Abstract
Background
The defective clearance of apoptotic bodies in juvenile-onset systemic lupus erythematosus (jSLE) potentially leads to the persistence of autoreactive lymphocytes and the perpetuation of the autoimmune response. These factors contribute to the disturbance in lymphocyte apoptosis and show potential as key determinants in the clinical course and severity of jSLE. This study evaluates the role of peripheral blood (PB) lymphocyte apoptosis in prognosis of jSLE and as a predictor for disease activity.
Methods
The study involved 100 jSLE patients and 50 healthy controls. Flow cytometry was used to analyze percentages of lymphocyte apoptosis in PB of all study participants. Plasma levels of pro-inflammatory cytokines were determined using ELISA.
Results
Our results showed that percentages of lymphocyte apoptosis in PB of jSLE patients are significantly higher than those of healthy controls. These percentages are significantly positively associated with disease activity of patients (SLEDAI-2 K). Furthermore, plasma cytokine levels (IL-17, IFN-γ and TNF-α) are significantly elevated in jSLE patients compared to their levels in healthy controls. Also, there are weak significant positive correlations between percentages of PB lymphocyte apoptosis and each of IL-17 and IFN-γ plasma levels in jSLE patients. Moreover, PB lymphocyte apoptosis percentages among jSLE patients are higher in the presence of some clinical and laboratory features than those in their absence.
Conclusion
Peripheral apoptotic lymphocytes could contribute to the prognosis of jSLE and could be used as a predictor for disease activity in jSLE patients.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with multiple immune dysregulation mechanisms. Juvenile-onset systemic lupus erythematosus (jSLE) represents a complex and multifactorial autoimmune disorder that predominantly affects children and adolescents, presenting distinct clinical and immunological challenges when compared to its adult-onset counterpart. It differs from adult-onset SLE (aSLE) in its higher disease activity, increased damage at an earlier stage and the need for use of immunosuppressive drugs at more frequent pattern [1]. The etiology of jSLE remains enigmatic, with the interplay of genetic predisposition, hormonal factors, and aberrant immune responses contributing to its pathogenesis. Among the intricate facets of jSLE pathophysiology, dysregulated lymphocyte apoptosis has emerged as a compelling central mechanism in recent years [2].
Lymphocyte apoptosis, a fundamental process of programmed cell death, plays a pivotal role in maintaining immune homeostasis by orchestrating the elimination of autoreactive and senescent lymphocytes, as well as the proper clearance of apoptotic bodies [3, 4]. In jSLE, the delicate balance between apoptosis and cell survival mechanisms, along with the defective clearance of apoptotic bodies, appears to be disrupted. This disruption potentially leads to the persistence of autoreactive lymphocytes and the perpetuation of the autoimmune response. Together, these factors contribute to the disturbance in lymphocyte apoptosis and show potential as key determinants in the clinical course and severity of jSLE [2, 5, 6].
Moreover, mounting evidence suggests that dysregulated lymphocyte apoptosis in jSLE may be closely connected with the pro-inflammatory milieu orchestrated by cytokines [7]. Inflammatory cytokines, including interleukin-17 (IL-17) [8, 9], interferon-gamma (IFN-γ) [10, 11], and tumor necrosis factor-alpha (TNF-α) [12,13,14], have been implicated as pivotal players in jSLE pathogenesis, contributing to tissue damage and immune dysregulation. The intricate crosstalk between aberrant lymphocyte apoptosis and these inflammatory mediators presents a compelling point for further exploration.
Identification of the molecular processes responsible for leukocyte apoptosis has gained significant consideration [15, 16]. Indeed, numerous research groups have linked various signaling pathway products, such as FasL, NF-κB [17], and Bcl-2 [18], to impaired apoptosis. This potential connection may ultimately contribute to hyperactivity of B and T lymphocytes resulting in excessive accumulation of autoantigens, polyclonal B cell activation and increases in autoantibody production [19, 20].
Lymphocytes themselves have a significant role in controlling the apoptosis of different cell types. For instance, cytotoxic T cells release enzymes like perforin and granzyme B, which can induce apoptosis in target cells. Additionally, T lymphocytes produce Fas ligand (FasL/CD95L), which interacts with Fas (Apo-1/CD95) on epithelial cells, leading to their apoptosis [20].
Unfortunately, the current understanding of immunopathological mechanisms and treatment protocols for jSLE is primarily derived from studies conducted on aSLE patients. This study is dedicated to unraveling the age-specific immunopathogenesis in jSLE and confirming whether it aligns with findings observed in aSLE patients, thereby addressing previous recommendations on this matter [1].
We evaluated the role of peripheral blood (PB) lymphocyte apoptosis in prognosis of jSLE and as a predictor for disease activity. The levels of apoptotic lymphocytes (AL) in PB of jSLE patients was compared to healthy controls. Also, we determined plasma levels of the pro-inflammatory cytokines in jSLE patients in comparison to healthy controls. We correlated PB lymphocyte apoptosis with the clinical manifestations, disease activity and inflammatory markers in jSLE patients.
Patients and methods
Ethics approval and consent to participate
This study was approved by the ethics committee of the National Research Centre (NRC), Egypt and has therefore been performed under the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All samples were obtained with the written informed consent of all patients involved in this study before their enrollment.
Study participants
This study included 50 healthy subjects and 100 juvenile-onset SLE patients (age of onset ≤ 16 years) recruited from Rheumatology and Rehabilitation outpatient clinic, Kasr Al Ainy Hospital, Cairo University from April to December 2022. All patients fulfilled the 2012 Systemic Lupus Collaborating Clinics (SLICC) classification criteria for SLE [21]. Demographic and cumulative clinical manifestations were recorded, and disease activity at the last visit was assessed through the Systemic Lupus Erythematosus Disease Activity Index-2 K (SLEDAI-2 K) [22]. Patients have been divided according to SLEDAI-2 K into active and inactive patients, patients with SLEDAI-2 K ≥ 5 are active while those with SLEDAI-2 K < 5 are inactive.
Determination of peripheral blood lymphocyte apoptosis using flow cytometry
Apoptosis of lymphocytes was determined in peripheral blood (PB) by Annexin V Apoptosis Detection kit (BD Biosciences, United States) using the BD Accuri™ C6 Flow Cytometer Instrument. The PB was collected from patients and healthy controls in tubes containing EDTA. 50 μL of EDTA‑treated PB were incubated for 30 min at 4 °C in the dark with Annexin V and propodium iodide. The red blood cells were lysed using BD FACS Lysing Solution (Becton Dickinson, USA). The stained cells were then washed and resuspended in buffer solution. Approximately 30,000 stained cells in each sample were analyzed using the BD Accuri™ C6 Flow Cytometer Instrument (BD Biosciences). The lymphocytes were gated by setting the appropriate forward scatter/side scatter axes. Data were acquired, and data analysis was performed by the BD Accuri™ C6 software program.
Enzyme‑linked immunosorbent assay (ELISA)
Plasma cytokine levels (IFN-γ, IL-17 and TNF-α) of all study subjects were determined using Human ELISA kit (Elabscience, Elabscience Biotechnology Co., Ltd) according to the manufacturer’s protocol.
Statistical analysis
Data were statistically analyzed using SPSS version 27.0 software (SPSS Inc., Chicago, Illinois, USA). Data were presented as mean ± SD or median (Interquartile range (IQR)). Non-parametric Mann–whitney U test was used to analyze the statistical differences of lymphocyte apoptosis and inflammatory markers between jSLE patients and healthy controls. Association analyses were examined using Spearman correlation analysis. A P value of less than 0.05 was considered statistically significant.
Results
Baseline manifestations of juvenile-onset SLE patients
The study involved 100 jSLE patients, of whom 96 were females (96%). The mean age at sampling time was 25 ± 8 years, and the median disease duration (IQR) was 140 (112) months. It was observed that 22 (22%) of patients had a family history of SLE and other autoimmune diseases. Cumulative clinical and laboratory patients’ manifestations, disease activity and disease damage and the medications received at sampling time are summarized in Table 1.
Peripheral blood lymphocyte apoptosis in juvenile-onset SLE patients
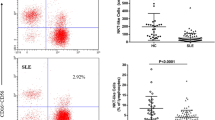
Our findings showed that percentages of lymphocyte apoptosis in peripheral blood (PB) of jSLE patients are significantly higher than those of healthy controls (p < 0.001) (Fig. 1a). These percentages are significantly positively correlated with disease activity of patients (SLEDAI-2 K) (p < 0.001) (Fig. 1b). Active group of jSLE patients have significantly higher percentages of PB lymphocyte apoptosis than those of the inactive group (p = 0.002) (Fig. 1c).
a. Comparison of percentages of PB lymphocyte apoptosis between jSLE patients and healthy controls (**: significant at p < 0.001, by Mann–Whitney U test). b Correlation between percentages of PB lymphocyte apoptosis and SLEDAI-2 K in jSLE patients (p < 0.001, by Spearman correlation). c Comparison between active and inactive jSLE patients regarding percentages of PB lymphocyte apoptosis (**: significant at p = 0.002, by Mann–Whitney U test)
Plasma levels of inflammatory cytokines in juvenile-onset SLE patients
We found that plasma levels of inflammatory cytokines (IL-17, IFN-γ and TNF-α) are significantly elevated in jSLE patients compared to their levels in healthy controls (p < 0.001) (Fig. 2).
Correlation between PB lymphocyte apoptosis and inflammatory markers in juvenile-onset SLE patients
Our correlation analysis indicated that there is a weak significant positive correlation between percentages of PB lymphocyte apoptosis and plasma levels of IL-17 (r = 0.329; p = 0.029) and IFN-γ (r = 0.382; p = 0.010) in jSLE patients, while no significant correlation was found between levels of TNF-α and percentages of PB lymphocyte apoptosis.
Comparison of PB lymphocyte apoptosis among jSLE patients based on the presence or absence of clinical and laboratory manifestations
Although there are no statistically significant differences, percentages of PB lymphocyte apoptosis among jSLE patients are higher in the presence of some clinical and laboratory features than those in their absence (Table 2).
Discussion
SLE is a systemic autoimmune disease whose pathogenesis implicates many immune mechanisms, interplaying in a rather complex way that has not been fully elucidated yet. The presence of autoantibodies is the hallmark of ‘classical’ SLE that are mainly directed toward nuclear antigens and autoreactive lymphocytes. This applies to most cases of jSLE except those with early onset disease that starts before 5 years of age [2]. The immunopathogenesis is even more complex in jSLE where genetic and hormonal factors contribute more centrally in the pathophysiology [1]. The release of autoantibodies was constantly suggested to be a result from the increase in lymphocyte apoptosis and failure of macrophage clearance of apoptotic cells [6].
This study aimed to evaluate the role of peripheral blood lymphocyte apoptosis in the prognosis of jSLE and as a predictor for disease activity as previously validated and demonstrated for aSLE [6, 23,24,25,26,27,28,29,30]. We also aimed to correlate percentage of AL with pro-inflammatory cytokines and clinical features to eventually reach age-specific laboratory findings elaborating immunopathological mechanisms characteristic for this age group upon which targeted treatment protocols and disease prognosis could address. We found that percentages of AL in jSLE patients were significantly higher than those of healthy controls and they were also significantly positively correlated with disease activity represented as SLEDAI-2 K. In addition, Active group of jSLE patients have significantly higher percentages of PB lymphocyte apoptosis than those of the inactive group. These results resemble findings observed in aSLE in both aspects; increased percentage of AL and their correlation to disease activity [6, 27]. However, other studies that were performed in aSLE showed significance only in the increased AL in aSLE patients while the correlation between their count and disease activity was not significant [28, 30].
The plasma levels of inflammatory cytokines (IL-17, IFN-γ and TNF-α) were significantly higher in jSLE patients in comparison to healthy controls. Similarly, previous observations have shown that IL-17 levels were significantly higher both in jSLE [31,32,33,34] and aSLE [35, 36] strongly suggesting its potential as a therapeutic target [37, 38]. Regarding IFN-γ and TNF-α, studies that were performed in pediatric and adolescent patients investigating their role in jSLE have focused on their gene expression levels and polymorphisms rather than their serum or plasma levels; reporting their overexpression in jSLE and correlation with disease activity and clinical manifestations [34]. Polymorphisms in TNF-α was also reported to influence susceptibility to jSLE [39]. Postal et al. found that serum levels of TNF-α was significantly higher in jSLE patients than controls, while serum levels of IFN-γ showed no significant difference. On the other hand, Cavalcanti et al. reported that they did not find significant difference in levels of IL-17, IFN-γ or TNF-α in jSLE [40]. We postulate, considering the smaller sample size included in the latter two studies, that significant differences may have appeared with larger representative samples. This controversy seen in studies done in jSLE patients investigating previous cytokines is not present in those performed in aSLE in which the significant increase in levels of IL-17, IFN-γ or TNF-α has been widely recognized for a considerable time [8, 10,11,12, 41, 42].
IL-17, IFN-γ and TNF-α are proinflammatory cytokines that are mainly secreted by T helper lymphocytes along with other innate and adaptive immune cells. TNF-α is also primarily secreted by activated macrophages [13], which contribute also to a lesser extent in the production of IFN-γ. IL-17 is specifically produced by Th17 cells derived from naive T cells under the effect of different combinations of other cytokines like TGF-β with IL-21 [43],or IL-6, IL-23, IL-1β and TNF-α [44]. A large fraction of the IL-17 found in SLE patients originates from CD4−CD8− double-negative (DN) T cells which also produces IFN-γ. These cells are infrequent in healthy individuals; however, they were found abundantly in PB of SLE patients [45]. IL-17 and IFN-γ impact SLE by modulating autoantibody production through acting directly on B cells [36], or indirectly through recruiting inflammatory cells and stimulating of macrophages, synoviocytes, fibroblasts and neutrophils [37, 46]. Stimulated macrophages and activated T cells produce TNF-α which is known to be a potent apoptotic inducer [13, 14]. The increase in apoptotic cells cause subsequent increase in autoantigen load which indirectly increase autoantibodies [27]. Our findings, which show a positive correlation between plasma levels of IL-17 and IFN-γ and the percentages of AL in peripheral PB of jSLE patients, align with the previous explanations of immunopathogenesis. This provides affirmation for investigating these factors in the specific context of jSLE. Although no significant association was found between the levels of TNF-α and the percentages of AL, its concurrent significant elevation in jSLE patients, along with IFN-γ, rules out the presence of an intrinsic defect in macrophage function as a possible cause for the defective clearance of apoptotic bodies. In accordance with this finding, a prior study has shown that lower ability of macrophage to phagocytose apoptotic bodies was secondary to serum factors rather than a primary defect in macrophages [27]. Complement deficiency was suggested to be among these factors [47]. Notably, hypocomplementemia was observed in fifty percent of our patients.
The main limitations of this study involve the limited number of individuals in each group. Furthermore, the drugs used by patients might have affected the percentages of lymphocyte apoptosis. However, studying the correlation between percentages of lymphocyte apoptosis and disease activity is highly important in exploring their role in the prognosis of lupus.
Conclusion
To the best of our knowledge, this is the first in vivo case control study that investigated the role of AL in jSLE in practical means that provide age-specific relevance for its prognostic performance in terms of evaluating its significance in differentiating active from inactive disease and furthermore, correlating with other inflammatory cytokines which showed evidence that can prove theory implicating defective macrophage clearance in immunopathogenesis. Further studies are needed to elucidate the sequence of events revealed in the results regarding the interplay between AL and inflammatory cytokines. These studies should determine whether AL is a consequence of systemic inflammation or if it acts as the driving force behind such events, possibly influenced by other serum factors. Identifying the trigger for these events should be the focus of future research aimed at exploring potential therapeutic solutions.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SLE:
-
Systemic lupus erythematosus
- jSLE:
-
Juvenile-onset systemic lupus erythematosus
- aSLE:
-
Adult-onset SLE
- PB:
-
Peripheral blood
- SLEDAI:
-
Systemic lupus erythematosus disease activity index
- IL-17:
-
Interleukin-17
- IFN-γ:
-
Interferon-gamma
- TNF-α:
-
Tumor necrosis factor-alpha
- FasL:
-
Fas ligand
- AL:
-
Apoptotic lymphocytes
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- ANA:
-
Anti-nuclear antibodies
- Anti-ds DNA:
-
Anti-double stranded deoxyribonucleic acid antibodies
- aPL:
-
Antiphospholipid antibodies
- SDI:
-
Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index
References
Smith EMD, Lythgoe H, Midgley A, Beresford MW, Hedrich CM. Juvenile-onset systemic lupus erythematosus: Update on clinical presentation, pathophysiology and treatment options. Clin Immunol. 2019;209:108274.
Hedrich CM, Smith EMD, Beresford MW. Juvenile-onset systemic lupus erythematosus (jSLE) - Pathophysiological concepts and treatment options. Best Pract Res Clin Rheumatol. 2017;31(4):488–504.
Navratil JS, Liu CC, Ahearn JM. Apoptosis and autoimmunity. Immunol Res. 2006;36(1–3):3–12.
Lleo A, Selmi C, Invernizzi P, Podda M, Gershwin ME. The consequences of apoptosis in autoimmunity. J Autoimmun. 2008;31(3):257–62.
Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–21.
Su YJ, Cheng TT, Chen CJ, et al. The association among leukocyte apoptosis, autoantibodies and disease severity in systemic lupus erythematosus. J Transl Med. 2013;11:261.
Graninger WB, Steiner CW, Graninger MT, Aringer M, Smolen JS. Cytokine regulation of apoptosis and Bcl-2 expression in lymphocytes of patients with systemic lupus erythematosus. Cell Death Differ. 2000;7(10):966–72.
Nalbandian A, Crispin JC, Tsokos GC. Interleukin-17 and systemic lupus erythematosus: current concepts. Clin Exp Immunol. 2009;157(2):209–15.
Koga T, Ichinose K, Kawakami A, Tsokos GC. The role of IL-17 in systemic lupus erythematosus and its potential as a therapeutic target. Expert Rev Clin Immunol. 2019;15(6):629–37.
Liu W, Zhang S, Wang J. IFN-gamma, should not be ignored in SLE. Front Immunol. 2022;13:954706.
Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 2001;3(3):136–41.
Weckerle CE, Mangale D, Franek BS, et al. Large-scale analysis of tumor necrosis factor alpha levels in systemic lupus erythematosus. Arthritis Rheum. 2012;64(9):2947–52.
Jang DI, Lee AH, Shin HY, et al. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int J Mol Sci. 2021;22(5):2719.
Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19(6):350–64.
Rose LM, Latchman DS, Isenberg DA. Apoptosis in peripheral lymphocytes in systemic lupus erythematosus: a review. Br J Rheumatol. 1997;36(2):158–63.
Nishioka K, Hasunuma T, Kato T, Sumida T, Kobata T. Apoptosis in rheumatoid arthritis: a novel pathway in the regulation of synovial tissue. Arthritis Rheum. 1998;41(1):1–9.
Kuhtreiber WM, Hayashi T, Dale EA, Faustman DL. Central role of defective apoptosis in autoimmunity. J Mol Endocrinol. 2003;31(3):373–99.
Tamura A, Katsumata M, Greene MI, Yui K. Inhibition of apoptosis and augmentation of lymphoproliferation in bcl-2 transgenic Fas/Fas ligand-defective mice. Cell Immunol. 1996;168(2):220–8.
Ren Y, Savill J. Apoptosis: the importance of being eaten. Cell Death Differ. 1998;5(7):563–8.
Trebeden-Negre H, Weill B, Fournier C, Batteux F. B cell apoptosis accelerates the onset of murine lupus. Eur J Immunol. 2003;33(6):1603–12.
Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–86.
Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
Li M, Luo L, Wu Y, et al. Elevated apoptosis and abnormal apoptosis signaling of regulatory T cells in patients with systemic lupus erythematosus. Lupus. 2022;31(12):1441–55.
Luo N, Wu Y, Chen Y, et al. Upregulated BclG(L) expression enhances apoptosis of peripheral blood CD4+ T lymphocytes in patients with systemic lupus erythematosus. Clin Immunol. 2009;132(3):349–61.
Xie L, Xu J. Role of MiR-98 and Its Underlying Mechanisms in Systemic Lupus Erythematosus. J Rheumatol. 2018;45(10):1397–405.
Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12(12):716–30.
Jin O, Sun LY, Zhou KX, et al. Lymphocyte apoptosis and macrophage function: correlation with disease activity in systemic lupus erythematosus. Clin Rheumatol. 2005;24(2):107–10.
Robak E, Sysa-Jedrzejowska A, Robak T, Smolewski P. Peripheral blood lymphocyte apoptosis and circulating dendritic cells in patients with systemic lupus erythematosus: correlation with immunological status and disease-related symptoms. Clin Rheumatol. 2006;25(2):225–33.
Eggleton P, Harries LW, Alberigo G, et al. Changes in apoptotic gene expression in lymphocytes from rheumatoid arthritis and systemic lupus erythematosus patients compared with healthy lymphocytes. J Clin Immunol. 2010;30(5):649–58.
Bengtsson AA, Sturfelt G, Gullstrand B, Truedsson L. Induction of apoptosis in monocytes and lymphocytes by serum from patients with systemic lupus erythematosus - an additional mechanism to increased autoantigen load? Clin Exp Immunol. 2004;135(3):535–43.
Hammad A, Osman E, Mosaad Y, Wahba M. Serum interleukin-17 in Egyptian children with systemic lupus erythematosus: is it related to pulmonary affection? Lupus. 2017;26(4):388–95.
Radwan N, Hamza MT, Ghareeb I, Hisham M. Serum interleukin-17 expression in a group of Egyptian patients with juvenile systemic lupus erythematosus %J. Egypt J Pediatr Allergy Immunol. 2021;19(2):97–103.
Zhang S, Li X, Tian Y, et al. Assay for interferon gamma release as a novel marker in pediatric patients with systemic lupus erythematosus. 2022.
Rana A, Minz RW, Aggarwal R, Anand S, Pasricha N, Singh S. Gene expression of cytokines (TNF-alpha, IFN-gamma), serum profiles of IL-17 and IL-23 in paediatric systemic lupus erythematosus. Lupus. 2012;21(10):1105–12.
Tang Y, Tao H, Gong Y, Chen F, Li C, Yang X. Changes of Serum IL-6, IL-17, and Complements in Systemic Lupus Erythematosus Patients. J Interferon Cytokine Res. 2019;39(7):410–5.
Alduraibi FK, Sullivan KA, Chatham WW, Hsu HC, Mountz JD. Interrelation of T cell cytokines and autoantibodies in systemic lupus erythematosus: A cross-sectional study. Clin Immunol. 2023;247:109239.
Koga T, Ichinose K, Kawakami A, Tsokos GC. Current insights and future prospects for targeting il-17 to treat patients with systemic lupus erythematosus. Front Immunol. 2020;11: 624971.
Robert M, Miossec P. Interleukin-17 and lupus: enough to be a target? For which patients? Lupus. 2020;29(1):6–14.
Tahghighi F, Ziaee V, Moradinejad MH, et al. Tumor necrosis factor-alpha single nucleotide polymorphisms in juvenile systemic lupus erythematosus. Hum Immunol. 2015;76(8):533–6.
Cavalcanti A, Santos R, Mesquita Z, Duarte AL, Lucena-Silva N. Cytokine profile in childhood-onset systemic lupus erythematosus: a cross-sectional and longitudinal study. Braz J Med Biol Res. 2017;50(4):e5738.
Kim T, Kanayama Y, Negoro N, Okamura M, Takeda T, Inoue T. Serum levels of interferons in patients with systemic lupus erythematosus. Clin Exp Immunol. 1987;70(3):562–9.
Sabry A, Sheashaa H, El-Husseini A, et al. Proinflammatory cytokines (TNF-alpha and IL-6) in Egyptian patients with SLE: its correlation with disease activity. Cytokine. 2006;35(3–4):148–53.
Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454(7202):350–2.
Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9(6):650–7.
Crispin JC, Oukka M, Bayliss G, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–6.
De George DJ, Ge T, Krishnamurthy B, Kay TWH, Thomas HE. Inflammation versus regulation: how interferon-gamma contributes to type 1 diabetes pathogenesis. Front Cell Dev Biol. 2023;11:1205590.
Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192(3):359–66.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was funded by their authors.
Author information
Authors and Affiliations
Contributions
E. E. and R. K. performed the laboratory and statistical analyses. D. D. was the doctor responsible for clinical examination of the patients eligible for the study and recording their history. R. G. performed the laboratory analyses and wrote the main manuscript text. N. K. contributed to the conception and design of the study and prepared tables and figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the National Research Centre (NRC), Egypt and has therefore been performed under the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All samples were obtained with the written informed consent of all patients involved in this study before their enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eissa, E., Kandil, R., Dorgham, D. et al. Lymphocyte apoptosis and its association with the inflammatory markers and disease severity in juvenile-onset systemic lupus erythematosus patients. Pediatr Rheumatol 22, 20 (2024). https://doi.org/10.1186/s12969-024-00953-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-024-00953-9