Abstract
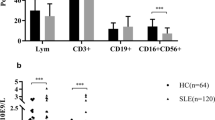
Increased lymphocyte apoptosis and defects in macrophage removal of apoptotic cells have been suggested to contribute to the development of systemic lupus erythematosus (SLE). The aim of this study was to investigate the relationship between peripheral lymphocyte apoptosis, macrophage function as determined by the serum levels of neopterin and interferon-γ (IFN-γ), and SLE disease activity. Peripheral apoptotic lymphocytes (AL) were detected by annexin V-fluorescein isothiocyanate (FITC) staining and flow cytometry. Serum levels of neopterin and IFN-γ were measured by enzyme-linked immunosorbent assay (ELISA). SLE disease activity was determined using the systemic lupus activity measure (SLAM) and the serum titer of anti-dsDNA antibodies. The percentage of AL in the peripheral blood of active SLE patients was significantly higher (13.07±7.39%, n=30) than that of the inactive SLE patients (4.08±3.55%, n=8, p<0.01) and normal controls (5.13±3.37%, n=11, p<0.01). Serum levels of neopterin in active SLE patients were significantly higher (1.39±1.10 μg/dl, n=22) than in controls (0.26±0.19 μg/dl, n=20, p<0.01). Serum levels of IFN-γ in active SLE patients were elevated (58.97±34.52 ng/l, n=15) when compared with controls (28.06±2.35 ng/l, n=16, p<0.05). The percentage of AL correlated significantly with serum levels of neopterin (r=0.446, p<0.05, n=22) and SLAM score (r=0.533, p<0.001, n=38), but not with the serum levels of IFN-γ. The SLAM score also correlated with the serum levels of neopterin (r=0.485, p<0.05, n=22), but not with those of IFN-γ. Our study supported the hypothesis that increased lymphocyte apoptosis has a pathogenic role in SLE. The increased levels of serum neopterin may suggest an attempt of the patients’ macrophage system to remove the apoptotic cell excess. Since serum levels of neopterin correlated with the overall lupus disease activity, they may be regarded as an index of SLE disease activity.


Similar content being viewed by others
References
Emlen W, Niebur J, Kadera R (1994) Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol 152:3685–3693
Perniok A, Wedekind F, Herrmann M, Specker C, Schneider M (1998) High levels of circulating early apoptotic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus 7:113–118
Courtney PA, Crockard AD, Williamson K, Irvine AE, Kennedy RJ, Bell AL (1999) Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann Rheum Dis 58:309–314
Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR (1998) Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum 41:1241–1250
Baumann I, Kolowos W, Voll RE, et al. (2002) Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum 46:191–201
Lorenz HM, Herrmann M, Winkler T, Gaipl U, Kalden JR (2000) Role of apoptosis in autoimmunity. Apoptosis 5:443–449
Taylor PR, Carugati A, Fadok VA, et al. (2000) A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med 192:359–366
Ren Y, Tang J, Mok MY, Chan AW, Wu A, Lau CS (2003) Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum 48:2888–2897
Huber C, Batchelor JR, Fuchs D, et al. (1984) Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med 160:310–316
Berdowska A, Zwirska-Korczala K (2001) Neopterin measurement in clinical diagnosis. J Clin Pharm Ther 26:319–329
Le Page C, Genin P, Baines MG, Hiscott J. Hiscott J (2000) Interferon activation and innate immunity. Rev Immunogenet 2:374–386
Liang MH, Socher SA, Larson MG, Schur PH (1989) Reliability and validity of six systems for the clinical assessment of six systems of disease activity in systemic lupus erythematosus. Arthritis Rheum 32:1107–1118
Navratil JS, Ahearn JM (2001) Apoptosis clearance mechanisms, and the development of systemic lupus erythematosus. Curr Rheumatol Rep 3:191–198
Herrmann M, Zoller OM, Hagenhofer M, Voll R, Kalden JR (1996) What triggers anti-dsDNA antibodies? Mol Biol Rep 23:265–267
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S (1992) Lymphoproliferative disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314–317
Takahashi T, Tanaka M, Brannan C, et al. (1994) Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76:969–976
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Bright J, Khar A (1994) Apoptosis: programmed cell death in health and disease. Biosci Rep 14:67–81
Loo DT, Rillema JR (1998) Measurement of cell death. Methods Cell Biol 57:251–264
Casciola-Rosen LA, Anhalt G, Rosen A (1994) Autoantigens targeted in SLE are clustered in two populations surface structures on apoptotic keratinocytes. J Exp Med 179:1317–1330
Huggins ML, Todd I, Cavers MA, Pavuluri SR, Tighe PJ, Powell RJ (1999) Antibodies from systemic lupus erythematosus (SLE) sera define differential release of autoantigens from cell lines undergoing apoptosis. Clin Exp Immunol 118:322–328
Licht R, Jacobs CW, Tax WJ, Berden JH (2001) No constitutive defect in phagocytosis of apoptotic cells by resident peritoneal macrophages from pre-morbid lupus mice. Lupus 10:102–107
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, O., Sun, Ly., Zhou, Kx. et al. Lymphocyte apoptosis and macrophage function: correlation with disease activity in systemic lupus erythematosus. Clin Rheumatol 24, 107–110 (2005). https://doi.org/10.1007/s10067-004-0972-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-004-0972-x