Abstract
Objective
Glioblastoma is an infiltrative malignancy that tends to extend beyond the MRI-defined tumor volume. We utilized positron emission tomography (PET) imaging with the radiotracer alpha-[11C]methyl-l-tryptophan (AMT) to develop a reliable high-risk gross tumor volume (HR-GTV) method for delineation of glioblastoma. AMT can detect solid tumor mass and tumoral brain infiltration by increased tumoral tryptophan transport and metabolism via the immunosuppressive kynurenine pathway.
Methods
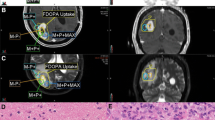
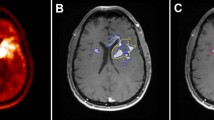
We reviewed all patients in our database with histologically proven glioblastoma who underwent preoperative AMT-PET scan prior to surgery and chemoradiation. Treated radiotherapy volumes were derived from the simulation CT with MRI fusion. High-GTV with contrast enhanced T1-weighted MRI alone (GTVMRI) was defined as the postoperative cavity plus any residual area of enhancement on postcontrast T1-weighted images. AMT-PET images were retrospectively fused to the simulation CT, and a high-risk GTVs generated by both AMT-PET alone (GTVAMT) was defined using a threshold previously established to distinguish tumor tissue from peritumoral edema. A composite volume of MRI and AMT tumor volume was also created (combination of MRI fused with AMT-PET data; GTVMRI + AMT). In patients with definitive radiographic progression, follow-up MRI demonstrating initial tumor progression was fused with the pretreatment images and a progression volume was contoured. The coverage of the progression volume by GTVMRI, GTVAMT, and GTVMRI + AMT was determined and compared using the Wilcoxon’s signed-rank test.
Results
Eleven patients completed presurgical AMT-PET scan, seven of whom had progressive disease after initial therapy. GTVMRI (mean, 50.2 cm3) and GTVAMT (mean, 48.9 cm3) were not significantly different. Mean concordance index of the volumes was 39 ± 15 %. Coverage of the initial recurrence volume by HR-GTVMRI (mean, 52 %) was inferior to both GTVAMT (mean, 68 %; p = 0.028) and GTVMRI + AMT (mean 73 %; p = 0.018). The AMT-PET-exclusive coverage was up to 41 % of the recurrent volume. There was a tendency towards better recurrence coverage with GTVMRI + AMT than with GTVAMT alone (p = 0.068). Addition of 5 mm concentric margin around GTVMRI, GTVAMT, and GTVMRI + AMT would have completely covered the initial progression volume in 14, 57, and 71 % of the patients, respectively.
Conclusion
We found that a GTV defined by AMT-PET produced similar volume, but superior recurrence coverage than the treated standard MRI-determined volume. A prospective study is necessary to fully determine the usefulness of AMT-PET for volume definition in glioblastoma radiotherapy planning.



Similar content being viewed by others
Abbreviations
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- AMT:
-
Alpha-[11C]methyl-l-tryptophan
- SUV:
-
Standardized uptake volume
- CI:
-
Concordance index
- GTV:
-
Gross tumor volume
- CTV:
-
Clinical target volume
- PTV:
-
Planning target volume
- LAT1:
-
l-type amino acid transporter
References
Ohgaki H, Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109:93–108
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Swanson KR, Alvord EC, Murray JD (2002) Virtual brain tumors (gliomas) enhance the reality of medical imaging and highlight the inadequacies of current therapy. Br J Cancer 86:14–18
Farace P, Giri MG, Meliado G, Amelio D, Widesott L, Ricciardi GK, Dall’Oglio S, Rizzotti A, Sbarbati A, Beltramello A, Maluta S, Amichetti M (2010) Clinical target volume delineation in glioblastomas: per-operative versus post-operative/pre-radiotherapy MRI. Br J Radiol 84:271–278
Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ (2010) Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol 9:906–920
Galldiks N, Ullrich R, Schroeter M, Fink GR, Jacobs AH, Kracht LW (2010) Volumetry of [(11)C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. Eur J Nucl Med Mol Imaging 37:84–92
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Pope WB, Young JR, Ellingson BM (2011) Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep 11:336–344
Grosu AL, Piert M, Weber WA, Jeremic B, Picchio M, Schratzenstaller U, Zimmermann FB, Schwaiger M, Molls M (2005) Positron emission tomography for radiation treatment planning. Strahlenther Onkol 181:483–499
Chiti A, Kirienko M, Gregoire V (2010) Clinical use of PET-CT data for radiotherapy planning: what are we looking for? Radiother Oncol 96:277–279
Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, Gumprecht H, Jaeger R, Schwaiger M, Molls M (2005) l-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 63(1):64–74
Weber DC, Zilli T, Buchegger F, Casanova N, Haller G, Rouzaud M, Nouet P, Dipasquale G, Ratib O, Zaidi H, Vees H, Miralbell R (2008) [(18)F]Fluoroethyltyrosine- positron emission tomography-guided radiotherapy for high-grade glioma. Radiat Oncol 3:44
Vees H, Senthamizhchelvan S, Miralbell R, Weber DC, Ratib O, Zaidi H (2009) Assessment of various strategies for 18F-FET PET-guided delineation of target volumes in high-grade glioma patients. Eur J Nucl Med Mol Imaging 36(2):182–193
Niyazi M, Geisler J, Siefert A, Schwarz SB, Ganswindt U, Garny S, Schnell O, Suchorska B, Kreth FW, Tonn JC, Bartenstein P, la Fougère C, Belka C (2011) FET-PET for malignant glioma treatment planning. Radiother Oncol 99:44–48
Chen W (2007) Clinical applications of PET in brain tumors. J Nucl Med 48:1468–1481
Isselbacher KJ (1972) Sugar and amino acid transport by cells in culture—differences between normal and malignant cells. N Engl J Med 286:929–933
Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL (1990) A new method to measure brain serotonin synthesis in vivo. I. Theory and basic data for a biological model. J Cereb Blood Flow Metab 10:1–12
Chugani DC, Heyes MP, Kuhn DM, Chugani HT (1998) Evidence of alpha[11C]methyl-l-tryptophan metabolism via the kynurenine pathway in tuberous sclerosis complex [abstract]. Neuroscience 24:692
Chugani DC, Muzik O (2000) Alpha[C-11]methyl-l-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab 20:2–9
Juhasz C, Chugani DC, Muzik O, Wu D, Sloan AE, Barger G, Watson C, Shah AK, Sood S, Ergun EL, Mangner TJ, Chakraborty PK, Kupsky WJ, Chugani HT (2006) In vivo uptake and metabolism of alpha-[11C]methyl-l-tryptophan in human brain tumors. J Cereb Blood Flow Metab 26:345–357
Batista CE, Juhász C, Muzik O, Kupsky WJ, Barger G, Chugani HT, Mittal S, Sood S, Chakraborty PK, Chugani DC (2009) Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol 11:460–466
Adams S, Braidy N, Bessesde A, Brew BJ, Grant R, Teo C, Guillemin GJ (2012) The kynurenine pathway in brain tumor pathogenesis. Cancer Res 72(22):5649–5657
Kamson DO, Juhasz C, Buth A, Kupsky WJ, Barger GR, Chakraborty PK, Muzik O, Mittal S (2013) Tryptophan PET in pretreatment delineation of newly-diagnosed gliomas: MRI and histopathologic correlates. J Neurooncol 112(1):121–132
Chakraborty PK, Mangner TJ, Chugani DC, Muzik O, Chugani HT (1996) A high-yield and simplified procedure for the synthesis of alpha-[11C]methyl-l-tryptophan. Nucl Med Biol 23:1005–1008
Alkonyi B, Barger GR, Mittal S, Muzik O, Chugani DC, Bahl G, Robinette NL, Kupsky WJ, Chakraborty PK, Juhász C (2012) Accurate differentiation of recurrent gliomas from radiation injury by kinetic analysis of alpha-11C-methyl-l-tryptophan PET. J Nucl Med 53:1058–1064
Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG (1989) Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 16:1405–1409
Liang BC, Thornton AF Jr, Sandler HM, Greenberg HS (1991) Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg 75:559–563
Hochberg FH, Pruitt A (1980) Assumptions in the radiotherapy of glioblastoma. Neurology 30:907–911
Juhász C, Chugani DC, Padhye UN, Muzik O, Shah A, Asano E, Mangner TJ, Chakraborty PK, Sood S, Chugani HT (2004) Evaluation with alpha-[11C]methyl-l-tryptophan positron emission tomography for reoperation after failed epilepsy surgery. Epilepsia 45(2):124–130
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, Zilles K, Coenen HH, Langen KJ (2005) O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128:678–687
Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, Klein JC, Herholz K, Heiss WD (2004) Delineation of brain tumor extent with [11C]l-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 10:7163–7170
Acknowledgments
The study was partially supported by a grant from the National Cancer Institute (R01 CA123451 to CJ). The authors are thankful to Geoffrey Barger, MD who performed the clinical follow-up of the patients after postsurgical chemoradiation. Also, the authors are also thankful to Janet Barger, RN; Melissa Burkett, CNMT; Jane Cornett, RN; Anna DeBoard, RN; Kelly Forcucci, RN; Cathie Germain, MA; Carole Klapko, CNMT; Mei-li Lee, MS; Xin Lu, MS; Marcia Lodej, RN; Andrew Mosqueda, CNMT; Karen Nichols, NP; Galina Rabkin, CNMT; and Angela Wigeluk, CNMT for their assistance in patient recruitment, scheduling, and preparation, as well as for their technical assistance in performing the PET studies. Finally, the authors are grateful to Pulak Chakraborty, PhD and Hancheng Cai, PhD for the reliable radiosynthesis of the PET radiotracer.
Compliance with ethical guidelines
This retrospective series does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christensen, M., Kamson, D.O., Snyder, M. et al. Tryptophan PET-defined gross tumor volume offers better coverage of initial progression than standard MRI-based planning in glioblastoma patients. J Radiat Oncol 3, 131–138 (2014). https://doi.org/10.1007/s13566-013-0132-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-013-0132-5