning. Their boost volume was defined on MET uptake and their results showed that the SIB had significant efficacy in controlling both regional and infiltrating tumour cells, without evidence of increased neurological toxicity. In subsequent studies hypo-IMRT and stereotactic IMRT showed a favourable survival outcomes for patients with GBM when there was a complementary use of 11C-MET PET [63, 64].
Despite apparent gross total resection the majority of patients may have residual disease detected by 11C-MET PET before chemoradiation, the persistent 11C-MET PET subvolume is a strong predictor for in field progression-free survival (PFS) and OS [65].
Other Investigators have used 18F-FET PET in dosimetric studies, in order to understand the potential of a PET based dose escalation, without increasing the dose to the OAR, with an approach similar to isotoxic dose escalation [66].
Piroth et al. investigated the use of 18F-FET PET for dose escalation, finding that the auto-contoured PTV led to complex geometric configurations limiting the achievable mean dose in the boost volume [67]. The same group conducted a phase II study that demonstrated that dose escalation based on 18F-FET PET did not lead to a survival benefit [68], probably due to the low resolution of PET scans and to the low contrast between healthy tissues and tumour periphery in terms of 18F-FET uptake.
In 2013 Rieken et al. [69] showed that the integration of both MRI and 18F-FET PET/CT may help to improve GTV coverage by avoiding larger incongruence between physical and biological imaging techniques. The integration of 18F-FET PET in recurrent glioblastoma has been analysed by Piroth et al. more recently [70]. The Authors analysed 13 patients with recurrent GBM and found that a simulated target volume, based on first FET-1 with 7 mm margin covered 100% of relapse volume in median and led to a significantly reduced PTV, compared to MRI-based PTVs. This approach may achieve similar therapeutic efficacy but lower side effects offering a broader window to intensify concomitant systemic treatment focusing distant failures. In the same context, Lohmann et al. enrolled 50 GBM patients [15] that underwent upfront 18F-FET PET and MRI. In 43 patients (86%), the PET tumour volume was significantly larger than the contrast enhancement MRI volume, and thus the information derived from both imaging modalities should be integrated into the management of patients with newly diagnosed glioblastoma.
Fleischmann et al. [71] also enrolled 36 GBM patients undergoing 18F-FET PET examination before primary radiochemotherapy, confirming that target volume delineation of GBM patients can be improved through metabolic imaging prior to primary radiation treatment, since vital tumour can be detected more accurately and at the same time suggesting that CTV margins could be reduced.
Albert et al. [33] used 18F-GE-180 and MRI in order to investigate the 18-kDa mitochondrial translocator protein (TSPO) that was reported to be upregulated in gliomas. In percentage difference, the PET volumes were on average 179%, 135% and 90% larger than the respective MRI volumes showing that 18F-GE-180 PET provides a remarkably high tumour contrast in untreated and pretreated glioblastoma even beyond contrast enhancement on MRI.
While 18F-FET PETs are based on the amino acid uptake of the tumour, other promising radiotracers are ligands of Fibroblast Activating Protein, FAPI-02 and FAPI-04, which detect tumours based on their expression of Fibroblast Activation Protein (FAP) in tumour stroma on cancer-activated fibroblasts [72].
Windisch et al. [73] compared FAP-specific PET to MRI for treatment planning in 13 GBM patients, finding that the GTV based on different technique was different. The resulting incongruent volumes could provide additional information for radiotherapy or biopsy planning. In 2015 Bell et al. [26] analysed in a review different approach, finding that 18F-FDOPA PET provides greater sensitivity and specificity in detection, grading, prognosis and validation of treatment success in both primary and recurrent gliomas.
Recently Laak et al. demonstrated in a phase 2 trial that that 18F-DOPA PET is sensitive and specific for identifying regions of high density and biologically aggressive glioblastoma. The authors analysed an image-based dose escalation approach in 75 GBM patients that was correlated with an increase of PFS, with no increase of toxicity [74].
These results suggests that the additional value of 18F-FDOPA should be considered when delineating target volumes to improve patient care, optimize outcome and deliver more focused therapies [75].
Finally, other metabolic approach that have been used consists in the evaluation of hypoxia that is closely related to the aggressiveness of the brain tumour [76]. 18F-FMISO is currently the most commonly used radiotracer for hypoxia imaging and 18F-FMISO PET can distinguish glioblastomas from lower-grade gliomas, and it can predict the micro-environment of tumours, including necrosis, vascularization, and permeability, survival and treatment response prediction [77].
In the recent years more efforts have been put in finding a way to improve RT plans by adding new type of imaging with computer-based models. Multiparametric MRI, using both DWI and ADC maps were correlated with 18F-FET PET in a cohort of 41 recurrent glioblastoma [78]. Lipkovà combined patient structural and metabolic scans from a single time point with a computational tumour growth model through a Bayesian inference framework [79], in order to obtain a more personalized RT plan for each patient.
Finally, other approach consists in the delivery of a subsequent boost with a stereotactic approach based on functional imaging, such as the protocol of Jacobo et al. [80]. GBM patients underwent a maximal safe resection, followed by the Stupp protocol and in the end a PET guided SRS (stereotactic radiosurgery). The results were encouraging but further studies are needed to confirm this hypothesis.
Several of the above-mentioned PET tracers were successfully correlated with the outcomes of GBM patients.
Graham et al. analysed 31 GBM patients and showed that qualitative FDG uptake was significantly associated with OS (p value 0.03), with a median OS of 9.0 months in non-avid patients versus 4.5 months in avid patients [81]. SUVmax, SUVpeak, TNR-WM and TLG were significantly associated with OS.
Similar results were obtained by other Researchers [82, 83]. A ratio of 2.0 or 2.5 between the residual lesion SUVmax and the healthy white matter SUVmax could be used as a cutoff to identify patients with reduced survival who may potentially benefit from intensive therapeutic strategies [84].
Kawasaki et al., conversely, analysed MET-PET before and after Stupp protocol in 30 newly diagnosed GBM patients who had undergone surgical resection [85]. A reduction in TBRmax of 36.6% or more, correlated to a longer OS of > 23 months.
18F-FDOPA is very useful for distinguishing radiation necrosis and glioblastoma recurrence. Hermann and collegues analysed 110 patients followed for glioblastoma and found that also 18F-FDOPA PET were prognostic of PFS. Patients with positive examinations had a 4.2 times shorter median OS than patients with negative examinations [86].
Conversely, Patel et al., conversely, demonstrated that age (p = 0.001) and the metabolic tumour volume on PET (p = 0.016, using a SUVmax T/N threshold) were correlated with the 2-year overall survival time [87]. Chen et al. demonstrated that increased 18F-FLT uptake in high-grade glioma was associated with reduced patient survival, in a study of 25 patients [88]. 18F-FLT SUVmax correlated more strongly with Ki-67 index (r = 0.84; p < 0.0001) than 18F-FDG SUVmax (r = 0.51; p = 0.07). 18F-FLT uptake also had more significant predictive power with respect to tumour progression and survival (p = 0.0005 and p = 0.001, respectively).
In summary, we report a table comparing different approaches of PET tracers for radiotherapy (see Table 1).
The role of NM after glioblastoma radiotherapy: response assessment, differential diagnosis
The aim of the present paragraph was to report the published data regarding the potential role of emerging PET techniques as useful tools of prognostic value in the setting of response-to-treatment evaluation and in differential diagnosis between true tumour progression and radiation-induced side effects, as compared with conventional MRI assessment.
After chemoradiotherapy for gliomas, conventional treatment response assessment is evaluated via T1-weighted MRI, T2- and/or FLAIR-weighted MRI signal [12, 89].
Although RECIST criteria are widely used for response assessment to therapy in most of cancers, its use in neuro-oncology has been limited because concerns that one-dimensional (1D) measurements may not accurately measure the irregular or asymmetric margins characteristic of high-grade gliomas (HGG). Then, MacDonald criteria were published in 1990 with regard of two-dimensional (2D) assessment of tumour size either on contrast-enhanced CT or contrast-enhanced MRI. With development and growing use of anti-angiogenic agents that affect vascular permeability and contrast enhancement, the limitations and shortcomings of RECIST and MacDonald criteria have become more apparent and necessitated changes. To address this issue, RANO-HGG criteria were introduced in 2010 [90].
However, MRI technique’s reliability is limited by several issues such as treatment-related effects, radiation necrosis and pseudoprogression. The assessment of treatment response in glioblastoma is difficult with MRI scans because reactive blood–brain barrier alterations with contrast enhancement can mimic tumour progression [91].
Radiologically, pseudoprogression (PsP) is defined as a new or enlarging area(s) of contrast agent enhancement occurring early after the end of radiotherapy (e.g. within 3–4 months), in the absence of true tumour growth, which subsides or stabilizes without a change in therapy [92].The RANO criteria attempts to address the phenomenon of pseudoprogression characterized by contrast enhancement in absence of true tumour progression typically following chemoradiation therapy.
In the absence of tissue confirmation, there is no available imaging modality which reliably distinguishes true from pseudoprogression. The RANO–HGG criteria suggest that within the first 3 months after completion of chemoradiation patients whose MRI shows increased enhancement should not be considered to have progressed and should not be considered eligible for clinical trials for recurrent disease. The exceptions are patients who develop new areas of enhancement outside of the radiation field (beyond the high-dose region or 80% iso-dose line) or if there is unequivocal evidence of tumour on histopathologic sampling. Because of the difficulty in differentiating pseudoprogression from true progression, there is the suggestion that the first post-radiation MRI, rather than the postoperative MRI, should be used as the baseline scan [90].
Radiation necrosis is another issue in terms of differential diagnosis. This phenomenon is an important side effect of radiotherapy and can be assessed as a radiation-induced change which usually occurs more than 6 months after radiotherapy even up to several years later. The rate of radiation necrosis following radiotherapy may vary considerably (approximately 5–25%) and depends on the irradiated volume, radiation dose and fractionation scheme as well as on concurrently applied therapies such as targeted therapy or immunotherapy using checkpoint inhibitors [12].
Nowadays, the emergence of advanced MRI techniques, MR spectroscopy and PET tracers has improved response assessment [89] and has helped in overcoming the limits in differential diagnosis.
18F-FDG PET value has proven limited in the brain in consideration of its high glucose metabolism in tumour target delineation [12], but of independent prognostic value pre- and post- radiotherapy in glioma patients [82, 93, 94]. In the setting of response assessment 18F-FDG PET has a controversial role: the evidence that 18F-FDG PET uptake pre- and post RT treatment in a setting of glioma patients showed no correlation between metabolic changes and survival of patients was demonstrated [95], but, on the other hand, the correlation of 18F-FDG PET scans with survival in a cohort of glioma patients was established, with patients with higher 18F-FDG PET scores more likely to progress clinically and to have lower overall actuarial survival times [96].
18F-FDG PET also has a potential role in distinguishing PsP from true tumour progression, in comparison to MRI scans at 1 month post the end of radiotherapy which were significant of tumour progression because of contrast enhancement even in the presence of clinical improvement [97].
18F-FDG PET has a rationale in discriminating between radiation necrosis and tumour recurrence, with a high positive predictive value (PPV) and negative predictive value (NPV), as compared to conventional MRI [98].
Of much more relevance in this context is the use of PET with radiolabelled aminoacids.
Several studies reported the emerging role of 18F-FET PET —particularly related to its early changes in tumour-to-brain uptake ratios— in the prediction of both PFS and OS in newly diagnosed glioma patients, compared to traditional MRI scans [91, 99, 100].
The prognostic impact of postoperative tumour volume and tumour/brain ratios (TBR) in 18F-FET PET using in comparison with MRI was evaluated in prospective studies with the results that 18F-FET PET could be helpful to determine the residual tumour volume after surgery of multiforme glioblastoma (GBM) and may serve as a valuable tool for optimal planning of radiation treatment [70].
18F-FET PET has also been investigated in terms of differential diagnosis between radiation-induced injuries (pseudoprogression and radiation necrosis) and true tumour progression with successful evidence.
Werner and colleagues analysed with 32 18F-FET PET scans 23 newly diagnosed glioblastoma patients following lomustine-temozolomide chemoradiation and with equivocal MRI scans. They defined maximum and mean tumour-to-brain ratios and other dynamic 18F-FET uptake parameters (e.g. time-to-peak). In patients with more than one 18F-FET PET scan, relative changes of TBR values were considered as follows: an increase or decrease of > 10% compared with the reference scan was considered as tumour progression or pseudoprogression. As a result, 18F-FET PET demonstrated a significant role in diagnosing pseudoprogression in this setting of patients [101].
To validate the emerging role of 18F-FET PET in comparison to MRI scans in detecting early PsP, we report Galldiks and Langen experience [102].
Pseudoprogression usually occurs within 3–4 months after the end of radiation therapy, but as a matter of fact it may occur later in the course of the disease and may then be particularly difficult to distinguish from true tumour progression.
In this regard, the role of 18F-FET PET in the diagnosis of rare late pseudoprogression was investigated in 26 glioblastoma patients that presented with increasing contrast-enhancing MRI lesions later than 3 months after completion of radiochemotherapy and who then underwent 18F-FET PET. TBRmax and TBRmean were significantly higher in patients with true progression than in patients with late pseudoprogression. So, 18F-FET PET provided valuable information in assessing the elusive phenomenon of late PsP [103].
18F-FET PET was also investigated for differentiating local recurrent brain metastasis from radiation necrosis after radiation therapy with the results that using tumour/brain ratios in combination with the evaluation of time–activity curves derived from the kinetic 18F-FET PET scans has a sensitivity and specificity of about 90% [104].
Among new aminoacid PET tracers, 11C-MET PET imaging of glioblastoma has proven useful for detecting postoperative residual disease and response to chemoradiation therapy; it may also have a role both in delineation of target volume and in response assessment. Indeed, 11C-MET PET scanning showed a significant decrease in metabolic signal at 1 month after chemoradiation compared with the immediate postoperative period, even when T2/fluid-attenuated inversion recovery changed little [105]. 11C-MET PET MTV (metabolic tumour volume) both in low- and in high-grade gliomas has proven to have a significant and independent prognostic value for patients’ survival [65, 106, 107].
For the differentiation of local brain metastasis recurrence from radiation related effects, the calculation of tumour-to-brain ratios in 11C-MET PET studies revealed a sensitivity and specificity of 70–80% [108,109,110].
18F-FDOPA PET may also be useful for diagnosing patients with pseudoprogression and differentiating them from those with true tumour progression. In this line, we report Hermann’s experience whose study on 110 glioblastoma patients revealed an accuracy of 82% for the correct diagnosis of tumour progression or recurrence [86]. A 18F-FDOPA PET study also revealed a sensitivity and a specificity of more than 80% for the differentiation of local brain metastasis recurrence from radiation-induced effects [111]. Another study compared 18F-FDOPA and 18F-FDG PET brain imaging with the conclusion that 18F-FDOPA PET was more accurate than 18F-FDG PET for imaging of low-grade tumours, for evaluating recurrent tumours and for distinguishing tumour recurrence from radiation necrosis [88].
18F-FMISO PET evaluates intratumoural hypoxia to deliver higher radiation dose to hypoxic subvolumes and overcome hypoxia-induced radioresistance. 18F-FMISO uptake is a mark of an aggressive tumour, almost always a glioblastoma. 18F-FMISO PET could be useful to guide glioma treatment, and in particular radiotherapy since hypoxia is a well-known factor of resistance [112]. Its role has been investigated also in the setting of response assessment. Leimgruber and colleagues showed in a cohort of 18 glioblastoma patients who underwent radiotherapy that patients with the longest overall survival showed non-detectable hypoxia in both pre-radiotherapy and post-radiotherapy 18F-FMISO PET [113].
The role of PET with radiolabelled aminoacids has also been evaluated for what concerns re-irradiation in glioma relapse. A small number of clinical trials have utilized PET for target volume delineation. Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity-modulated radiation therapy has recently been explored with good tolerance and a median survival time of 11 months after treatment completion [64]. A randomized phase II trial compared MRI-guided and 18F-FET PET-guided reirradiation in patients with recurrent GBM with the result that stereotactic fractionated RT is associated with improved survival when aminoacid-PET is integrated in tumour target delineation [114].
In summary, we report a table comparing diagnostic value of new AA tracers with standard MRI-scan (see Table 2).
The future role of NM in glioblastoma radiotherapy: trials in progress
Several trials are currently evaluating different approaches of nuclear medicine in the field of GBM research (see Table 3).
Some trials are investigating the differential diagnosis of GBM, with the use of PSMA PET (NCT04588454) in the visualisation of GBM, the use of 18F-FDG PET for the diagnosis of GBM, the use of 18F-FDOPA PET for the demonstration of functional brain abnormalities and the 18F-DASA-23 and PET Scan in the evaluation of Pyruvate Kinase M2 Expression in patients with intracranial tumours. Other trials in this context are evaluating Tryptophan Metabolism in Human Brain Tumours, the use of [68 Ga]-FF58 in Patients with selected solid tumours expected to overexpress selective Integrins, the assessment of Brain Tumour Hypoxia With Fluoromisonidazole, FDG and Water, the use of mpMRI/Fluorine-18 Fluciclovine PET-CT in GBM and the use of 11C-MET PET as a Post-surgery Baseline Scan for GBM.
Other trials are currently investigating the role of nuclear medicine in the response assessment after standard therapies for GBM, in order to differentiate pseudoprogression with 11C-MET PET or with different tracers, such as 68 Ga-PSMA PET-CT and 18F-FDOPA PET-CT, 18F-FDG PET/CT. Several trials are currently investigating F18 Fluciclovine PET/CT, either alone or in combination with mpMRI.
In the context of radiotherapy planning, other Investigators are enrolling patients for different protocols, such as Fluciclovine or 18F-FET PET guided radiotherapy, or the use of 18F-FDOPA PET/MRI scan for the investigation of proton beam therapy for elderly GBM patients. Other Investigators are currently enrolling GBP patients for TTFields and radiosurgery based on 18F-FET PET for recurrent glioblastoma, or for amino-acid PET guided reirradiation.
Finally, several other trials are currently evaluating different endpoints, such as the PARP-1 expression with 18F-FluorThanatrace PET, the predictive role of PET and perfusion CT in GBM patients undergoing anti-angiogenics, the role of 18F-FDG PET in EGFR positive GBM patients undergoing osimertinib, or laser interstitial thermal therapy treatment response assessment with Fluciclovine PET.
Future directions and conclusions
All the above mentioned studies have shown promising results of different application of nuclear medicine in the field of GBM. Nevertheless, the clinical approach of GBM patients remains the same from the Stupp trial [2]. In this context, considering the poor OS, several efforts must be taken in near future in order to increase the therapeutic efficacy of different therapies.
Conversely, despite an impressive number of retrospective studies, the number of prospective clinical trials investigating the potential role of nuclear medicine in GBM patients remains somewhat low (see Table 3).
There are still some limitations to resolve before nuclear medicine techniques can be successfully applied in the clinical management of GBM patients. More specifically, current major pitfalls in nuclear medicine are the big heterogeneity of tracers adopted, the lack of image standardization and the lack of standardization of volumes definition to be used in the treatment planning.
Nuclear medicine actually represents one of the most interesting approaches of tailored medicine in this disease. Future research will also need to focus on big data analysis and artificial intelligence in order to facilitate the clinical application of nuclear medicine in the management of GBM patients.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33. https://doi.org/10.3322/caac.21654
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330
Smoll NR, Schaller K, Gautschi OP (2013) Long-term survival of patients with glioblastoma multiforme (GBM). J Clin Neurosci 20(5):670–675
Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17(1):98–110
Brennan Cameron W, Verhaak Roel GW, McKenna A, Campos B, Noushmehr H, Salama Sofie R, Zheng S, Chakravarty D, Sanborn JZ, Berman Samuel H, Beroukhim R, Bernard B, Wu C-J, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla Sachet A, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner Darell D, Van Meir EG, Prados M, Sloan A, Black Keith L, Eschbacher J, Finocchiaro G, Friedman W, Andrews David W, Guha A, Iacocca M, O’Neill Brian P, Foltz G, Myers J, Weisenberger Daniel J, Penny R, Kucherlapati R, Perou Charles M, Hayes DN, Gibbs R, Marra M, Mills Gordon B, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird Peter W, Haussler D, Getz G, Chin L, Benz C, Barnholtz-Sloan J, Barrett W, Ostrom Q, Wolinsky Y, Black Keith L, Bose B, Boulos Paul T, Boulos M, Brown J, Czerinski C, Eppley M, Iacocca M, Kempista T, Kitko T, Koyfman Y, Rabeno B, Rastogi P, Sugarman M, Swanson P, Yalamanchii K, Otey Ilana P, Liu Yingchun S, Xiao Y, Auman JT, Chen P-C, Hadjipanayis A, Lee E, Lee S, Park Peter J, Seidman J, Yang L, Kucherlapati R, Kalkanis S, Mikkelsen T, Poisson Laila M, Raghunathan A, Scarpace L, Bernard B, Bressler R, Eakin A, Iype L, Kreisberg Richard B, Leinonen K, Reynolds S, Rovira H, Thorsson V, Shmulevich I, Annala Matti J, Penny R, Paulauskis J, Curley E, Hatfield M, Mallery D, Morris S, Shelton T, Shelton C, Sherman M, Yena P, Cuppini L, DiMeco F, Eoli M, Finocchiaro G, Maderna E, Pollo B, Saini M, Balu S, Hoadley Katherine A, Li L, Miller CR, Shi Y, Topal Michael D, Wu J, Dunn G, Giannini C, O’Neill Brian P, Aksoy BA, Antipin Y, Borsu L, Berman Samuel H, Brennan Cameron W, Cerami E, Chakravarty D, Ciriello G, Gao J, Gross B, Jacobsen A, Ladanyi M, Lash A, Liang Y, Reva B, Sander C, Schultz N, Shen R, Socci Nicholas D, Viale A, Ferguson Martin L, Chen Q-R, Demchok John A, Dillon Laura AL, Shaw Kenna RM, Sheth M, Tarnuzzer R, Wang Z, Yang L, Davidsen T, Guyer Mark S, Ozenberger Bradley A, Sofia Heidi J, Bergsten J, Eckman J, Harr J, Myers J, Smith C, Tucker K, Winemiller C, Zach Leigh A, Ljubimova Julia Y, Eley G, Ayala B, Jensen Mark A, Kahn A, Pihl Todd D, Pot David A, Wan Y, Eschbacher J, Foltz G, Hansen N, Hothi P, Lin B, Shah N, Yoon J-g, Lau C, Berens M, Ardlie K, Beroukhim R, Carter Scott L, Cherniack Andrew D, Noble M, Cho J, Cibulskis K, DiCara D, Frazer S, Gabriel Stacey B, Gehlenborg N, Gentry J, Heiman D, Kim J, Jing R, Lander Eric S, Lawrence M, Lin P, Mallard W, Meyerson M, Onofrio Robert C, Saksena G, Schumacher S, Sougnez C, Stojanov P, Tabak B, Voet D, Zhang H, Zou L, Getz G, Dees Nathan N, Ding L, Fulton Lucinda L, Fulton Robert S, Kanchi K-L, Mardis Elaine R, Wilson Richard K, Baylin Stephen B, Andrews David W, Harshyne L, Cohen Mark L, Devine K, Sloan Andrew E, VandenBerg Scott R, Berger Mitchel S, Prados M, Carlin D, Craft B, Ellrott K, Goldman M, Goldstein T, Grifford M, Haussler D, Ma S, Ng S, Salama Sofie R, Sanborn JZ, Stuart J, Swatloski T, Waltman P, Zhu J, Foss R, Frentzen B, Friedman W, McTiernan R, Yachnis A, Hayes DN, Perou Charles M, Zheng S, Vegesna R, Mao Y, Akbani R, Aldape K, Bogler O, Fuller Gregory N, Liu W, Liu Y, Lu Y, Mills G, Protopopov A, Ren X, Sun Y, Wu C-J, Yung WKA, Zhang W, Zhang J, Chen K, Weinstein John N, Chin L, Verhaak Roel GW, Noushmehr H, Weisenberger Daniel J, Bootwalla Moiz S, Lai Phillip H, Triche Timothy J, Van Den Berg DJ, Laird Peter W, Gutmann David H, Lehman Norman L, VanMeir EG, Brat D, Olson Jeffrey J, Mastrogianakis Gena M, Devi Narra S, Zhang Z, Bigner D, Lipp E, McLendon R (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477
Tini P, Belmonte G, Toscano M, Miracco C, Palumbo S, Pastina P, Battaglia G, Nardone V, Butorano MAGM, Masucci A, Cerase A, Pirtoli L (2015) Combined epidermal growth factor receptor and beclin1 autophagic protein expression analysis identifies different clinical presentations, responses to chemo- and radiotherapy, and prognosis in glioblastoma. Biomed Res Int. https://doi.org/10.1155/2015/208076
Tini P, Nardone V, Pastina P, Battaglia G, Miracco C, Carbone SF, Sebaste L, Rubino G, Cerase A, Pirtoli L (2018) Epidermal growth factor receptor expression predicts time and patterns of recurrence in patients with glioblastoma after radiotherapy and temozolomide. World Neurosurg. https://doi.org/10.1016/j.wneu.2017.10.052
Tini P, Pastina P, Nardone V, Sebaste L, Toscano M, Miracco C, Cerase A, Pirtoli L (2016) The combined EGFR protein expression analysis refines the prognostic value of the MGMT promoter methylation status in glioblastoma. Clin Neurol Neurosurg. https://doi.org/10.1016/j.clineuro.2016.07.023
Detti B, Scoccianti S, Teriaca MA, Maragna V, Lorenzetti V, Lucidi S, Bellini C, Greto D, Desideri I, Livi L (2021) Bevacizumab in recurrent high-grade glioma: a single institution retrospective analysis on 92 patients. Radiol Med 126(9):1249–1254. https://doi.org/10.1007/s11547-021-01381-5
Pasqualetti F, Montemurro N, Desideri I, Loi M, Giannini N, Gadducci G, Malfatti G, Cantarella M, Gonnelli A, Montrone S, Visani L, Scatena C, Naccarato AG, Perrini P, Gambacciani C, Santonocito O, Morganti R, Paiar F (2021) Impact of recurrence pattern in patients undergoing a second surgery for recurrent glioblastoma. Acta Neurol Belg. https://doi.org/10.1007/s13760-021-01765-4
Scoccianti S, Perna M, Olmetto E, Delli Paoli C, Terziani F, Ciccone LP, Detti B, Greto D, Simontacchi G, Grassi R, Scoccimarro E, Bonomo P, Mangoni M, Desideri I, Di Cataldo V, Vernaleone M, Casati M, Pallotta S, Livi L (2021) Local treatment for relapsing glioblastoma: a decision-making tree for choosing between reirradiation and second surgery. Crit Rev Oncol Hematol 157:103184. https://doi.org/10.1016/j.critrevonc.2020.103184
Galldiks N, Niyazi M, Grosu AL, Kocher M, Langen KJ, Law I, Minniti G, Kim MM, Tsien C, Dhermain F, Soffietti R, Mehta MP, Weller M, Tonn JC (2021) Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients—a report of the PET/RANO group. Neuro Oncol 23(6):881–893. https://doi.org/10.1093/neuonc/noab013
Galldiks N, Langen KJ, Albert NL, Chamberlain M, Soffietti R, Kim MM, Law I, Le Rhun E, Chang S, Schwarting J, Combs SE, Preusser M, Forsyth P, Pope W, Weller M, Tonn JC (2019) PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol 21(5):585–595. https://doi.org/10.1093/neuonc/noz003
Treglia G, Sadeghi R, Del Sole A, Giovanella L (2014) Diagnostic performance of PET/CT with tracers other than F-18-FDG in oncology: an evidence-based review. Clin Transl Oncol 16(9):770–775. https://doi.org/10.1007/s12094-014-1168-8
Lohmann P, Stavrinou P, Lipke K, Bauer EK, Ceccon G, Werner JM, Neumaier B, Fink GR, Shah NJ, Langen KJ, Galldiks N (2019) FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging 46(3):591–602. https://doi.org/10.1007/s00259-018-4188-8
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, Levin VA (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217(2):377–384. https://doi.org/10.1148/radiology.217.2.r00nv36377
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, la Fougère C, Pope W, Law I, Arbizu J, Chamberlain MC, Vogelbaum M, Ellingson BM, Tonn JC (2016) Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 18(9):1199–1208. https://doi.org/10.1093/neuonc/now058
Palmedo H, Urbach H, Bender H, Schlegel U, Schmidt-Wolf IG, Matthies A, Linnebank M, Joe A, Bucerius J, Biersack HJ, Pels H (2006) FDG-PET in immunocompetent patients with primary central nervous system lymphoma: correlation with MRI and clinical follow-up. Eur J Nucl Med Mol Imaging 33(2):164–168. https://doi.org/10.1007/s00259-005-1917-6
Birsen R, Blanc E, Willems L, Burroni B, Legoff M, Le Ray E, Pilorge S, Salah S, Quentin A, Deau B, Franchi P, Vignon M, Mabille L, Nguyen C, Kirova Y, Varlet P, Edjlali M, Dezamis E, Hoang-Xuan K, Soussain C, Houillier C, Damotte D, Pallud J, Bouscary D, Tamburini J (2018) Prognostic value of early 18F-FDG PET scanning evaluation in immunocompetent primary CNS lymphoma patients. Oncotarget 9(24):16822–16831. https://doi.org/10.18632/oncotarget.24706
Katsanos AH, Alexiou GA, Fotopoulos AD, Jabbour P, Kyritsis AP, Sioka C (2019) Performance of 18F-FDG, 11C-methionine, and 18F-FET PET for glioma grading: a meta-analysis. Clin Nucl Med 44(11):864–869. https://doi.org/10.1097/rlu.0000000000002654
Hayes AR, Jayamanne D, Hsiao E, Schembri GP, Bailey DL, Roach PJ, Khasraw M, Newey A, Wheeler HR, Back M (2018) Utilizing 18F-fluoroethyltyrosine (FET) positron emission tomography (PET) to define suspected nonenhancing tumor for radiation therapy planning of glioblastoma. Pract Radiat Oncol 8(4):230–238. https://doi.org/10.1016/j.prro.2018.01.006
Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, Klein JC, Herholz K, Heiss WD (2004) Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 10(21):7163–7170. https://doi.org/10.1158/1078-0432.Ccr-04-0262
Lopez WO, Cordeiro JG, Albicker U, Doostkam S, Nikkhah G, Kirch RD, Trippel M, Reithmeier T (2015) Correlation of (18)F-fluoroethyl tyrosine positron-emission tomography uptake values and histomorphological findings by stereotactic serial biopsy in newly diagnosed brain tumors using a refined software tool. Onco Targ Ther 8:3803–3815. https://doi.org/10.2147/ott.S87126
Treglia G, Muoio B, Trevisi G, Mattoli MV, Albano D, Bertagna F, Giovanella L (2019) Diagnostic performance and prognostic value of PET/CT with different tracers for brain tumors: a systematic review of published meta-analyses. Int J Mol Sci. https://doi.org/10.3390/ijms20194669
Muoio B, Giovanella L, Treglia G (2018) Recent developments of 18F-FET PET in neuro-oncology. Curr Med Chem 25(26):3061–3073. https://doi.org/10.2174/0929867325666171123202644
Bell C, Dowson N, Puttick S, Gal Y, Thomas P, Fay M, Smith J, Rose S (2015) Increasing feasibility and utility of (18)F-FDOPA PET for the management of glioma. Nucl Med Biol 42(10):788–795. https://doi.org/10.1016/j.nucmedbio.2015.06.001
Bertagna F, Biasiotto G, Giubbini R (2013) The role of F-18-fluorothymidine PET in oncology. Clin Transl Imaging 1(2):77–97. https://doi.org/10.1007/s40336-013-0014-2
Saga T, Kawashima H, Araki N, Takahashi JA, Nakashima Y, Higashi T, Oya N, Mukai T, Hojo M, Hashimoto N, Manabe T, Hiraoka M, Togashi K (2006) Evaluation of primary brain tumors with FLT-PET: usefulness and limitations. Clin Nucl Med 31(12):774–780. https://doi.org/10.1097/01.rlu.0000246820.14892.d2
Jacobs AH, Thomas A, Kracht LW, Li H, Dittmar C, Garlip G, Galldiks N, Klein JC, Sobesky J, Hilker R, Vollmar S, Herholz K, Wienhard K, Heiss WD (2005) 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med 46(12):1948–1958
Treglia G, Giovannini E, Di Franco D, Calcagni ML, Rufini V, Picchio M, Giordano A (2012) The role of positron emission tomography using carbon-11 and fluorine-18 choline in tumors other than prostate cancer: a systematic review. Ann Nucl Med 26(6):451–461. https://doi.org/10.1007/s12149-012-0602-7
Langen KJ, Galldiks N, Hattingen E, Shah NJ (2017) Advances in neuro-oncology imaging. Nat Rev Neurol 13(5):279–289. https://doi.org/10.1038/nrneurol.2017.44
Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M (2006) Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27(8):402–409. https://doi.org/10.1016/j.tips.2006.06.005
Albert NL, Unterrainer M, Fleischmann DF, Lindner S, Vettermann F, Brunegraf A, Vomacka L, Brendel M, Wenter V, Wetzel C, Rupprecht R, Tonn JC, Belka C, Bartenstein P, Niyazi M (2017) TSPO PET for glioma imaging using the novel ligand (18)F-GE-180: first results in patients with glioblastoma. Eur J Nucl Med Mol Imaging 44(13):2230–2238. https://doi.org/10.1007/s00259-017-3799-9
Laperriere N, Zuraw L, Cairncross G (2002) Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol 64(3):259–273. https://doi.org/10.1016/s0167-8140(02)00078-6
van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmström PO, Collette L, Piérart M, Mirimanoff R, Karim AB (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366(9490):985–990. https://doi.org/10.1016/s0140-6736(05)67070-5
Shaw E, Arusell R, Scheithauer B, O’Fallon J, O’Neill B, Dinapoli R, Nelson D, Earle J, Jones C, Cascino T, Nichols D, Ivnik R, Hellman R, Curran W, Abrams R (2002) Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol 20(9):2267–2276. https://doi.org/10.1200/jco.2002.09.126
Karim AB, Maat B, Hatlevoll R, Menten J, Rutten EH, Thomas DG, Mascarenhas F, Horiot JC, Parvinen LM, van Reijn M, Jager JJ, Fabrini MG, van Alphen AM, Hamers HP, Gaspar L, Noordman E, Pierart M, van Glabbeke M (1996) A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys 36(3):549–556. https://doi.org/10.1016/s0360-3016(96)00352-5
Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, Brandes AA, Kantor G, Taphoorn MJB, Hassel MB, Hartmann C, Ryan G, Capper D, Kros JM, Kurscheid S, Wick W, Enting R, Reni M, Thiessen B, Dhermain F, Bromberg JE, Feuvret L, Reijneveld JC, Chinot O, Gijtenbeek JMM, Rossiter JP, Dif N, Balana C, Bravo-Marques J, Clement PM, Marosi C, Tzuk-Shina T, Nordal RA, Rees J, Lacombe D, Mason WP, Stupp R (2016) Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 17(11):1521–1532. https://doi.org/10.1016/s1470-2045(16)30313-8
Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA (2009) Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol 27(22):3598–3604. https://doi.org/10.1200/jco.2008.20.9494
Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31(3):337–343. https://doi.org/10.1200/jco.2012.43.2674
van den Bent MJ, Tesileanu CMS, Wick W, Sanson M, Brandes AA, Clement PM, Erridge S, Vogelbaum MA, Nowak AK, Baurain JF, Mason WP, Wheeler H, Chinot OL, Gill S, Griffin M, Rogers L, Taal W, Rudà R, Weller M, McBain C, Reijneveld J, Enting RH, Caparrotti F, Lesimple T, Clenton S, Gijtenbeek A, Lim E, Herrlinger U, Hau P, Dhermain F, de Heer I, Aldape K, Jenkins RB, Dubbink HJ, Kros JM, Wesseling P, Nuyens S, Golfinopoulos V, Gorlia T, French P, Baumert BG (2021) Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053–22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol 22(6):813–823. https://doi.org/10.1016/s1470-2045(21)00090-5
van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, Brandes AA, Clement PM, Baurain JF, Mason WP, Wheeler H, Chinot OL, Gill S, Griffin M, Brachman DG, Taal W, Rudà R, Weller M, McBain C, Reijneveld J, Enting RH, Weber DC, Lesimple T, Clenton S, Gijtenbeek A, Pascoe S, Herrlinger U, Hau P, Dhermain F, van Heuvel I, Stupp R, Aldape K, Jenkins RB, Dubbink HJ, Dinjens WNM, Wesseling P, Nuyens S, Golfinopoulos V, Gorlia T, Wick W, Kros JM (2017) Interim results from the CATNON trial (EORTC study 26053–22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet 390(10103):1645–1653. https://doi.org/10.1016/s0140-6736(17)31442-3
Shapiro WR, Young DF (1976) Treatment of malignant glioma. A controlled study of chemotherapy and irradiation. Arch Neurol 33(7):494–450. https://doi.org/10.1001/archneur.1976.00500070036007
Andersen AP (1978) Postoperative irradiation of glioblastomas. Results in a randomized series. Acta Radiol Oncol Radiat Phys Biol 17(6):475–484. https://doi.org/10.3109/02841867809128178
Kristiansen K, Hagen S, Kollevold T, Torvik A, Holme I, Nesbakken R, Hatlevoll R, Lindgren M, Brun A, Lindgren S, Notter G, Andersen AP, Elgen K (1981) Combined modality therapy of operated astrocytomas grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: a prospective multicenter trial of the Scandinavian Glioblastoma Study Group. Cancer 47(4):649–652. https://doi.org/10.1002/1097-0142(19810215)47:4<649::aid-cncr2820470405>3.0.co;2-w
Niyazi M, Brada M, Chalmers AJ, Combs SE, Erridge SC, Fiorentino A, Grosu AL, Lagerwaard FJ, Minniti G, Mirimanoff RO, Ricardi U, Short SC, Weber DC, Belka C (2016) ESTRO-ACROP guideline “target delineation of glioblastomas.” Radiother Oncol 118(1):35–42. https://doi.org/10.1016/j.radonc.2015.12.003
Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C, Husain S, Murtha A, Petruk K, Stewart D, Tai P, Urtasun R, Cairncross JG, Forsyth P (2004) Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 22(9):1583–1588. https://doi.org/10.1200/jco.2004.06.082
Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13(9):916–926. https://doi.org/10.1016/s1470-2045(12)70265-6
Koukourakis GV, Kouloulias V, Zacharias G, Papadimitriou C, Pantelakos P, Maravelis G, Fotineas A, Beli I, Chaldeopoulos D, Kouvaris J (2009) Temozolomide with radiation therapy in high grade brain gliomas: pharmaceuticals considerations and efficacy; a review article. Molecules 14(4):1561–1577. https://doi.org/10.3390/molecules14041561
Amelio D, Lorentini S, Schwarz M, Amichetti M (2010) Intensity-modulated radiation therapy in newly diagnosed glioblastoma: a systematic review on clinical and technical issues. Radiother Oncol 97(3):361–369. https://doi.org/10.1016/j.radonc.2010.08.018
Kotecha R, Tom MC, Mehta MP (2021) Novel radiation approaches. Neurosurg Clin N Am 32(2):211–223. https://doi.org/10.1016/j.nec.2020.12.007
Weller M, Le Rhun E, Preusser M, Tonn JC, Roth P (2019) How we treat glioblastoma. ESMO Open 4(Suppl 2):e000520. https://doi.org/10.1136/esmoopen-2019-000520
Iuchi T, Hatano K, Uchino Y, Itami M, Hasegawa Y, Kawasaki K, Sakaida T, Hara R (2015) Methionine uptake and required radiation dose to control glioblastoma. Int J Radiat Oncol Biol Phys 93(1):133–140. https://doi.org/10.1016/j.ijrobp.2015.04.044
Mahasittiwat P, Mizoe JE, Hasegawa A, Ishikawa H, Yoshikawa K, Mizuno H, Yanagi T, Takagi R, Pattaranutaporn P, Tsujii H (2008) l-[METHYL-(11)C] methionine positron emission tomography for target delineation in malignant gliomas: impact on results of carbon ion radiotherapy. Int J Radiat Oncol Biol Phys 70(2):515–522. https://doi.org/10.1016/j.ijrobp.2007.06.071
Suchorska B, Jansen NL, Linn J, Kretzschmar H, Janssen H, Eigenbrod S, Simon M, Pöpperl G, Kreth FW, la Fougere C, Weller M, Tonn JC (2015) Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 84(7):710–719. https://doi.org/10.1212/wnl.0000000000001262
Susheela SP, Revannasiddaiah S (2016) Radiotherapy to volumes defined by metabolic imaging in gliomas: time to abandon monstrous margins? Ann Transl Med 4(3):55. https://doi.org/10.3978/j.issn.2305-5839.2016.01.19
Moreau A, Febvey O, Mognetti T, Frappaz D, Kryza D (2019) Contribution of different positron emission tomography tracers in glioma management: focus on glioblastoma. Front Oncol. https://doi.org/10.3389/fonc.2019.01134
Tralins KS, Douglas JG, Stelzer KJ, Mankoff DA, Silbergeld DL, Rostomily RC, Hummel S, Scharnhorst J, Krohn KA, Spence AM (2002) Volumetric analysis of 18F-FDG PET in glioblastoma multiforme: prognostic information and possible role in definition of target volumes in radiation dose escalation. J Nucl Med 43(12):1667–1673
Spence AM, Muzi M, Mankoff DA, O’Sullivan SF, Link JM, Lewellen TK, Lewellen B, Pham P, Minoshima S, Swanson K, Krohn KA (2004) 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med 45(10):1653–1659
Horky LL, Hsiao EM, Weiss SE, Drappatz J, Gerbaudo VH (2011) Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J Neurooncol 103(1):137–146. https://doi.org/10.1007/s11060-010-0365-8
Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, Gumprecht H, Jaeger R, Schwaiger M, Molls M (2005) L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 63(1):64–74. https://doi.org/10.1016/j.ijrobp.2005.01.045
Douglas JG, Stelzer KJ, Mankoff DA, Tralins KS, Krohn KA, Muzi M, Silbergeld DL, Rostomily RC, Scharnhorst J, Spence AM (2006) [F-18]-fluorodeoxyglucose positron emission tomography for targeting radiation dose escalation for patients with glioblastoma multiforme: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys 64(3):886–891. https://doi.org/10.1016/j.ijrobp.2005.08.013
Miwa K, Matsuo M, Ogawa S, Shinoda J, Asano Y, Ito T, Yokoyama K, Yamada J, Yano H, Iwama T (2014) Hypofractionated high-dose irradiation with positron emission tomography data for the treatment of glioblastoma multiforme. Biomed Res Int 2014:407026. https://doi.org/10.1155/2014/407026
Miwa K, Matsuo M, Ogawa S, Shinoda J, Yokoyama K, Yamada J, Yano H, Iwama T (2014) Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol 9:181. https://doi.org/10.1186/1748-717x-9-181
Miller S, Li P, Schipper M, Junck L, Piert M, Lawrence TS, Tsien C, Cao Y, Kim MM (2020) Metabolic tumor volume response assessment using (11)C-methionine positron emission tomography identifies glioblastoma tumor subregions that predict progression better than baseline or anatomic magnetic resonance imaging alone. Adv Radiat Oncol 5(1):53–61. https://doi.org/10.1016/j.adro.2019.08.004
Rickhey M, Koelbl O, Eilles C, Bogner L (2008) A biologically adapted dose-escalation approach, demonstrated for 18F-FET-PET in brain tumors. Strahlenther Onkol 184(10):536–542. https://doi.org/10.1007/s00066-008-1883-6
Piroth MD, Pinkawa M, Holy R, Stoffels G, Demirel C, Attieh C, Kaiser HJ, Langen KJ, Eble MJ (2009) Integrated-boost IMRT or 3-D-CRT using FET-PET based auto-contoured target volume delineation for glioblastoma multiforme—a dosimetric comparison. Radiat Oncol 4:57. https://doi.org/10.1186/1748-717x-4-57
Piroth MD, Pinkawa M, Holy R, Klotz J, Schaar S, Stoffels G, Galldiks N, Coenen HH, Kaiser HJ, Langen KJ, Eble MJ (2012) Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Results of a prospective phase II study. Strahlenther Onkol 188(4):334–339. https://doi.org/10.1007/s00066-011-0060-5
Rieken S, Habermehl D, Giesel FL, Hoffmann C, Burger U, Rief H, Welzel T, Haberkorn U, Debus J, Combs SE (2013) Analysis of FET-PET imaging for target volume definition in patients with gliomas treated with conformal radiotherapy. Radiother Oncol 109(3):487–492. https://doi.org/10.1016/j.radonc.2013.06.043
Piroth MD, Galldiks N, Pinkawa M, Holy R, Stoffels G, Ermert J, Mottaghy FM, Shah NJ, Langen KJ, Eble MJ (2016) Relapse patterns after radiochemotherapy of glioblastoma with FET PET-guided boost irradiation and simulation to optimize radiation target volume. Radiat Oncol 11:87. https://doi.org/10.1186/s13014-016-0665-z
Fleischmann DF, Unterrainer M, Schön R, Corradini S, Maihöfer C, Bartenstein P, Belka C, Albert NL, Niyazi M (2020) Margin reduction in radiotherapy for glioblastoma through (18)F-fluoroethyltyrosine PET?—a recurrence pattern analysis. Radiother Oncol 145:49–55. https://doi.org/10.1016/j.radonc.2019.12.005
Dendl K, Koerber SA, Kratochwil C, Cardinale J, Finck R, Dabir M, Novruzov E, Watabe T, Kramer V, Choyke PL, Haberkorn U, Giesel FL (2021) FAP and FAPI-PET/CT in malignant and non-malignant diseases: a perfect symbiosis? Cancers (Basel). https://doi.org/10.3390/cancers13194946
Windisch P, Röhrich M, Regnery S, Tonndorf-Martini E, Held T, Lang K, Bernhardt D, Rieken S, Giesel F, Haberkorn U, Debus J, Adeberg S (2020) Fibroblast activation protein (FAP) specific PET for advanced target volume delineation in glioblastoma. Radiother Oncol 150:159–163. https://doi.org/10.1016/j.radonc.2020.06.040
Laack NN, Pafundi D, Anderson SK, Kaufmann T, Lowe V, Hunt C, Vogen D, Yan E, Sarkaria J, Brown P, Kizilbash S, Uhm J, Ruff M, Zakhary M, Zhang Y, Seaberg M, Wan Chan Tseung HS, Kabat B, Kemp B, Brinkmann D (2021) Initial results of a phase 2 trial of (18)F-DOPA PET-guided dose-escalated radiation therapy for glioblastoma. Int J Radiat Oncol Biol Phys 110(5):1383–1395. https://doi.org/10.1016/j.ijrobp.2021.03.032
Sipos D, László Z, Tóth Z, Kovács P, Tollár J, Gulybán A, Lakosi F, Repa I, Kovács A (2021) Additional value of 18F-FDOPA amino acid analog radiotracer to irradiation planning process of patients with glioblastoma multiforme. Front Oncol 11:699360. https://doi.org/10.3389/fonc.2021.699360
Chédeville AL, Madureira PA (2021) The role of hypoxia in glioblastoma radiotherapy resistance. Cancers (Basel). https://doi.org/10.3390/cancers13030542
Hirata K, Yamaguchi S, Shiga T, Kuge Y, Tamaki N (2019) The roles of hypoxia imaging using (18)F-fluoromisonidazole positron emission tomography in glioma treatment. J Clin Med. https://doi.org/10.3390/jcm8081088
Popp I, Bott S, Mix M, Oehlke O, Schimek-Jasch T, Nieder C, Nestle U, Bock M, Yuh WTC, Meyer PT, Weber WA, Urbach H, Mader I, Grosu AL (2019) Diffusion-weighted MRI and ADC versus FET-PET and GdT1w-MRI for gross tumor volume (GTV) delineation in re-irradiation of recurrent glioblastoma. Radiother Oncol 130:121–131. https://doi.org/10.1016/j.radonc.2018.08.019
Lipkova J, Angelikopoulos P, Wu S, Alberts E, Wiestler B, Diehl C, Preibisch C, Pyka T, Combs SE, Hadjidoukas P, Van Leemput K, Koumoutsakos P, Lowengrub J, Menze B (2019) Personalized radiotherapy design for glioblastoma: integrating mathematical tumor models, multimodal scans, and bayesian inference. IEEE Trans Med Imaging 38(8):1875–1884. https://doi.org/10.1109/tmi.2019.2902044
Jacobo JA, Buentello M, Del Valle R (2021) C-methionine-PET-guided Gamma Knife radiosurgery boost as adjuvant treatment for newly diagnosed glioblastomas. Surg Neurol Int 12:247. https://doi.org/10.25259/sni_706_2020
Graham MS, Krebs S, Bale T, Domfe K, Lobaugh SM, Zhang Z, Dunphy MP, Kaley T, Young RJ (2020) Value of [(18)F]-FDG positron emission tomography in patients with recurrent glioblastoma receiving bevacizumab. Neurooncol Adv 2(1):vdaa050. https://doi.org/10.1093/noajnl/vdaa050
Colavolpe C, Metellus P, Mancini J, Barrie M, Béquet-Boucard C, Figarella-Branger D, Mundler O, Chinot O, Guedj E (2012) Independent prognostic value of pre-treatment 18-FDG-PET in high-grade gliomas. J Neurooncol 107(3):527–535. https://doi.org/10.1007/s11060-011-0771-6
Santra A, Kumar R, Sharma P, Bal C, Julka PK, Malhotra A (2011) F-18 FDG PET-CT for predicting survival in patients with recurrent glioma: a prospective study. Neuroradiology 53(12):1017–1024. https://doi.org/10.1007/s00234-011-0898-3
Leiva-Salinas C, Schiff D, Flors L, Patrie JT, Rehm PK (2017) FDG PET/MR imaging coregistration helps predict survival in patients with glioblastoma and radiologic progression after standard of care treatment. Radiology 283(2):508–514. https://doi.org/10.1148/radiol.2016161172
Kawasaki T, Miwa K, Shinoda J, Asano Y, Takei H, Ikegame Y, Yokoyama K, Yano H, Iwama T (2019) Dissociation between 11C-methionine-positron emission tomography and gadolinium-enhanced magnetic resonance imaging in longitudinal features of glioblastoma after postoperative radiotherapy. World Neurosurg 125:93–100. https://doi.org/10.1016/j.wneu.2019.01.129
Herrmann K, Czernin J, Cloughesy T, Lai A, Pomykala KL, Benz MR, Buck AK, Phelps ME, Chen W (2014) Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol 16(4):603–609. https://doi.org/10.1093/neuonc/not166
Patel CB, Fazzari E, Chakhoyan A, Yao J, Raymond C, Nguyen H, Manoukian J, Nguyen N, Pope W, Cloughesy TF, Nghiemphu PL, Czernin J, Lai A, Ellingson BM (2018) (18)F-FDOPA PET and MRI characteristics correlate with degree of malignancy and predict survival in treatment-naïve gliomas: a cross-sectional study. J Neurooncol 139(2):399–409. https://doi.org/10.1007/s11060-018-2877-6
Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, Satyamurthy N, Schiepers C, Cloughesy T (2006) 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47(6):904–911
Le Fèvre C, Constans JM, Chambrelant I, Antoni D, Bund C, Leroy-Freschini B, Schott R, Cebula H, Noël G (2021) Pseudoprogression versus true progression in glioblastoma patients: a multiapproach literature review. Part 2—Radiological features and metric markers. Crit Rev Oncol Hematol 159:103230. https://doi.org/10.1016/j.critrevonc.2021.103230
Chukwueke UN, Wen PY (2019) Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol 8(1):Cns28. https://doi.org/10.2217/cns-2018-0007
Galldiks N, Langen KJ, Holy R, Pinkawa M, Stoffels G, Nolte KW, Kaiser HJ, Filss CP, Fink GR, Coenen HH, Eble MJ, Piroth MD (2012) Assessment of treatment response in patients with glioblastoma using O-(2–18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med 53(7):1048–1057. https://doi.org/10.2967/jnumed.111.098590
Thust SC, van den Bent MJ, Smits M (2018) Pseudoprogression of brain tumors. J Magn Reson Imaging 48(3):571–589. https://doi.org/10.1002/jmri.26171
Padma MV, Said S, Jacobs M, Hwang DR, Dunigan K, Satter M, Christian B, Ruppert J, Bernstein T, Kraus G, Mantil JC (2003) Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol 64(3):227–237. https://doi.org/10.1023/a:1025665820001
Hölzer T, Herholz K, Jeske J, Heiss WD (1993) FDG-PET as a prognostic indicator in radiochemotherapy of glioblastoma. J Comput Assist Tomogr 17(5):681–687. https://doi.org/10.1097/00004728-199309000-00002
Spence AM, Muzi M, Graham MM, O’Sullivan F, Link JM, Lewellen TK, Lewellen B, Freeman SD, Mankoff DA, Eary JF, Krohn KA (2002) 2-[(18)F]Fluoro-2-deoxyglucose and glucose uptake in malignant gliomas before and after radiotherapy: correlation with outcome. Clin Cancer Res 8(4):971–979
Pardo FS, Aronen HJ, Fitzek M, Kennedy DN, Efird J, Rosen BR, Fischman AJ (2004) Correlation of FDG-PET interpretation with survival in a cohort of glioma patients. Anticancer Res 24(4):2359–2365
Oborski MJ, Laymon CM, Lieberman FS, Mountz JM (2013) Distinguishing pseudoprogression from progression in high-grade gliomas: a brief review of current clinical practice and demonstration of the potential value of 18F-FDG PET. Clin Nucl Med 38(5):381–384. https://doi.org/10.1097/RLU.0b013e318286c148
Prat R, Galeano I, Lucas A, Martínez JC, Martín M, Amador R, Reynés G (2010) Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion, and 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. J Clin Neurosci 17(1):50–53. https://doi.org/10.1016/j.jocn.2009.02.035
Ceccon G, Lohmann P, Werner JM, Tscherpel C, Dunkl V, Stoffels G, Rosen J, Rapp M, Sabel M, Herrlinger U, Schäfer N, Shah NJ, Fink GR, Langen KJ, Galldiks N (2021) Early treatment response assessment using (18)F-FET PET compared with contrast-enhanced MRI in glioma patients after adjuvant temozolomide chemotherapy. J Nucl Med 62(7):918–925. https://doi.org/10.2967/jnumed.120.254243
Baguet T, Verhoeven J, De Vos F, Goethals I (2019) Cost-effectiveness of [(18)F] fluoroethyl-L-tyrosine for temozolomide therapy assessment in patients with glioblastoma. Front Oncol 9:814. https://doi.org/10.3389/fonc.2019.00814
Werner JM, Weller J, Ceccon G, Schaub C, Tscherpel C, Lohmann P, Bauer EK, Schäfer N, Stoffels G, Baues C, Celik E, Marnitz S, Kabbasch C, Gielen GH, Fink GR, Langen KJ, Herrlinger U, Galldiks N (2021) Diagnosis of pseudoprogression following lomustine-temozolomide chemoradiation in newly diagnosed glioblastoma patients using FET-PET. Clin Cancer Res 27(13):3704–3713. https://doi.org/10.1158/1078-0432.Ccr-21-0471
Galldiks N, Langen KJ (2016) Amino acid PET—an imaging option to identify treatment response, posttherapeutic effects, and tumor recurrence? Front Neurol 7:120. https://doi.org/10.3389/fneur.2016.00120
Kebir S, Fimmers R, Galldiks N, Schäfer N, Mack F, Schaub C, Stuplich M, Niessen M, Tzaridis T, Simon M, Stoffels G, Langen KJ, Scheffler B, Glas M, Herrlinger U (2016) Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Cancer Res 22(9):2190–2196. https://doi.org/10.1158/1078-0432.Ccr-15-1334
Galldiks N, Stoffels G, Filss CP, Piroth MD, Sabel M, Ruge MI, Herzog H, Shah NJ, Fink GR, Coenen HH, Langen KJ (2012) Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 53(9):1367–1374. https://doi.org/10.2967/jnumed.112.103325
Wang Y, Rapalino O, Heidari P, Loeffler J, Shih HA, Oh K, Mahmood U (2018) C11 methionine PET (MET-PET) imaging of glioblastoma for detecting postoperative residual disease and response to chemoradiation therapy. Int J Radiat Oncol Biol Phys 102(4):1024–1028. https://doi.org/10.1016/j.ijrobp.2018.06.011
Yoo MY, Paeng JC, Cheon GJ, Lee DS, Chung JK, Kim EE, Kang KW (2015) Prognostic value of metabolic tumor volume on (11)C-methionine PET in predicting progression-free survival in high-grade glioma. Nucl Med Mol Imaging 49(4):291–297. https://doi.org/10.1007/s13139-015-0362-0
Nuutinen J, Sonninen P, Lehikoinen P, Sutinen E, Valavaara R, Eronen E, Norrgård S, Kulmala J, Teräs M, Minn H (2000) Radiotherapy treatment planning and long-term follow-up with [(11)C]methionine PET in patients with low-grade astrocytoma. Int J Radiat Oncol Biol Phys 48(1):43–52. https://doi.org/10.1016/s0360-3016(00)00604-0
Terakawa Y, Tsuyuguchi N, Iwai Y, Yamanaka K, Higashiyama S, Takami T, Ohata K (2008) Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49(5):694–699. https://doi.org/10.2967/jnumed.107.048082
Tsuyuguchi N, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Takami T, Otsuka Y, Sakamoto S, Ohata K, Goto T, Hara M (2003) Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg 98(5):1056–1064. https://doi.org/10.3171/jns.2003.98.5.1056
Nihashi T, Dahabreh IJ, Terasawa T (2013) Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol 34(5):944–950. https://doi.org/10.3174/ajnr.A3324
Lizarraga KJ, Allen-Auerbach M, Czernin J, DeSalles AA, Yong WH, Phelps ME, Chen W (2014) (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med 55(1):30–36. https://doi.org/10.2967/jnumed.113.121418
Bekaert L, Valable S, Lechapt-Zalcman E, Ponte K, Collet S, Constans JM, Levallet G, Bordji K, Petit E, Branger P, Emery E, Manrique A, Barré L, Bernaudin M, Guillamo JS (2017) [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging 44(8):1383–1392. https://doi.org/10.1007/s00259-017-3677-5
Leimgruber A, Hickson K, Lee ST, Gan HK, Cher LM, Sachinidis JI, O’Keefe GJ, Scott AM (2020) Spatial and quantitative mapping of glycolysis and hypoxia in glioblastoma as a predictor of radiotherapy response and sites of relapse. Eur J Nucl Med Mol Imaging 47(6):1476–1485. https://doi.org/10.1007/s00259-020-04706-0
Oehlke O, Mix M, Graf E, Schimek-Jasch T, Nestle U, Götz I, Schneider-Fuchs S, Weyerbrock A, Mader I, Baumert BG, Short SC, Meyer PT, Weber WA, Grosu AL (2016) Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA)—protocol of a randomized phase II trial (NOA 10/ARO 2013-1). BMC Cancer 16(1):769. https://doi.org/10.1186/s12885-016-2806-z
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by VN, ID, LD, IM, LV, EG. The first draft of the manuscript was written by VN, ID, LD, IM, LV, EG, FC, AC. The Review and Editing was performed by CG, AC, MPB, FC, MS, CV, LM, AR, SC. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the Authors (Valerio Nardone, Isacco Desideri, Luca D’Ambrosio, Ilaria Morelli, Luca Visani, Eugenio Di Giorgio, Cesare Guida, Alfredo Clemente, Maria Paola Belfiore, Fabrizio Cioce, Marco Spadafora, Claudia Vinciguerra, Luigi Mansi, Alfonso Reginelli and Salvatore Cappabianca) declare no conflicts of interest.
Research involving human and animal participants
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nardone, V., Desideri, I., D’Ambrosio, L. et al. Nuclear medicine and radiotherapy in the clinical management of glioblastoma patients. Clin Transl Imaging 10, 477–493 (2022). https://doi.org/10.1007/s40336-022-00495-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-022-00495-8