Abstract
Background
Healthcare-associated infections (HAIs) are often caused by multidrug-resistant (MDR) bacteria contaminating hospital environments which can cause outbreaks as well as sporadic transmission.
Methods
This study systematically sampled and utilized standard bacteriological culture methods to determine the numbers and types of MDR Enterococcus faecalis/faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter species, and Escherichia coli (ESKAPEE) from high-touch environments of five Kenyan hospitals; level 6 and 5 hospitals (A, B, and C), and level 4 hospitals (D and E), in 2018. Six hundred and seventeen high-touch surfaces across six hospital departments; surgical, general, maternity, newborn, outpatient and pediatric were sampled.
Results
78/617 (12.6%) of the sampled high-touch surfaces were contaminated with MDR ESKAPEE; A. baumannii, 23/617 (3.7%), K. pneumoniae, 22/617 (3.6%), Enterobacter species, 19/617 (3.1%), methicillin resistant S. aureus (MRSA), 5/617 (0.8%), E. coli, 5/617 (0.8%), P. aeruginosa, 2/617 (0.3%), and E. faecalis and faecium, 2/617 (0.3%). Items found in patient areas, such as beddings, newborn incubators, baby cots, and sinks were the most frequently contaminated. Level 6 and 5 hospitals, B, 21/122 (17.2%), A, 21/122 (17.2%), and C, 18/136 (13.2%), were more frequently contaminated with MDR ESKAPEE than level 4 hospitals; D, 6/101 (5.9%), and E, 8/131 (6.1%). All the sampled hospital departments were contaminated with MDR ESKAPEE, with high levels observed in newborn, surgical and maternity. All the A. baumannii, Enterobacter species, and K. pneumoniae isolates were non-susceptible to piperacillin, ceftriaxone and cefepime. 22/23 (95.6%) of the A. baumannii isolates were non-susceptible to meropenem. In addition, 5 K. pneumoniae isolates were resistant to all the antibiotics tested except for colistin.
Conclusion
The presence of MDR ESKAPEE across all the hospitals demonstrated gaps in infection prevention practices (IPCs) that should be addressed. Non-susceptibility to last-line antibiotics such as meropenem threatens the ability to treat infections.
Similar content being viewed by others
Background
Healthcare-associated infections (HAIs) are among the leading threats to patient safety. Hospital patients are often predisposed to infections because of exposure to invasive devices during surgical procedures and possibly impaired or underdeveloped immunity [1]. In Kenya, the prevalence of HAIs is estimated to be 4.4 per 100 patient admissions, with the highest rates observed in medical, 5.1%, and pediatric, 4.9%, departments [2]. High rates of surgical site infections of up to 9.3% have been reported [3].
Enterococcus faecalis/faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter species, and Escherichia coli (ESKAPEE) pathogens are the leading causes of HAIs globally and in Kenya [4, 5]. Consequently, the World Health Organization lists antibiotic-resistant ESKAPEE pathogens as high to critical priority pathogens for research and development of new antibiotics [6]. Infections caused by multi-drug resistant (MDR) ESKAPEE pathogens are of particular concern as they are associated with increased mortality and treatment costs [7, 8].
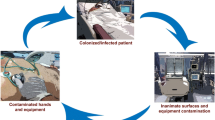
HAIs are frequently caused by bacterial pathogens that contaminate hospital environments[9]. These bacteria persist in hospital environments through the formation of biofilms and can withstand desiccation and resist disinfection [9, 10]. HAIs arising from contamination of frequently handled hospital surfaces or equipment regarded as high-touch surfaces[11], such as sinks, patients’ beds, and linens by bacterial pathogens, including; Acinetobacter baumannii, Staphylococcus aureus, carbapenem-resistant Enterobacterales, and vancomycin-resistant Enterococcus species, have been reported[12,13,14]. Microbial monitoring of these high-touch hospital environments can help determine the presence of contaminating pathogens and thus aid in implementing targeted infection prevention practices (IPCs) that may reduce HAIs. Our previous study determined the overall bacteria levels in Kenyan hospital environments and identified modifiable risk factors for improved infection control [15]. This study systematically sampled and characterized MDR ESKAPEE pathogens contaminating high-touch environments in five Kenyan hospitals and identified the highest-risk departments to target to reduce the risk of HAIs to patients in these hospitals.
Materials and methods
Study design
This descriptive laboratory-based study was conducted in five hospitals in Kenya, as previously described [15]. Briefly, one level six hospital (B), two level five hospitals (A and C), and two level four hospitals (D and E) were sampled. Hospital B is a 450-bed capacity national referral and teaching hospital. Hospitals A and C, with 168 and 270-bed capacities, respectively, are county referral hospitals, while Hospitals D and E, with 158 and 54-bed capacities, respectively, are level four facilities. Three departments identified by the hospital administration as having high levels of HAIs were selected for sampling in each hospital. In addition, the outpatient departments of the five hospitals were sampled. In total, five outpatient departments (hospitals A, B, C, D, and E), five pediatric departments (hospitals A, B, C, D and E), four surgical departments (hospitals B and E, and two surgical departments in hospital A), three maternity departments (hospitals C, D and E), three newborn departments (hospitals A, B, and D) and two general departments (hospitals D and E) were sampled.
Sampling strategy
Sampling was carried out twice in each of the hospitals, between February and September.
2018. Swabs in neutralizing buffer (NB) (Puritan ESK sampling kit, Guilford, ME, USA) were used to sample 617 selected high-touch areas. High-touch areas, as classified in the guidelines for environmental infection control in healthcare facilities [16], are surfaces or equipment frequently handled by patients and clinicians, thus carrying a high risk for transmission of HAIs. For each surface, one swab was used. The surfaces sampled included items and areas close to the patient, such as; intravenous pole steering handles, intravenous tubing, patient bedding, bed rails, newborn incubators, tray tabletops, baby cots, bedside tabletops, baby weighing scale, room light switch plates, room inner doorknobs and clinician gowns. Surfaces in the bathroom, such as sinks, handrails, and toilet flush handles, were also sampled. In addition, equipment, including computer keyboards and mice, blood pressure cuffs, clinician cell phones, stethoscopes, and thermometers, were sampled. A sterile square frame measuring 500cm2 was used to define the swabbed area, while for smaller objects or surfaces, the surface area was approximated, and the whole surface area was swabbed. Samples were collected from clinician uniforms by swabbing the abdominal region and sleeve cuffs of the uniform. The swabs were then shipped at 4℃ to the testing lab at the Kenya Medical Research Institute (KEMRI), Nairobi, where they were processed within 36 h.
Isolation and detection of MDR ESKAPEE pathogens
The NB solution containing the swab was vortexed and 100 µl was inoculated on respective chromogenic agars (CHROMagar, Paris, France) for isolation and detection of ESKAPEE pathogens; CHROMagar ESBL for Enterobacterales, CHROMagar MRSA for methicillin resistant Staphylococcus aureus (MRSA), CHROMagar VRE for vancomycin-resistant Enterococcus species and CHROMagar Acinetobacter for Acinetobacter baumannii. The inoculated plates were incubated aerobically at 37 °C for 24 h. The target MDR ESKAPEE was identified by observing the typical growth characteristics on the respective chromogenic agar. Three colonies of the target organisms were sub-cultured on Mueller Hinton agar (HIMEDIA, Mumbai, India) and incubated for 24 h to obtain pure bacterial isolates. Gram stain was performed for each isolate. Bacterial identification and antimicrobial susceptibility testing were performed on the VITEK 2 system per Clinical and Laboratory Standards Institute (CLSI) 2017 guidelines. AST-XN05 and AST-P580 cards were used for gram-negative and gram-positive antimicrobial susceptibility testing, respectively. Staphylococcus aureus 25,923 and Escherichia coli 25,922 were used for quality control. Bacterial isolates were categorized as MDR if they were non-susceptible to at least one antimicrobial drug in three or more therapeutically relevant antibacterial classes [17].
Statistical analysis
Data were captured in excel sheets. All statistical analyses were performed on STATA (StataCorp. 2013. College Station, TX, USA). Descriptive statistics were expressed as percentages. Chi-square Fisher’s exact test was used to determine associations between the numbers and type of contaminating MDR ESKAPEE and the study hospitals and their departments. A P-value of ≤ 0.05 was considered statistically significant. The hospitals were classified as either higher level; level 6 hospitals (B), level 5 hospitals (A and C), or lower level; level 4 hospitals (D and E).
Results
Recovery of MDR ESKAPEE from the high-touch surfaces
A total of 617 hospital high-touch surfaces were sampled across the five study hospitals. Six hospital departments were sampled, including; surgical, general, maternity, newborn, outpatient and pediatric. 78/617 (12.6%) of the sampled high-touch surfaces across the study hospitals were contaminated with MDR ESKAPEE. The isolated MDR ESKAPEE pathogens included; A. baumannii, 23/617 (3.7%), K. pneumoniae, 22/617 (3.6%), Enterobacter species, 19/617 (3.1%), MRSA, 5/617 (0.8%), E. coli, 5/617 (0.8%), P. aeruginosa, 2/617 (0.3%), and Enterococcus faecalis and faecium, 2/617 (0.3%), (Table 1). A. baumannii, K. pneumoniae and Enterobacter species contaminated the widest range of hospital surfaces. The most frequently contaminated items were those found in patient areas, including patient beddings, newborn incubators and baby cots, department sinks, door knobs, and tray table tops. MDR ESKAPEE pathogens were also isolated from equipment such as intravenous pole steering handles, light switch plates, and a dial pad for a surgical table. Bathroom surfaces, bathroom sinks and a saline bathtub were also contaminated with MDR ESKAPEE.
Distribution of MDR ESKAPEE pathogens across the different hospital levels
Higher-level hospitals were more frequently contaminated with MDR ESKAPEE than lower-level hospitals; level 6 hospital B, 21/122 (17.2%), level 5 hospitals A and C, 21/122 (17.2%), and 18/136 (13.2%), respectively, and level 4 hospitals, D, 6/101 (5.9%), and E, 8/131 (6.1%),. There was no significant association between the rates of contamination of each of the leading contaminants, MDR A. baumannii, MDR K. pneumoniae and MDR Enterobacter species, with the level of the hospital facility, P = 0.097, P = 0.721 and P = 0.729, respectively.
Distribution of MDR ESKAPEE pathogens across the different hospital departments
Newborn and surgical departments were the most frequently contaminated with MDR ESKAPEE, 18/70 (25.0%) and 21/110 (19.1%), respectively (Table 2). A. baumannii, K. pneumoniae, and Enterobacter species accounted for 30/37 (81.1%) of MDR ESKAPEE contamination in these two departments. Conversely, the outpatient departments, 8/174 (4.6%), and general departments, 3/64 (4.7%), were the least frequently contaminated with MDR ESKAPEE pathogens.
Antibiotic susceptibility profiles of the isolated MDR ESKAPEE isolates
All the 23 A. baumannii isolates were non-susceptible to piperacillin, 23/23 (100.0%), cefepime, 23/23 (100.0%), and ceftriaxone, 23/23 (100%) (Table 3). 22/23 (95.6%) of the A. baumannii isolates were non-susceptible to meropenem. All the 22/22 (100.0%) K. pneumoniae isolates were non-susceptible to; piperacillin, cefepime, cefuroxime, cefuroxime-axetil, ceftriaxone, and aztreonam. Five of the twenty-two (22.7%) K. pneumoniae isolates were resistant to all the antibiotics tested except colistin. In addition, one of the twenty-three (4.4%) A. baumannii isolates was also resistant to all antibiotics tested except colistin. All the 19/19 (100.0%) Enterobacter species were non-susceptible to piperacillin, cefepime, cefuroxime, cefuroxime-axetil, ceftriaxone, and aztreonam. 3/5 (60%) of the isolated E. coli were non-susceptible to antibiotics piperacillin, cefepime, cefuroxime, cefuroxime-axetil, ceftriaxone, aztreonam, minocycline, tetracycline and sulfamethoxazole. All five E. coli isolates were susceptible to meropenem and tigecycline. Both the P. aeruginosa isolates were non-susceptible to ticarcillin-clavulanic acid and meropenem. At least 3/5 (60%) of the isolated MRSA were non-susceptible to cefoxitin, benzylpenicillin, oxacillin, erythromycin, clindamycin, gentamycin, levofloxacin, moxifloxacin, tetracycline and trimethoprim-sulfamethoxazole. Both the E. faecium and faecalis isolates were non-susceptible to erythromycin, levofloxacin, tetracycline and nitrofurantoin (Table 4).
Discussion
This study sampled high-touch surfaces in five Kenyan hospitals and found that more than 12.0% were contaminated with MDR ESKAPEE pathogens. There was a risk of HAIs by MDR ESKAPEE pathogens in all the hospital departments sampled. In particular, items found in patient areas, such as newborn incubators and patient beddings, were frequently contaminated, posing a high risk of HAIs. Four departments, newborn, surgical, maternity, and pediatrics, had particularly worrying contamination rates of at least 10%, with the surgical and newborn having the highest rates of 25.0% and 19.1%, respectively.
Surgical site infections comprise the largest proportion of HAIs in developing countries, with an estimated incidence of 5.6 per 100 surgical procedures [18]. Caesarian sections are the most common surgical procedure in Kenya[19]. Longer durations of labor have been associated with a higher incidence of surgical site infections following caesarian-section in Kenyan hospitals [20], suggesting a role of the hospital environments in the acquisition of HAIs. The high contamination rate of the surgical and maternity departments by MDR ESKAPEE in this study supports this hypothesis. Newborn populations are particularly vulnerable to HAIs because of their immature immunity [1] and the high rate of contamination of newborn departments by the MDR ESKAPEE in this study may explain, in part, the high prevalence of HAIs observed in Kenyan pediatric departments [2]. The higher levels of MDR ESKAPEE contamination in higher-level hospitals than in lower-level hospitals may be linked to increased antibiotics selection pressure resulting from extensive use, particularly in critical care units or specialized departments such as the surgical departments. Patients with severe infections, trauma and those referred from lower-level hospitals often seek medical care in higher-level hospitals. These patients often require surgical interventions and the administration of antibiotics for patient care.
MDR A. baumannii was the most frequently isolated ESKAPEE pathogen, 23/617 (3.7%) and was found across all departments. Its ability to form biofilms and withstand desiccation [21, 22] enables it to persist in the hospital environment for long periods. Furthermore, it can maintain virulence even after prolonged desiccation and starvation [23], which partly explains its ability to cause frequent and prolonged hospital outbreaks [21]. As a result, A. baumannii has been linked to a wide range of HAIs, including ventilator-associated pneumoniae, skin and soft tissue infections, urinary tract infections, secondary meningitis and bloodstream infections that often affect critically ill patients [24], all of which have been associated with high mortality rates [25]. Data on the burden of A. baumannii infections in Kenya is not readily available. The available data is from facility-based studies, which show that A. baumannii is an important cause of infections in Kenya [26, 27]. A. baumannii has also been implicated in outbreaks in a teaching hospital in Kenya [28]. The high frequency of A. baumannii isolation in this study suggests an impending threat of hospital acquired A. baumannii infections. Typically, A. baumannii is intrinsically resistant to commonly prescribed antibiotics, such as first and second-generation cephalosporins, chloramphenicol, and aminopenicillins [25]. Carbapenem antibiotics are the main treatment option for MDR A. baumannii infections [25], yet in this study, 22/23 isolates were carbapenem non-susceptible. The use of quaternary ammonium compounds-based disposable wipes by in-house staff and chlorine-based disinfection is recommended to reduce contamination of hospital surfaces by carbapenem-resistant A. baumannii [29]. Terminal cleaning and enhanced cleaning of the high-touch surfaces [30] can help reduce the risk of A. baumannii HAIs at the study hospitals. None of the contaminating A. baumannii isolates were resistant to colistin and 22/23 were susceptible to minocycline, leaving these agents as treatment options for carbapenem-resistant A. baumannii strains [31]. The high rate of carbapenem resistance inA. baumanniiisolates reflects an overreliance on carbapenem, a last-line antibiotic, for treatingA. baumannii, while disregarding first- and second-line options such as tetracyclines and fluoroquinolones at the study hospitals.
K. pneumoniae is a significant cause of opportunistic HAIs, including; pneumoniae, urinary tract infections, skin and soft tissue infections, septicemia and endocarditis [32]. It is the most common cause of HAIs, including hospital outbreaks, in Kenya and Africa [4, 33]. In this study, MDR K. pnuemoniae was the second most frequently isolated MDR ESKAPEE pathogen, 22/617 (3.6%). It was isolated from newborn, surgical and maternity departments from high-touch items in near-patient areas, bathroom areas, and hospital equipment, reflecting its ubiquitous nature [32] and ability to persist in the hospital environment by forming biofilms [10, 34]. Equally important, K. pneumoniae has been implicated in several outbreaks and HAIs in neonatal units [35,36,37,38]. In Kenya, multi-drug resistant K. pneumoniae has been implicated in an outbreak in a neonatal critical care unit of a referral hospital in which six of the thirteen patients succumbed, a 46% case fatality rate [39]. Multi-drug-resistant K. pneumoniae was also identified as the common cause of blood-borne infections in newborn units of another referral hospital in Kenya [40]. Indeed, these reports highlight multi-drug-resistant K. pneumoniae as an important cause of HAIs in Kenyan hospitals. Therefore, the high contamination rate of surfaces and equipment in newborn departments in this study is of grave concern. All the K. pneumoniae isolates were resistant to aztreonam and third and fourth-generation cephalosporins, ceftriaxone and cefepime, respectively, which infers the production of extended-spectrum beta-lactamases and/or AmpC β-lactamases in addition to other resistance mechanisms [41]. Additionally, 22.7% of the K. pneumoniae isolates were also resistant to meropenem which infers the production of carbapenemases [42][43]. Resistance to last-line antibiotics such as meropenem threatens the ability to treat K. pneumoniae HAIs. Enforcing effective terminal cleaning of newborn incubators and enhanced cleaning of high-touch areas can help reduce the risk of K. pneumoniae HAIs in the newborn departments of the study hospitals. Additionally, safe disposal of diapers, cleaning of soiled articles with water and appropriately diluted disinfectants and soap, adherence to hand hygiene procedures, and limited staff rotations have been successfully employed to contain an outbreak in a neonatal unit [44].
Enterobacter species are associated primarily with HAIs [45] and were the third most frequently isolated MDR ESKAPEE pathogen in this study, 19/617 (3.1%). It was isolated across all the departments. Third-generation cephalosporins are known to induce variants of AmpC beta-lactamases in Enterobacter species, resulting in resistance [46], and their widespread use in Kenyan hospitals [47, 48] could be providing a selective pressure that favors Enterobacter species and the emergence of cephalosporin resistance. Because of innate resistance to first and second-generation cephalosporins, treatment of Enterobacter infections is often limited to carbapenems, fluoroquinolones, and aminoglycosides [45]. Fourth-generation cephalosporins have been used to treat Enterobacter infections due to their relative stability to AmpC beta-lactamases in the absence of extended-spectrum beta-lactamases [45]. This study, however, found all the Enterobacter isolates resistant to cefepime, a fourth-generation cephalosporin, which further limits treatment options for Enterobacter infections in the study hospitals. There was, however, a low rate of resistance to fluoroquinolones, moxifloxacin, and levofloxacin, while meropenem was active against all the Enterobacter isolates from the study hospitals. Interestingly, one isolate was resistant to colistin while retaining susceptibility to other drug classes, which may be attributed to chromosomal mutations or acquired resistance genes. However, a more reliable colistin test will be necessary to rule out any false resistance often observed with colistin testing in automated platforms.
This study isolated two isolates of P. aeruginosa from high-touch surfaces. P. aeruginosa is a common contaminant in hospital environments, particularly moist surfaces such as sinks and taps [49, 50], which were sampled in this study. The media used to detect P. aeruginosa was not the optimal selective media which could have limited the ability to detect it from the hospital environment. Similar to A. baumannii, the two isolates of P. aeruginosa in this study were resistant to meropenem but retained susceptibility to the fluoroquinolone moxifloxacin further highlighting an overreliance on carbapenem for treatment at the study hospitals. Putting carbapenems on watch lists and limiting access without approval can help limit increasing levels of carbapenem resistance in the study hospitals.
At least 3/5 E. coli isolates were non-susceptible to third-generation cephalosporins, which may infer the production of extended-spectrum beta-lactamases. However, all five isolates of E. coli were susceptible to last-line antibiotics such as meropenem and tigecycline.
The Gram-positive MDR ESKAPEE pathogens, MRSA and Enterococcus faecalis/faecium, had high levels of resistance to first-line antibiotics, including erythromycin, levofloxacin, and tetracycline, while they were susceptible to last line antibiotics; vancomycin, teicoplanin, and tigecycline. This study isolated MRSA and Enterococcus faecalis/faecium mainly from the newborn and pediatric departments, which signifies a high risk of HAIs to these younger patient populations. CHROMagar VRE should ideally limit the growth of contaminating bacteria and vancomycin-susceptible Enterococcus species. Despite this, in this study, the detected isolates of the Enterococcus faecalis/faecium were susceptible to vancomycin, indicating that the media may be permissive to their growth and antimicrobial sensitivity testing is necessary to confirm whether the recovered isolates are indeed vancomycin-resistant. Gram-negative MDR ESKAPEE pathogens were isolated in greater numbers than Gram-positive MDR ESKAPEE, which may be linked to IPC practices such as using contaminated cleaning materials, which has been found to mainly replaceram-positive cocci with Gram-negative bacilli [51]. In a previous study conducted in the same hospitals [15], storing wet mops was predictive of increased bacterial loads, reflecting that this poor cleaning practice contributes to the spread of bacterial contamination.
Study limitations
Molecular characterization of the isolates from this study would provide more information on the MDR ESKAPEE strain types found within these hospitals. Further, a comparison with clinical strains could confirm the transmission patterns and ascertain that these pathogens are causes of HAIs in the study hospitals. The low rate of detection of Pseudomonas aeruginosa, a common cause of HAIs [52, 53], may be due to the failure to use a suitable culture media for its detection in the study.
Conclusion
The widespread presence of MDR ESKAPEE contamination in the study hospital environments suggests low compliance to IPC practices in Kenyan hospitals, which are already outstretched by challenges such as poor water supply, frequent electricity outages, sporadic supply of critical cleaning reagents and personnel, among others. There was a high risk of HAIs by MDR ESKAPEE pathogens across all the sampled hospitals. Non-susceptibility to last-line antibiotics such as meropenem threatens the ability to treat HAIs. The study hospitals could benefit from strengthening diagnostic capacity for antimicrobial sensitivity testing for judicious antibiotics prescription practices. In addition, enforcing terminal cleaning of patients’ beds and newborn incubators, hospital environment biomonitoring around high-touch areas, antibiotic stewardship programs, enforced hand hygiene, and adequate and frequent cleaning of high-touch areas, as previously described [15] can help reduce the risk of HAIs at the study hospitals.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- HAIs:
-
Healthcare-associated infections
- MDR:
-
Multidrug-resistant
- ESKAPEE:
-
Enterococcus faecalis/faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter species, and Escherichia coli
- IPCs:
-
Infection prevention control practices
- NB:
-
Neutralizing buffer
- KEMRI:
-
Kenya Medical Research Institute
- CLSI:
-
Clinical and Laboratory Standards Institute
- WRAIR:
-
Walter Reed Army Institute of Research
- R:
-
Resistant
- NS:
-
Non-susceptible (Resistant or Intermediate)
- S:
-
Susceptible
- nd:
-
Antimicrobial sensitivity testing not done
References
Catania VD, Boscarelli A, Lauriti G, Morini F, Zani A. Risk factors for surgical site infection in neonates: a systematic review of the literature and meta-analysis. Front Pediatr. 2019;7(MAR):1–11.
Ndegwa L. Hospital-Acquired Infections Surveillance in Three Kenyan Hospitals, 2010–2012. Open Forum Infect Dis. 2015;2(suppl_1):2014–5.
Ntumba P, Mwangi C, Barasa J, Aiken A, Kubilay Z, Allegranzi B. Multimodal approach for surgical site infection prevention – results from a pilot site in Kenya. Antimicrob Resist Infect Control. 2015;4(S1):2015.
Fraser JL, Mwatondo A, Alimi YH, Varma JK, Vilas VJDR. Healthcare-associated outbreaks of bacterial infections in Africa, 2009–2018: a review. Int J Infect Dis. 2021;103:469–77.
Aiken AM, Mturi N, Njuguna P, Mohammed S, Berkley JA, Mwangi I, et al. Risk and causes of paediatric hospital-acquired bacteraemia in Kilifi District Hospital, Kenya: a prospective cohort study. The Lancet. 2011;378(9808):2021–7.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27.
Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(SUPPL 2):82–9.
Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with healthcare-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2010;54(1):109–15.
Otter JA, Yezli S, Salkeld JAG, French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control. 2013 May;41(5 Suppl):6–11.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108.
Huslage K, Rutala WA, Sickbert-Bennett E, Weber DJ. A quantitative approach to defining “high-touch” surfaces in hospitals. Infect Control Hosp Epidemiol. 2010 Aug;31(8):850–3.
Creamer E, Humphreys H. The contribution of beds to healthcare-associated infection: the importance of adequate decontamination. J Hosp Infect. 2008;69(1):8–23.
Leitner E, Zarfel G, Luxner J, Herzog K, Pekard-Amenitsch S, Hoenigl M, et al. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother. 2015;59(1):714–6.
Suleyman G, Alangaden G, Bardossy AC. The Role of Environmental Contamination in the Transmission of Nosocomial Pathogens and Healthcare-Associated Infections. Curr Infect Dis Rep. 2018;20(6).
Odoyo E, Matano D, Georges M, Tiria F, Wahome S, Kyany’a C, et al. Ten thousand fold higher than acceptable bacterial loads detected in kenyan hospital environments: targeted approaches to reduce contamination levels. Int J Environ Res Public Health. 2021;18(13):6810.
Sehulster L, Chinn RRY, CDC HICPAC. Guidelines for environmental infection control in healthcare facilities. Recommendations of CDC and the Healthcare infection control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR–10):1–42.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81.
Allegranzi B, Nejad SB, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. The Lancet. 2011;377(9761):228–41.
Aiken AM, Wanyoro AK, Mwangi J, Juma F, Mugoya IK, Scott JAG. Changing use of surgical antibiotic prophylaxis in Thika Hospital, Kenya: A quality improvement intervention with an interrupted time series design. PLoS One. 2013;8(11).
Koigi-Kamau R, Kabare LW, Wanyoike-Gichuhi J. Incidence of wound infection after caesarean delivery in a district hospital in central Kenya. East Afr Med J. 2005;82(7):357–61.
Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol. 1998;36(7):1938–41.
Espinal P, Martí S, Vila J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect. 2012;80(1):56–60.
Chapartegui-González I, Lázaro-Díez M, Bravo Z, Navas J, Icardo JM, Ramos-Vivas J. Acinetobacter baumannii maintains its virulence after long-time starvation. PLoS One. 2018;13(8).
Aofie H, Michael O, Audrey F, Roy DS. Acinetobacter baumannii, an emerging opportunistic pathogen. Virulence. 2012;3(3):243–50.
Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–82.
Revathi G, Siu LK, Lu PL, Huang LY. First report of NDM-1-producing Acinetobacter baumannii in East Africa.International Journal of Infectious Diseases. 2013 Dec;17(12).
Musila L, Kyany’a C, Maybank R, Stam J, Oundo V, Sang W. Detection of diverse carbapenem and multi-drug resistance genes and high-risk strain types among carbapenem non-susceptible clinical isolates of target gram-negative bacteria in Kenya.PLoS One. 2021 Feb 1;16(2 February).
Huber CA, Sartor AL, McOdimba F, Shah R, Shivachi P, Sidjabat HE et al. Outbreaks of multidrug-resistant Acinetobacter baumannii strains in a Kenyan teaching hospital. J Glob Antimicrob Resist [Internet]. 2014;2(3):190–3. Available from: https://www.sciencedirect.com/science/article/pii/S2213716514000393
Casini B, Righi A, de Feo N, Totaro M, Giorgi S, Zezza L et al. Improving cleaning and disinfection of high-touch surfaces in intensive care during carbapenem-resistant acinetobacter baumannii endemo-epidemic situations. Int J Environ Res Public Health. 2018 Oct 19;15(10).
Odoyo E, Kyanya C, Mutai W, Musila L. High levels of toxigenic Clostridioides difficile contamination of hospital environments: a hidden threat in hospital-acquired infections in Kenya. Access Microbiol. 2020;2(12).
Lashinsky JN, Henig O, Pogue JM, Kaye KS. Minocycline for the Treatment of Multi-drug and Extensively Drug-Resistant A. baumannii: A Review. Vol. 6, Infectious Diseases and Therapy. 2017. p.199–211.
Podschun R, Pietsch S, Höller C, Ullmann U. Incidence of Klebsiella Species in Surface Waters and their expression of virulence factors. Appl Environ Microbiol. 2001;67(7):3325–7.
Irek EO, Amupitan AA, Obadare TO, Aboderin AO. A systematic review of healthcare-associated infections in Africa: An antimicrobial resistance perspective. Afr J Lab Med. 2018;7(2).
Anderl JN, Zahller J, Roe F, Stewart PS. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2003;47(4):1251–6.
Jarvis WR, Munn VP, Highsmith AK, Culver DH, Hughes JM. The epidemiology of nosocomial infections caused by Klebsiella pneumoniae. Infect Control. 1985;6(2):68–74.
Fernández-Prada M, Martínez-Ortega C, Santos-Simarro G, Morán-Álvarez P, Fernández-Verdugo A, Costa-Romero M. Outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit: risk factors and key preventive measures for eradication in record time. An Pediatr (Engl Ed). 2019;91(1):13–20.
Artelt T, Kaase M, Bley I, Eiffert H, Mellmann A, Küster H et al. Transmission Risk on a Neonatal Intensive Care Unit: Escherichia coli versus Klebsiella pneumoniae. Canadian Journal of Infectious Diseases and Medical Microbiology. 2018;2018.
Ali S, Abbasi SA, Mirza IA, Khan IU, Fayyaz M, Hussain A, et al. Case Series Outbreak of Multidrug-Resistant Klebsiella Pneumoniae Sepsis in neonatal intensive care unit. Gomal J Med Sci. 2012;10(1):154–7.
Ministry of Health, Kenya. https://www.health.go.ke/ministry-of-health-issues-new-guidelines-targeting-multidrug-resistant-pneumonia/.
Apondi OE, Oduor OC, Gye BK, Kipkoech MK. High prevalence of multi-drug resistant Klebsiella Pneumoniae in a tertiary teaching hospital in western Kenya. Afr J Infect Dis. 2016;10(2):89–95.
Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-Type-lactamases. Antimicrob Agents Chemother. 2002;46(1):1–11.
Musila L, Kyany’a C, Maybank R, Stam J, Oundo V, Sang W. Detection of diverse carbapenem and multi-drug resistance genes and high-risk strain types among carbapenem non-susceptible clinical isolates of target gram-negative bacteria in Kenya. PLoS One [Internet]. 2021;16(2 February):1–18. Available from: https://doi.org/10.1371/journal.pone.0246937
Arnold RS, Thom KA, Sharma S, Phillips M, Kristie Johnson J, Morgan DJ. Emergence of Klebsiella pneumoniae carbapenemase-producing bacteria. South Med J. 2011;104(1):40–5.
Mavroidi A, Liakopoulos A, Gounaris A, Goudesidou M, Gaitana K, Miriagou V et al. Successful control of a neonatal outbreak caused mainly by ST20 multidrug-resistant SHV-5-producing Klebsiella pneumoniae, Greece. BMC Pediatr. 2014 Apr 17;14(1).
Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol. 2012;7(7):887–902.
Jacoby GA. AmpC Β-Lactamases. Clin Microbiol Rev. 2009;22(1):161–82.
Giorgia S, Benjamin D, Ada K, Gandra S, Amrita D, Jishnu D et al. Antibiotic overuse in the primary health care setting: a secondary data analysis of standardised patient studies from India, China and Kenya. BMJ Glob Health. 2020
Momanyi L, Opanga S, Nyamu D, Oluka M, Kurdi A, Godman B. Antibiotic prescribing patterns at a leading referral hospital in Kenya: a point prevalence survey. J Res Pharm Pract. 2019;8(3):149.
de Abreu PM, Farias PG, Paiva GS, Almeida AM, Morais PV. Persistence of microbial communities including Pseudomonas aeruginosa in a hospital environment: A potential health hazard. BMC Microbiol. 2014 May 8;14(1).
Sukhum K, Newcomer EP, Cass C, Wallace MA, Johnson C, Fine J et al. Antibiotic-resistant organisms establish reservoirs in new hospital built environments and are related to patient blood infection isolates. Communications Medicine. 2022 Jun 1;2(1).
Dharan S, Mourouga P, Copin P, Bessmer G, Tschanz B, Pittet D. Routine disinfection of patients’ environmental surfaces. Myth or reality? J Hosp Infect. 1999;42(2):113–7.
Pachori P, Gothalwal R, Gandhi P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019;6(2):109–19.
Spagnolo AM, Sartini M, Cristina ML. Pseudomonas aeruginosa in the health-care facility setting. Reviews in Medical Microbiology. 2021;32(3).
Acknowledgements
The authors appreciate the collaboration and support of the study hospital administration. The authors also appreciate Cliff Momanyi, Ruth Kiage, Mitsanze Thoya, Ruth Mupa, Charles Adega, Gladys Biwott, Alfred Odindo and Catherine Muriuki for sample collection.
Funding
This work was funded by the Armed Forces Health Surveillance Division (AFHSD), Global Emerging Infections Surveillance (GEIS) Branch [PROMIS ID 17_KY_1.3.1]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Funding acquisition: L.M. Conceptualization: L.M., E.O. Investigation: E. O., D.M., F.T., M.G., C. K., W. M. Data Analysis: E. O. and S.W. Writing – original draft: E. O. Writing – review and editing: E. O., L. M. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declared that they have no competing interests.
Ethical statement
This study was approved by the KEMRI Scientific and Ethical Review Board, protocol #3482, and the Walter Reed Army Institute of Research (WRAIR), protocol #2416, institutional review boards. Written approval was also obtained from the county and hospital administration. No human subjects or animals were involved in this study. Permission has been granted for publication of this manuscript by the Director KEMRI. Material has been reviewed by WRAIR. There is no objection to its publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the true views of the Department of Defense.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Odoyo, E., Matano, D., Tiria, F. et al. Environmental contamination across multiple hospital departments with multidrug-resistant bacteria pose an elevated risk of healthcare-associated infections in Kenyan hospitals. Antimicrob Resist Infect Control 12, 22 (2023). https://doi.org/10.1186/s13756-023-01227-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-023-01227-x