Abstract
Introduction
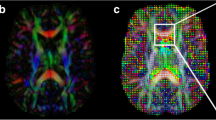
Diffusion tensor imaging (DTI) is an MR-based technique that may better detect the peritumoural region than MRI. Our aim was to explore the feasibility of using DTI for target volume delineation in glioblastoma patients.
Materials and methods
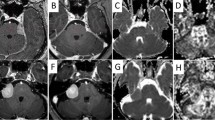
MR tensor tracts and maps of the isotropic (p) and anisotropic (q) components of water diffusion were coregistered with CT in 13 glioblastoma patients. An in-house image processing program was used to analyse water diffusion in each voxel of interest in the region of the tumour. Tumour infiltration was mapped according to validated criteria and contralateral normal brain was used as an internal control. A clinical target volume (CTV) was generated based on the T1-weighted image obtained using contrast agent (T1Gd), tractography and the infiltration map. This was compared to a conventional T2-weighted CTV (T2-w CTV).
Results
Definition of a diffusion-based CTV that included the adjacent white matter tracts proved highly feasible. A statistically significant difference was detected between the DTI-CTV and T2-w CTV volumes (p < 0.005, t = 3.480). As the DTI-CTVs were smaller than the T2-w CTVs (tumour plus peritumoural oedema), the pq maps were not simply detecting oedema. Compared to the clinical planning target volume (PTV), the DTI-PTV showed a trend towards volume reduction. These diffusion-based volumes were smaller than conventional volumes, yet still included sites of tumour recurrence.
Conclusion
Extending the CTV along the abnormal tensor tracts in order to preserve coverage of the likely routes of dissemination, whilst sparing uninvolved brain, is a rational approach to individualising radiotherapy planning for glioblastoma patients.
Zusammenfassung
Einführung
Die Diffusions-Tensor-Bildgebung (DTI) ist eine MR-Technik, die dank der Erfassung des peritumoralen Bereichs eine Verbesserung bezüglich MRI bringt. Unser Ziel war die Prüfung der Machbarkeit der Verwendung der DTI für die Zielvolumenabgrenzung für Patienten mit Glioblastomen.
Material und Methoden
MR-Tensor-Traktate und Karten von isotroper (p) und anisotroper (q) Diffusion von Wasser wurden bei 13 Patienten mit Glioblastomen mit der CT-Bildgebung koregistriert. Ein eigenes Bildverarbeitungsprogramm wurde verwendet, um die Diffusion von Wasser in jedem Voxel in der Umgebung des Tumors zu analysieren. Die Tumorinfiltration wurde nach validierten Kriterien abgebildet. Das kontralaterale normale Gehirn wurde als interne Kontrolle verwendet. Ein klinisches Zielvolumen (CTV) wurde auf Basis des T1-basierten Bilds (T1Gd), der Traktographie und des Infiltrationsmusters generiert. Dieses Zielvolumen wurde mit einem konventionellen T2-basierten CTV verglichen.
Ergebnisse
Die Erstellung eines diffusionsbasierten klinischen Zielvolumens, welches die benachbarte weiße Substanz enthält, ist sehr gut möglich. Zwischen dem DTI-CTV und dem T2-basierten CTV wurde eine statistisch signifikante Differenz nachgewiesen (p < 0,005; t = 3,480). Da die DTI-CTVs kleiner sind als die T2-basierten CTVs (Tumor plus peritumorales Ödem), werden die pq-Karten ein Ödem nicht einfach erkennen. Die DTI-PTV zeigt den Trend einer Volumenreduktion im Vergleich mit der PTV auf. Dieses diffusionsbasierte Volumen war kleiner als das herkömmlich definierte (konventionelle) Volumen.
Schlussfolgerung
Das Erweitern des CTV entlang der abnormalen Tensorabschnitte, um die Deckung der voraussichtlichen Verbreitungsrouten zu bewahren und unbeteiligtes Gehirn zu schonen, ist ein rationaler Ansatz, um die Strahlentherapieplanung für Glioblastompatienten zu individualisieren.

Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Price SJ, Jena R, Burnet NG, Carpenter TA, Pickard JD, Gillard JH (2007) Predicting patterns of glioma recurrence using diffusion tensor imaging. Eur Radiol 17:1675–1684
Shapiro WR (1999) Current therapy for brain tumours: back to the future. Arch Neurol 56:429–432
Scherer HJ (1938) Structural development in gliomas. Am J Cancer 34:333–351
Scherer HJ (1940) The forms of growth in gliomas and their practical significance. Brain 63:1–35
Creak AL, Tree A, Saran F (2011) Radiotherapy planning in high-grade gliomas: a survey of current UK practice. Clin. Oncol (R Coll Radiol) 23:189–198
Piroth MD; Pinkawa M, Hoy R et al (2012) Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Results of a prospective phase II study. Strahlenther Onkol 188:334–9
Witwer BP, Moftakhar R, Hasan KM et al (2002) Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg 97:568–575
Field AS, Alexander AL, Wu YC, Hasan KM, Witwer B, Badie B (2004) Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumour. J Magn Reson Imaging 20:555–562
Price SJ, Pena A, Burnet NG et al (2004) Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol 14:1909–1917
Price SJ, Jena R, Burnet NG et al (2006) Improved delineation of glioma margins and regions of infiltration using diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol 27:1969–1974
Price SJ, Burnet NG, Donovan T et al (2003) Diffusion tensor imaging of brain tumours at 3T: a potential tool for assessing white matter tract invasion? Clin Radiol 58:455–462
Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D (2004) Peritumoural brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 232:451–460
Price SJ, Pena A, Burnet NG, Pickard JD, Gillard JH (2004) Detecting glioma invasion of the corpus callosum using diffusion tensor imaging. Br J Neurosurg 18:391–395
Zhou XJ, Leeds NE, Poonawalla AH et al (2003) Assessment of tumour cell infiltration along white-matter fiber tracts using diffusion tensor imaging. Proc Intl Soc Mag Reson Med 11:2238
Intven M, Reerink O, Philippens ME (2013) Diffusion-weighted MRI in locally advanced rectal cancer: pathological response prediction after noe-adjuvant radiochemotherapy. Strahlenther Onkol 189:117–122
Server A, Kulle B, Maehlen J et al (2009) Quantitative apparent diffusion coefficients in the characterization of brain tumours and associated peritumoural oedema. Acta Radiol 50:682–689
Van Westen D, Lätt J, Englund E, Brockstedt S, Larsen EM (2006) Tumour extension in high-grade gliomas assessed with diffusion magnetic resonance imaging: values and lesion-to-brain ratios of apparent diffusion coefficient and fractional anisotropy. Acta Radiol 47:311–319
Berberat J, Eberle B, Rogers S et al (2013) Anisotropic phantom measurements for quality assured use of diffusion tensor imaging in clinical practice. Acta Radiol 54:576–580
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254
McDonald MW, Shu HK, Curran WJ Jr, Crocker IR (2011) Pattern of failure after limited margin radiotherapy and temozolamide for glioblastoma. Int J Radiat Oncol Biol Phys 79:130–136
Minniti G, Amelio D, Amichetti M et al (2010) Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol 97:377–381
Jamal M, Rath BH, Tsang PS, Camphausen K, Tofilon PJ (2012) The brain microenvironment prefentially enhances the radioresistance of CD133(+) glioblastoma stem-like cells. Neoplasia 14:150–158
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Sundgren PC, Cao Y (2009) Brain irradiation: effects on normal brain parenchyma and radiation injury. Neuroimaging Clin N Am 19:657–668
Jena R, Price SJ, Baker C et al (2005). NG Diffusion tensor imaging: possible implications for radiotherapy treatment planning of patients with highgrade glioma. Clin Oncol (R Coll Radiol) 17:581–590
Stummer W, Pichlmeier U, Meinel T et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Lee P, Eppinga W, Lagerwaard F et al (2013) Evaluation of high ipsilateral subventricualr zone radiation therapy dose in glioblastoma: a pooled analysis. Int J Radiat Oncol Biol Phys 86:609–615
Jones DK, Cercignani M (2010) Twenty-five Pitfalls in the Analysis of Diffusion MRI Data. NMR Biomed 23:803–820
Compliance with ethical guidelines
Acknowledgements
This work was partially funded by a grant from Krebsforschung Schweiz KFS 02779-02-2011.
Conflict of interest
J. Berberat, J. McNamara, L. Remonda, S. Bodis and S. Rogers state that there are no conflicts of interest. The accompanying manuscript does not include studies on humans or animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berberat, J., McNamara, J., Remonda, L. et al. Diffusion tensor imaging for target volume definition in glioblastoma multiforme. Strahlenther Onkol 190, 939–943 (2014). https://doi.org/10.1007/s00066-014-0676-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0676-3