Abstract
Background
Patients admitted to intensive care units (ICU) are at risk of Gram-negative bacteria (GNB) infections, especially those caused by multidrug-resistant (MDR) isolates, increasing morbidity, mortality, and healthcare costs. However, epidemiological surveillance data on MDR bacteria to inform infection prevention and control (IPCs) interventions is limited in our study setting. Here we assessed the prevalence and factors associated with GNB infections in ICU- patients admitted in our study setting.
Methods
This was a hospital-based cross-sectional study among patients admitted to ICU at the Nairobi West Hospital, Kenya, between January and October 2022. Altogether, we recruited 162 patients, excluding those hospitalized for less than 48 h and declining consent, and collected demographics and clinical data by case report form. Blood, wound and throat swab, ascetic tap, stool, urine, tracheal aspirate, and sputum samples were collected cultured. Isolates identity and antimicrobial susceptibility were elucidated using the BD Phoenix system.
Results
The prevalence of GNB infections was 55.6%, predominated by urinary tract infections (UTIs). We recovered 13 GNB types, with Escherichia coli (33.3%) and Klebsiella pneumoniae (31.1%) as the most common isolates. Factors associated with GNB infections were a history of antibiotic use (aOR = 4.23, p = 0.001), nasogastric tube use (NGT, aOR = 3.04, p = 0.013), respiratory tract (RT, aOR = 5.3, p = 0.005) and cardiovascular (CV, aOR = 5.7, p = 0.024) conditions. 92% of the isolates were MDR,predominantly Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Conclusion
We report a high prevalence of MDR-GNB infections, predominated by UTI, in ICU, whereby patients with a history of antibiotic use, using the NGT, and having RT and CV conditions were at increased risk. To improve the management of ICU-admitted patients, continuous education, training, monitoring, evaluation and feedback on infection prevention and control are warranted in our study setting.
Similar content being viewed by others
Introduction
Gram-negative bacteria (GNB) infections, including respiratory tract, urinary tract, wound or surgical site, and bloodstream infections, are among the leading causes of morbidity, mortality, and increased healthcare costs in patients admitted to intensive care units (ICUs) [1,2,3]. ICU-admitted patients are more vulnerable to GNB infections because of frequent invasive medical procedures, including intubation, mechanical ventilation, and vascular access [4]. Additionally, reduced immune response due to trauma, surgery, and sepsis and impaired protective mechanisms, such as cough, swallowing reflexes, gastric acid secretion, and normal flora, predispose ICU-admitted patients to infections [5].
ICUs are often the epicenter of multidrug-resistant (MDR) GNB, mainly arising from the frequent and inappropriate or incorrect use of broad-spectrum antibiotics that drive drug-resistant strains evolution [1, 2] and bacterial exchange of resistance traits, including plasmid-encoded β-lactamases, aminoglycosides modifying enzymes, quinolone resistance gene, in the environment through horizontal gene transfer [3]. Additionally, poor adherence to infection prevention and control (IPC) policies substantially contributes to the high burden of MDR infections in ICUs [4].
MDR-GNB infections, especially those caused by extended-spectrum beta-lactamases (ESBL)- and carbapenemases-producing Enterobacteriaceae and non-fermenters, such as Pseudomonas aeruginosa, Acinetobacter baumannii and Stenotrophomonas maltophilia, are present clinically with limited therapeutic options [5] ICU-admitted patients with antibiotic-resistant bacterial infections have worse clinical outcomes than non-resistant strains and have a significant economic burden [6]. Those with cardiovascular disease, urinary catheterization and inappropriate empirical antibiotic therapy show increased mortality [7].
Even though a given organism antibiograms should guide the choice of antibiotics for MDR infection [8, 9], in resource-constrained settings, clinical laboratories are inadequately equipped and poorly supplied, and the personnel capacity is underdeveloped. For instance, in a point prevalence survey across 14 Kenyan public hospitals, only 2 (0.1%) of 1505 patients received treatment based on antibiogram, and 697 (46.4%) were inappropriately prescribed antibiotics [10] with a potential negative impact on antimicrobial resistance [11]. MDR bacterial infections pose a substantial clinical challenge in Kenya [12, 13]. In the Kenyatta National Hospital’s 2015 annual antimicrobial surveillance data, 88% of pathogens isolated were MDR, whereas 26% were extensively drug-resistant [14]. Continuous and systematic antimicrobial resistance surveillance in line with local and global AMR control action plans is warranted. Here, we determined the prevalence and factors associated with MDR-GNB infections and mortality in a Kenyan tertiary hospital ICU. This information is critical for antimicrobial therapy selection and evaluating the effectiveness of AMR infection prevention and control strategies.
Materials and methods
Study setting, design and population
We conducted this study at The Nairobi West Hospital (TNWH), a 400-bed capacity, including an 18-bed intensive care unit (ICU), a private tertiary healthcare facility in Nairobi City, Kenya. This was a hospital-based cross-sectional study among patients admitted to the ICU at the Nairobi West Hospital, Kenya, between January and October 2022. A total of 162 patients were recruited, excluding those hospitalized for less than 48 h and declining consent. We obtained informed consent for study participation for each patient through a close relative or a family legal representative, carried out the research project in accordance with the Declaration of Helsinki, observed participants well-being, and ensured the doctors in charge of the patients got results timely on all critical findings. The Kenyatta University Ethical Review Committee (Protocol no. PKU/2395/11531), National Commission for Science and Innovation (License No. NACOSTI/P/22/15238), and TNWH management approved the research project.
Sample collection
We collected the participant’s demographics and clinical presentation data using a structured questionnaire and case report forms. The samples collected depended on the patient’s clinical presentation. A qualified nurse collected the tracheal aspirate and ascitic tap samples into sterile containers. Swab samples were collected using sterile swabs (Delta lab, Spain), whereas urine samples were collected aseptically from a catheter collection port using a needle into 20 mL sterile screw-capped universal containers (Delta lab, Spain). A 2-inch of catheter distal tip was clipped directly into a sterile container and transported at room temperature to microbiology laboratory within 15 min to avoid drying. For blood samples, we obtained 8–10 mL of participants' blood using a needle and syringe into BD BACTEC™ Blood Culture Media (BD Diagnostics, Sparks, MD, USA). All samples were transported to TNWH Microbiology laboratory in a cool box and processed within 2 h.
Bacterial isolation, identification, and antimicrobial susceptibility testing
We used standard bacteriological methods for bacterial isolation [15, 16]. Briefly, urine samples were inoculated on cysteine–lactose electrolyte deficient agar (CLED) (HI Media Laboratories LLC, India) and incubated aerobically at 37 °C overnight. Pus swab, ascetic tap, sputum, and tracheal aspirate samples were inoculated on MacConkey agar (Hi Media Laboratories LLC, India), sheep blood agar (Hi Media Laboratories LLC, India), and chocolate blood agar (CBA) (Hi Media Laboratories LLC, India), and incubated at 37 °C overnight at both ambient air and 5% CO2. We loaded blood samples in the BD BACTEC™ Automated Blood Culture System (BD Diagnostics, Sparks, MD, USA) at 36 °C for up to 5 days, and positive-flagged samples sub-cultured on MacConkey agar (Hi Media Laboratories LLC, India), sheep blood agar (Hi Media Laboratories LLC, India), and CBA (Hi Media Laboratories LLC, India), and incubated at 37 °C overnight at both ambient air and 5% CO2.
Isolates’ identity and antimicrobial susceptibility were elucidated using the BD Phoenix system (BD Diagnostics, Sparks, MD, USA), following the manufacturer’s instructions. Clinical and laboratory standards institute guidelines [17] informed the choice of test antibiotic and inhibition zones interpretation. The antibiotic panels were: amoxicillin/clavulanic acid (4/2–16/2 μg/ml), ampicillin (4–16 μg/ml), piperacillin/tazobactam (4/4–64/4 μg/ml), trimethoprim/sulfamethoxazole (1/19–4/76 μg/ml), nitrofurantoin (16–64 μg/ml), gentamicin (2–8 μg/ml), amikacin (8–32 μg/ml), ceftriaxone (1–32 μg/ml), cefazolin (4–16 μg/ml), cefotaxime (4–16 μg/ml), ceftolozane/tazobactam (1/4–8/4 μg/ml), ceftazidime (2–16 μg/ml), cefepime (1–16 μg/ml), tigecycline (1–4 μg/ml), ciprofloxacin (0.5–2 μg/ml), levofloxacin (1–4 μg/ml), meropenem (0.5–4 μg/ml), ertapenem (0.25–2 μg/ml), imipenem (0.25–4 μg/ml) and colistin (1–4 μg/ml). Pseudomonas aeruginosa (ATCC 27853) and Escherichia coli (25922) were used as the standard control organisms.
We defined carbapenem resistance as resistance to either ertapenem (≥ 2 μg/ml), imipenem (≥ 4 μg/ml), or meropenem (≥ 4 μg/ml), whereas resistance to either ceftriaxone (≥ 4 μg/ml) or ceftazidime (≥ 16 μg/ml) as third-generation cephalosporin resistance [21]. Isolates resistant to three or more antibiotic classes were considered multidrug-resistant (MDR) [13].
Statistical analysis
We analyzed the data using the Statistical Package for the Social Sciences (SPSS) version 17.0 for Windows (IBM SPSS Statistics, IBM Corporation, Armonk, NY, USA). We also analyzed the data for normality and presented it in figures and tables, with categorical data in frequencies and percentages and continuous data in means and medians. We used binomial logistic regression analysis to determine the association between GNB infections, ICU admission outcomes, and patients’ socio-demographic and clinical characteristics. Any association with p-value ≤ 0.2 were further analyzed by multinomial logistic regression, with the statistical significance level set at p < 0.05 ((95% Confidence Interval (95% CI)) and statistically significant associations bolded in Table 2.
Results
Socio-demographic and clinical characteristics of study participants
We sampled 162 critically ill patients admitted to an intensive care unit at the Nairobi West Hospital in Kenya. The study participants constituted a diverse group, aged between 1 and 88 years, with a mean of 44.2 years and a 17.42 standard deviation. The majority were males (64.2%, 104/ 162)-adults (92.6%, 150/162)-not referrals from other healthcare facilities (69.8%, 113/162). The median length of stay was 6 (IQR:4 – 9) days. Additionally, most of the patients had a history of prior hospital admission (56.8%, 92/162) with no ICU admission (98.8% 160/162), antibiotic use (65.4%, 106/162), invasive procedure (71.6%, 116/162), no nasogastric tube (NGT) use (67.3%, 109/162), and the majority were discharged alive from the ICU (80.9%, 131/160), Table 1.
Gram-negative bacteria spectrum and infections
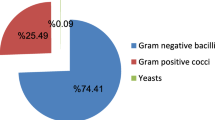
Ninety patients (90/162; 55.6%) had Gram-negative bacteria infections, with urinary tract infections (UTI) (35/90, 39%) being predominant, Fig. 1a. Escherichia coli (18/35, 51%) and Klebsiella pneumoniae (7/10, 70%) were the leading cause of urinary tract and lower respiratory tract infections, respectively. Other infections caused by K. pneumonia and E. coli were ascites, gastrointestinal and upper respiratory tract infections, and accounted for 3% of all GNB infections. Overall, we recovered thirteen [13] Gram-negative bacteria (GNB) types, whereby Escherichia coli (30/90, 33.3%) and Klebsiella pneumoniae (28/90, 31.1%) were the most common isolates, Fig. 1b.
Factors associated with Gram -negative bacterial infections
Patients referred from other hospitals were more than two times at risk of GNB infection when compared to those admitted directly to our study site (cOR = 2.23, 95% CI 1.13–4.70, p = 0.025). Participants with a history of antibiotic use were four times more likely to have GNB infection(aOR = 4.23, 95% CI 1.77–10.11, p =0.001). Those on nasogastric tube were three times at risk of harbouring GNB (aOR = 3.04, 95% CI 1.26–7.32, p = 0.013). Patients with respiratory tract were five times more likely to have GNB infection (aOR = 5.3, 95% CI 1.67–16.75, p= 0.005), while those with cardiovascular conditions were six times at risk (aOR = 5.7, 95% CI 1.25–25.81, p = 0.024), Table 2.
Antimicrobial susceptibility profiles of GNB isolates
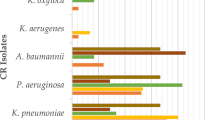
Generally, Enterobacteriaceae isolates showed resistance to third-generation cephalosporins (3GCs), ranging from 50 to 100%, Table 3. Further, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, and Proteus mirabilis exhibited tigecycline resistance (13 to 100%). Klebsiella pneumoniae (46% to 54%) and Escherichia coli (10 to 27%) dominated carbapenem-resistant Enterobacteriaceae (CRE), and the highest carbapenem resistance (CR) (60% to 100%) was among non-fermenting GNB, including Acinetobacter baumannii and Pseudomonas aeruginosa. Escherichia coli and Klebsiella pneumoniae were also resistant to colistin (17 to 46%). Colistin resistance in Acinetobacter baumannii and P. aeruginosa and S. maltophilia, ranging from 60 to 92%, was recorded, but A. baumannii remained susceptible to tigecycline, Table 3.
The antimicrobial susceptibility profile of isolates less than three, including Stenotrophomonas maltophilia, Salmonella typhi, Klebsiella oxytoca, Klebsiella aerogenes, Burkholderia cepacia complex, and Citrobacter freundii, was not presented.
Multidrug resistance
Ninety two percent (92%) of the GNB isolates in this study were multidrug-resistant (MDR), with Escherichia coli (27/30, 90%), Klebsiella pneumoniae (25/28, 89.3%), and Pseudomonas aeruginosa (13/13, 100%) as the most frequent isolates, Table 4. Salmonella typhi was non-MDR.
Discussion
In this study, 56% of patients admitted to Intensive care units (ICUs) had Gram-negative bacterial infections, a prevalence higher than reported in Tanzania [18] Nigeria [19], Nepal [20], Ethiopia [21] and Mexico [22] but lower than documented in the city of Sakaka in Turkey and Saudi Arabi [23]. Frequently ICU-admitted patients require medical interventions involving invasive procedures and mechanical devices, and they have induced immunosuppression and comorbidities that increase their risk for nosocomial infections (NI) [24]. Up to 30% of patients admitted to ICU in developed countries acquire at least one NI, whereas, in low and medium-income countries (LMICs), the frequency is at least 2–3 times higher [25], and mortality is reportedly higher (33.6%) than in high-income countries (< 20%) [26].
Urinary tract infections (UTI, 39%) and wound infections (26%) were the most common condition in ICU-admitted patients in the current study, and overall, Escherichia coli (33.3%) and Klebsiella pneumoniae (31.1%) were the most common isolates. The distribution of infections and their leading etiologies differ widely in the published literature. Sadar et al. found pneumonia (61.4%) as the most common infection in ICU-admitted patients from United States hospitals (2018 − 2020), with Pseudomonas aeruginosa (23.5%), Escherichia coli (18.8%), and Klebsiella pneumoniae (14.4%) as the predominant isolates. Elsewhere in an adult ICU of University Hospital Center in Marrakesh-Morocco, El mekes and others reported pneumonia (39%), bacteremia (29%), and catheter-related blood-stream infections (17%) as the most common infections [27]. Siwakoti and others reported Acinetobacter species (41%) as the leading cause of GNB infections, followed by Klebsiella pneumoniae (28%) and Pseudomonas spp (21%), in a Nepalian ICU [20]. Agaba and others found Klebsiella pneumoniae (30%) and Acinetobacter species (22%) as the most predominant GNB infections in Ugandan ICUs [28], whereas, in Mexican ICUs, P. aeruginosa, K. pneumoniae, E. coli, and A. baumannii were the most prevalent pathogens [22]. In a scoping review of infections and antimicrobial resistance in ICUs in LMICs, Saharman and colleagues found that Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae were the predominant isolates [1]. Klebsiella pneumoniae was the predominant isolate reported from January 2021 to March 2022 in the ICU of the Southern Medical University of Shunde Hospital, Foshan City, Guangdong Province, China [29].
In ICUs, the epidemiology of GNB infections may vary based on adherence to infection prevention and control policies and the patient's demographic and clinical characteristics. According to the Tripartite Antimicrobial Resistance Country Self-assessment Survey or TrACSS), the system established to monitor country's progress towards the implementation of the AMR global action plan, 11% of 162 countries did not have an IPC programme or an operational plan in 2021–2022, 54% had either unimplemented national IPC programmes/operational plans or implementation was taking place in selected health facilities, and only 34% of countries were having an IPC programme implemented nationwide. Less than 25% of countries in the World Health Organization (WHO) African region had an IPC programme, national and facility-level IPC guidelines, IPC education and training, and IPC monitoring, evaluation and feedback [30].
Here, patients with a history of antibiotic use were four times more likely to have GNB infections when compared to those without. Antibiotic overuse can increase the risk of more severe, prolonged and recurrent infections due to antimicrobial-resistant pathogens [31] and the antimicrobials associated-negative health effects may vary, ranging from direct drug toxicity to dysbiosis and immune cell dysfunction to idiosyncratic drug reactions [32]. Our study finding underscores the importance of balancing patients’ antibiotics-associated harm with the need for prompt and appropriate therapy [32] and emphasizing strict adherence to antimicrobial stewardship policies. Further, patients using nasogastric tubes (NGT) were three times at risk of harbouring a GNB infection in this study. This finding is consistent with that of Despotovic and others in a Serbian ICU [33] that NGT can predispose patients to pathogenic GNB colonization within 48 to 72 h [34] or even within the first day of the tube insertion [35], suggesting pre-insertion contamination. These tubes are reportedly associated with aspiration pneumonia in artificially ventilated patients [29, 36] and increase mortality [33].
In this study, participants with respiratory tract and cardiovascular conditions were five and six times, respectively, more likely to have a GNB infection. Bacterial infections play a crucial role in the pathogenesis of cardiovascular diseases (CVD). For instance, Chlamydia pneumoniae, Porphyromonas gingivalis, and Helicobacter pylori infections increase the risk of CVD [37]. Simonsen and others reported an increased risk of cardiovascular disease in bacterial infections among individuals with type 1 diabetes [38]. Further, patients with pulmonary comorbidity are especially prone to GNB [39], mainly due to impaired innate immunity that predispose them to bacterial colonization and infection of the respiratory tract [40]. Generally, patients with chronic diseases, such as coronary and respiratory diseases, have prolonged mechanical ventilation time, length of stay, and suppressed immunity making the patient more vulnerable to infection [41].
In the current study, Enterobacteriaceae isolates showed third-generation cephalosporins (3GCs) resistance, ranging from 50 to 100%. Third-generation cephalosporins-resistant Enterobacteriaceae (3GCRE), including Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae, were recently reported in Kenya among severely ill COVID-19 patients [13], from ‘high-touch’ sites in multiple hospital departments [42], and in communities and hospitals settings [43]. These bugs appear among the top World Health Organization (WHO) global priority pathogens (GPP), along with carbapenem-resistant- Enterobacteriaceae, -Pseudomonas, and -Acinetobacter baumannii, categorized as critical due to drug resistance and the need to discovery and development of new antimicrobial agents [44], In Enterobacterales, 3GC resistance is predominantly due to the production of extended-spectrum β-lactamases (ESBL) [45] and 3GCRE-strains pose higher disease burden than carbapenem-resistant ones [46]. Further, ESBL-producing Enterobacteriaceae often exhibit multidrug resistance and increase in their prevalence favour over-prescription of carbapenems, the drugs of ‘last-resort’ for treatment of multidrug-resistant (MDR) GNB infections, with consequent exacerbation of carbapenem resistance in our study setting.
Here, Klebsiella pneumoniae and Escherichia coli dominated carbapenem-resistant Enterobacteriaceae (CRE), whereas among non-fermenting GNB, Acinetobacter baumannii and Pseudomonas aeruginosa were the leading carbapenem-resistant isolates. Additionally, 90% of Escherichia coli (27/30), 89.3% of Klebsiella pneumoniae (25/28), and 100% of Pseudomonas aeruginosa (13/13) were MDR. CR genes can rapidly spread in clinical isolates via horizontal gene transfer involving plasmids, transposons, and integrons, and these elements often harbour multiple antibiotic-resistance genes [47]. Multidrug-resistant and carbapenem-resistant pathogens present a critical global health challenge [46] and are currently a growing clinical problem in Kenya [12, 43, 47, 48]. They cause community- and hospital-acquired pneumonia and complicated urinary tract infections, bloodstream infections, and complicated intra-abdominal infections. With limited antibiotic options for infections caused by CR pathogens, polymyxins are the mainstay therapy. However, reports on colistin-resistant clinical isolates are increasing globally, suggesting diminishing treatment options for CR-GNB infections and a high risk of difficult-to-treat (DTT) pathogens [20, 49]. We observed colistin resistance, ranging from 60 to 100%, among A. baumannii and P. aeruginosa isolates. Widespread antibiotic use in agriculture and pisciculture is among the leading drivers of drug resistance [50]. In a study by Kariuki and others on antibiotic use by poultry farmers in Kiambu County, Kenya, 13% of farmers used colistin in poultry feeds [51]. The public health implications of colistin-resistant pathogens in our setting remain critical because newer treatment options for CR bacterial infections, including ceftazidime/avibactam and meropenem/vaborbactam, are costly and largely unavailable.
Conclusion
We report a high prevalence of MDR-GNB infections, predominated by urinary tract infections, in ICU, whereby patients with a history of antibiotic use, using the nasogastric tube, and having respiratory tract and cardiovascular conditions were at increased risk. To improve the management of ICU-admitted patients, continuous education, training, monitoring, evaluation and feedback on infection prevention and control (IPCs) are warranted in our study setting.
Study limitation
This was a single hospital-based study, and bacteria isolates molecular characteristics were not elucidated due to limited resources. However, the data presented show a high burden of MDR-GNG infections in a country where most healthcare facilities lack microbiology laboratories or laboratories inadequately equipped and poorly supplied, with most antibiotic prescriptions not guided by antibiograms.
Availability of data and materials
This study’s primary data will be available on request.
Abbreviations
- CLSI:
-
Clinical and Laboratory Standard Institute
- AMR:
-
Antimcrobial resistance
- GNB:
-
Gram negative bacteria
- MDR-GNB:
-
Multidrug resistant Gram negative bacteria
- ICU:
-
Intensive care unit
- UTI:
-
Urinary tract infections
- CR:
-
Carbapenem Resistant
- WBC:
-
White blood cells
- NGT:
-
Nasogastric tube
- CRE:
-
Carbapenem-resistant Enterobacteriaceae
- CRPA:
-
Carbapenem-resistant Pseudomonas aeruginosa
- CRAB:
-
Carbapenem-resistant Acinetobacter baumannii
References
Saha M, Sarkar A. Review on multiple facets of drug resistance: a rising challenge in the 21st century. J Xenobiot. 2021;11(4):197–214.
Abraham EP. The antibiotics. Compr Biochem. 1963;11(4):181–224.
Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25(6):1340.
Sengupta S, Barman P, Lo J. Opportunities to overcome implementation challenges of infection prevention and control in low-middle income countries. Curr Treat Options Infect Dis. 2019;11(3):267–80.
Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR gram-negative bacteria. Front Med. 2019;6(April):1–10.
Hanberger H, Antonelli M, Holmbom M, Lipman J, Pickkers P, Leone M, et al. Infections, antibiotic treatment and mortality in patients admitted to ICUs in countries considered to have high levels of antibiotic resistance compared to those with low levels. BMC Infect Dis. 2014. https://doi.org/10.1186/1471-2334-14-513.
Kadri SS, Lai YL, Warner S, Strich JR, Babiker A, Ricotta EE, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21(2):241–51.
Hawkey PM, Warren RE, Livermore DM, McNulty CAM, Enoch DA, Otter JA, et al. Treatment of infections caused by multidrug-resistant gram-negative bacteria: Report of the British society for antimicrobial chemotherapy/healthcare infection society/british infection association joint working party. J Antimicrob Chemother. 2015;2018(73):2–78.
Crooks NH, Snaith C, Webster D, Gao F, Hawkey P. Clinical review: probiotics in critical care. Critical Care. 2012;16(6):237.
Maina M, Mwaniki P, Odira E, Kiko N, McKnight J, Schultsz C, et al. Antibiotic use in Kenyan public hospitals: prevalence, appropriateness and link to guideline availability. Int J Infect Dis. 2020;99:10–8.
Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903–10.
Musila L, Kyany’a C, Maybank R, Stam J, Oundo V, Sang W. Detection of diverse carbapenem and multidrug resistance genes and high-risk strain types among carbapenem non-susceptible clinical isolates of target gram-negative bacteria in Kenya. PLoS ONE. 2021;16:e0246937.
Mutua JM, Njeru JM, Musyoki AM. Multidrug resistant bacterial infections in severely ill COVID-19 patients admitted in a national referral and teaching hospital. Kenya BMC Infect Dis. 2022;22(1):1–12.
Wangai FK, Masika MM, Lule GN, Karari EM, Maritim MC, Jaoko WG, et al. Bridging antimicrobial resistance knowledge gaps: the East African perspective on a global problem. PLoS ONE. 2019;14(2):e0212131.
Bruno L. Standard operating procedures bacteriology. J Chem Inf Model. 2019;53(9):1689–99.
Lewis SM, Kumari S. Guidelines on Standard Operating Procedures for Haemathology. 2000;1–80.
Team C. M100 Performance Standards for Antimicrobial Susceptibility Testing. Vol. 8, Journal of Services Marketing. 2021. 18–260 p.
Silago V, Kovacs D, Msanga DR, Seni J, Matthews L, Oravcová K, et al. Bacteremia in critical care units at Bugando Medical Centre, Mwanza, Tanzania: the role of colonization and contaminated cots and mothers’ hands in cross-transmission of multidrug resistant gram-negative bacteria. Antimicrob Resist Infect Control. 2020;9(1):58.
Iwuafor AA, Ogunsola FT, Oladele RO, Oduyebo OO, Desalu I, Egwuatu CC, et al. Incidence, clinical outcome and risk factors of intensive care unit infections in the lagos university teaching hospital (LUTH), Lagos. Nigeria PLoS ONE. 2016;11(10):1–15.
Siwakoti S, Subedi A, Sharma A, Baral R, Bhattarai NR, Khanal B. Incidence and outcomes of multidrug-resistant gram-negative bacteria infections in intensive care unit from Nepal—a prospective cohort study. Antimicrob Resist Infect Control. 2018;7:114.
Sebre S, Abegaz WE, Seman A, Awoke T, Desalegn Z, Mihret W, et al. Bacterial profiles and antimicrobial susceptibility pattern of isolates from inanimate hospital environments at Tikur Anbessa specialized teaching hospital, Addis Ababa. Ethiopia Infect Drug Resist. 2020;13:4439–48.
Uc-Cachón AH, Gracida-Osorno C, Luna-Chi IG, Jiménez-Guillermo JG, Molina-Salinas GM. High prevalence of antimicrobial resistance among gram-negative isolated bacilli in intensive care units at a tertiary-care hospital in Yucatán Mexico. Medicina. 2019;55:588.
Wani FA, Bandy A, Alenzi MJS, Alzarea AI, Alanazi AS, Sayeed MU, et al. Resistance patterns of gram-negative bacteria recovered from clinical specimens of intensive care patients. Microorganisms. 2021;9(11):1–11.
Blot S, Ruppé E, Harbarth S, Asehnoune K, Poulakou G, Luyt CE, et al. Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive Crit Care Nurs. 2022;1:70.
WHO. World health statistics 2016: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization; 2016. 121 p. https://apps.who.int/iris/handle/10665/206498. Accessed 8 Apr 2023.
Saharman YR, Karuniawati A, Severin JA, Verbrugh HA. Infections and antimicrobial resistance in intensive care units in lower—middle income countries : a scoping review. Antimicrob Resist Infect Control. 2021;10:1–19.
Mekes ElA, Zahlane K, Ait SL, Tadlaoui OA, Barakate M. The clinical and epidemiological risk factors of infections due to multi-drug resistant bacteria in an adult intensive care unit of university hospital center in Marrakesh-Morocco. J Infect Public Health. 2020;13(4):637–43.
Agaba P, Tumukunde J, Tindimwebwa JVB, Kwizera A. Nosocomial bacterial infections and their antimicrobial susceptibility patterns among patients in Ugandan intensive care units: a cross sectional study. BMC Res Notes. 2017;10(1):349.
Wu C, Lu J, Ruan L, Yao J. Tracking epidemiological characteristics and risk factors of multi-drug resistant bacteria in intensive care units. Infect Drug Resist. 2023;16:1499–509.
WHO. Global report on infection prevention and control executive summary. Geneva: WHO; 2022.
Llor C, Bjerrum L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Safety. 2014;5(6):229–41.
Arulkumaran N, Routledge M, Schlebusch S, Lipman J, Conway MA. Antimicrobial-associated harm in critical care: a narrative review. Intensive Care Med. 2020;46(2):225–35.
Despotovic A, Milosevic B, Milosevic I, Mitrovic N, Cirkovic A, Jovanovic S, et al. Hospital-acquired infections in the adult intensive care unit—Epidemiology, antimicrobial resistance patterns, and risk factors for acquisition and mortality. Am J Infect Control. 2020;48(10):1211–5.
Thomas S, Raman R, Idikula J, Brahmadathan N. Alterations in oropharyngeal flora in patients with a nasogastric tube: a cohort study. Crit Care Med. 1992;20(12):1677–80.
Petersen SM, Greisen G, Krogfelt KA. Nasogastric feeding tubes from a neonatal department yield high concentrations of potentially pathogenic bacteria—even 1 d after insertion. Pediatr Res. 2016;80(3):395–400.
Teramoto S. Nasogastric tube feeding is a cause of aspiration pneumonia in ventilated patients. Eur Respir J. 2006;27(2):436–7.
Rosenfeld M, Ann CL. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011;106(11):858–67.
Simonsen JR, Järvinen A, Harjutsalo V, Forsblom C, Groop P-H, Lehto M. The association between bacterial infections and the risk of coronary heart disease in type 1 diabetes. J Intern Med. 2020;288(6):711–24.
Arancibia F, Bauer TT, Ewig S, Mensa J, Gonzalez J, Niederman MS, et al. Community-acquired pneumonia due to gram-negative bacteria and pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162(16):1849.
Lange P. Chronic obstructive pulmonary disease and risk of infection. Pneumonol Alergol Pol. 2009;77(3):284–8.
Wu C, Lu J, Ruan L, Yao J. Tracking epidemiological characteristics and risk factors of multi-drug resistant bacteria in intensive care units. Infection and Drug Resistance. 2023;16:1499–509.
Odoyo E, Matano D, Tiria F, Georges M, Kyanya C, Wahome S, et al. Environmental contamination across multiple hospital departments with multidrug-resistant bacteria pose an elevated risk of healthcare-associated infections in Kenyan hospitals. Antimicrob Resist Infect Control. 2023;12(1):22.
Ita T, Luvsansharav UO, Smith RM, Mugoh R, Ayodo C, Oduor B, et al. Prevalence of colonization with multidrug-resistant bacteria in communities and hospitals in Kenya. Sci Rep. 2022;12(1):22290.
Asokan G, Ramadhan T, Nursing, College of Health Sciences, WHO Collaborating Centre for Nursing Development University of Bahrain, Manama, Bahrain, Ahmed E, Nursing, College of Health Sciences, WHO Collaborating Centre for Nursing Development University of Bahrain, Manama, Bahrain, Sanad H, et al. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. OMJ. 2019;34(3):184–93.
Kaye KS, Belley A. Third-generation cephalosporin-resistant Enterobacterales are critical priority pathogens, too! Antimicrob Agents Chemother. 2022;66(4):e00213-e222.
CDC. Antibiotic resistance threats in The United States. 2019.
Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54(3):969–76.
WHO. Kenya national action plan on antimicrobial resistance. Geneva: WHO; 2022.
Di Francesco A, Salvatore D, Sakhria S, Bertelloni F, Catelli E, Ben Yahia S, et al. Colistin resistance genes in broiler chickens in Tunisia. Animals. 2023;13(8):1409.
Devan SS, Aklilu E, Zakaria Z, Hamdan RH, Lemlem M, Kamaruzzaman NF, et al. Emergence of MCR -1, -3, -6, -8 AND -9) in Escherichia Coli isolated from live chickens, raw chicken meat and vegetables from Kelantan Malaysia. J microb biotech food sci. 2023;6: e9829.
Kariuki JW, Jacobs J, Ngogang MP, Howland O. Antibiotic use by poultry farmers in Kiambu county, Kenya: exploring practices and drivers of potential overuse. Antimicrob Resist Infect Control. 2023;12(1):3.
Acknowledgements
We acknowledge the Nairobi West Hospital Microbiology Laboratory staff, Jeff Kwasi, Daniel Warui and Christopher Wambua, for their technical support during sample collection and processing.
Funding
There was no funding received for the fulfilment of this study.
Author information
Authors and Affiliations
Contributions
JWM and AMM conceived and designed the study. JWM collected the data directed by AMM and PKS. JWM and AMM analyzed and interpreted the data, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study ethical approval was obtained from Kenyatta University Ethical Review Board and written consent sought from all the participants.
Consent for publication
Informed consent was obtained from all the participants in this study as well as the co-authors.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maina, J.W., Onyambu, F.G., Kibet, P.S. et al. Multidrug-resistant Gram-negative bacterial infections and associated factors in a Kenyan intensive care unit: a cross-sectional study. Ann Clin Microbiol Antimicrob 22, 85 (2023). https://doi.org/10.1186/s12941-023-00636-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-023-00636-5