Abstract
Background
Nchelenge District in northern Zambia suffers from holoendemic malaria transmission despite a decade of yearly indoor residual spraying (IRS) and insecticide-treated net (ITN) distributions. One hypothesis for this lack of impact is that some vectors in the area may forage in the early evening or outdoors. Anopheles gibbinsi specimens were identified in early evening mosquito collections performed in this study area, and further insight was gleaned into this taxon, including characterizing its genetic identity, feeding preferences, and potential role as a malaria vector.
Methods
Mosquitoes were collected in July and August 2019 by CDC light traps in Nchelenge District in indoor sitting rooms, outdoor gathering spaces, and animal pens from 16:00–22:00. Host detection by PCR, COI and ITS2 PCR, and circumsporozoite (CSP) ELISA were performed on all samples morphologically identified as An. gibbinsi, and a subset of specimens were selected for COI and ITS2 sequencing. To determine risk factors for increased abundance of An. gibbinsi, a negative binomial generalized linear mixed-effects model was performed with household-level variables of interest.
Results
Comparison of COI and ITS2 An. gibbinsi reference sequences to the NCBI database revealed > 99% identity to “Anopheles sp. 6” from Kenya. More than 97% of specimens were morphologically and molecularly consistent with An. gibbinsi. Specimens were primarily collected in animal pen traps (59.2%), followed by traps outdoors near where humans gather (24.3%), and traps set indoors (16.5%). Host DNA detection revealed a high propensity for goats, but 5% of specimens with detected host DNA had fed on humans. No specimens were positive for Plasmodium falciparum sporozoites. Animal pens and inland households > 3 km from Lake Mweru were both associated with increased An. gibbinsi abundance.
Conclusions
This is the first report of An. gibbinsi in Nchelenge District, Zambia. This study provided a species identity for unknown “An. sp. 6” in the NCBI database, which has been implicated in malaria transmission in Kenya. Composite data suggest that this species is largely zoophilic and exophilic, but comes into contact with humans and the malaria parasites they carry. This species should continue to be monitored in Zambia and neighbouring countries as a potential malaria vector.
Similar content being viewed by others
Background
After 15 years of global reductions in malaria cases, progress has slowed since 2015, with an estimated 229 million malaria cases in 2019 [1]. Progress made has been heterogeneous across Africa, with some countries still experiencing a high burden of malaria, and others approaching elimination. In regions where major vectors have been successfully controlled, species that were once secondary vectors or had remained unrecognized have become an increasing concern, and may be responsible for more transmission than previously documented [2,3,4,5,6,7,8]. Many of these anopheline species tend to be exophilic or display behavioural plasticity in their foraging and resting behaviours, and may not choose humans as their preferential host [9, 10]. It is unclear if these species always contributed to transmission, or if they fill an open niche when primary vector populations are reduced [4, 5, 8, 10,11,12]. To fully understand the importance of these implicated vectors, their roles in transmission must be characterized not only when a region is close to elimination but when other well-studied anophelines are primarily driving transmission.
Nchelenge District is located in Luapula Province, northern Zambia and lies along the eastern shore of Lake Mweru. Many streams and tributaries in this district empty into the lake, creating a marshy ecology for malaria vector breeding sites that drives high year-round malaria transmission [13,14,15,16]. Well-recognized vectors in the area include Anopheles funestus sensu stricto (s.s.), with peak abundance during the dry season, and Anopheles gambiae s.s. with persistent low-level abundance. Both species reproduce year-round in this region [14]. High transmission in Nchelenge District has continued even after annual indoor residual house spraying (IRS) and multiple mass insecticide treated net (ITN) campaigns across the district, leading to the hypothesis that vectors may be biting outside or before people go under their mosquito nets at night [15,16,17,18].
To better characterize additional potential vectors, mosquito collections were performed to identify early-foraging anopheline species in Nchelenge District, Zambia, during the cool dry season in 2019. A large proportion of specimens collected as part of this study were Anopheles gibbinsi, a species which has not previously been reported in Zambia. Literature on this species is sparse, though it has been documented in the highlands of eastern Africa, from Ethiopia down to the Democratic Republic of the Congo (DRC) [2, 19,20,21,22,23]. Importantly, 7.7% of morphologically-identified An. gibbinsi captured in an early-foraging study in Kenya were determined to be positive for Plasmodium falciparum sporozoites, suggesting that this species is a vector of malaria [2]. In this study, further insight was gleaned into this understudied species, including characterizing its genetic identity, feeding preferences, and potential role as a malaria vector.
Methods
Study area and household selection
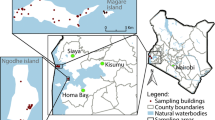
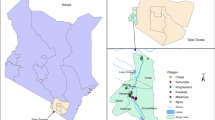
Data were collected in July and August 2019 in Nchelenge District, Zambia (Fig. 1). This region experiences three seasons: a rainy season from December to April, a cold dry season from May to August, and a hot dry season from September to November [14]. Nchelenge District lies along Lake Mweru and has several streams and tributaries that persist during the dry season, creating an expanse of marshy mosquito breeding sites.
Twenty-four households were selected for this study: twelve located < 3 km from the lake’s shore and twelve located > 3 km from the lake (lakeside and inland, respectively). Sampled households were required to have at least one animal pen outside of the main sleeping structure, and inland households were required to be located within 0.6 km of a major stream.
Environmental covariates
During the study visits, household coordinates were uploaded into ArcGIS Pro (ESRI, Redlands, CA, USA). Streams were previously mapped and categorized [17], and distance to the nearest major stream was calculated using the “near” tool in ArcGIS Pro (ESRI, Redlands, CA, USA).
Entomological sampling
Three lakeside and three inland households were sampled each day, and a Latin-square method was used to rotate the trap placement in each sampled household [24]. Mosquitoes were collected using miniature CDC Light Traps (John W. Hock Co., Gainesville) from 16:00 to 22:00. The head of household was provided a watch at the time of set up and was asked to turn the trap on at 16:00 and tie the collection bag at 22:00. Traps were hung between 1.5: 1.8 m above the ground, either in the indoor sitting room of a household, an outdoor area where people gather in the evening, or outdoors next to an animal pen. Other than the incandescent light on the trap and any people or animals that spent time near the trap, no other lures were used. Surveys regarding household features and human behaviour were conducted at the time of enrollment and additionally for each trap collection. The head of household answered all the enrollment questions, and if they were not present for the follow-up questionnaire, the spouse or eldest child responded.
Mosquito processing
Captured mosquitoes were killed by freezing, morphologically identified using Gillies and Coetzee [25], and stored individually in a microfuge tube on silica gel to desiccate in the field. Desiccated samples were returned to the Johns Hopkins Bloomberg School of Public Health (BSPH) in Baltimore, Maryland, USA, where each specimen was split into head/thorax and abdomen. Prior to splitting, 17 samples that were morphologically identified as An. gibbinsi were sent to the University of the Witwatersrand to confirm morphological identity.
DNA from female anopheline mosquito abdomens were crushed in lysis buffer using a Qiagen TissueLyser II (Qiagen, Hilden, Germany) and extracted using an automated DNA extraction method with a QIACube HT (Qiagen, Hilden, Germany) at Purdue University [26]. After DNA extraction, each specimen underwent a PCR assay to amplify a fragment of the internal transcribed spacer region 2 (ITS2) of the nuclear genome as described previously [27,28,29]. A random representative subset of 5.4% of An. gibbinsi specimens were selected for Sanger sequencing of the ITS2 target and the Barcode of Life fragment of the cytochrome oxidase I (COI) gene as previously described [27,28,29]. Sequencing for specimens returned to BSPH was conducted at the Johns Hopkins Medical Institutions (JHMI) Synthesis and Sequencing Facility. Forward and reverse sequences were imported into Geneious Prime (version 2021.2.2, Biomatters, Ltd, Auckland, New Zealand, https://www.geneious.com), trimmed to remove low-quality reads and primer sequences, and aligned to create a consensus sequence for each specimen. Consensus sequences were compared to the National Center for Biotechnology Information (NCBI) database and reference samples using BLASTn, and final identifications were confirmed if they had > 99% identity to an NCBI sequence. Data were submitted to NCBI’s GenBank and accession numbers were acquired for both ITS2 (OM459737-OM459768) and COI (OM456780-OM456806) sequences.
Host detection analyses
A host DNA detection assay was created to determine host preference. Primers from Kent & Norris [30], Izadpanah et al. [31], and Kumar et al. [32] were combined into a multiplexed PCR assay to detect individual and mixed blood meals from human, cow, pig, dog, chicken, and goat DNA, producing differential product sizes for each host animal (Additional file 1: Table S1). Each 25 μL PCR reaction consisted of 1 × buffer, 1.0 mM dNTPs, 0.625 units of Taq polymerase (New England Biolabs #M0273S), 50 pmol of each primer, and 1.0 μL extracted abdomen DNA (Additional file 1: Table S1). Thermocycler conditions consisted of an initial denaturation of 5 min at 95 °C, followed by 35 cycles at 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 45 s. The final extension step was at 72 °C for 5 min. 12.0 μL of product was then run on an agarose gel for visualization and host determination.
Detection of sporozoites
Head/thoraces were homogenized at BSPH in a buffer of boiled casein and Nonidet P-40 [18]. Circumsporozoite protein (CSP) ELISAs were performed using controls and protocols from BEI Resources Malaria Research and Reference Reagent Resource Center (MR4) to detect the presence of CSP from P. falciparum sporozoites [18, 28]. Samples were run in duplicate pools of five mosquito homogenates for the first ELISA, and then run individually in duplicate if the pool was positive as previously described [3, 29, 33]. Specimens were considered ELISA positive if the absorbance of the well for the individual mosquito was two times the absorbance of a negative insectary control mosquito.
Risk factor analysis
Ten of the 216 collections were excluded from the analysis because the battery failed on the trap while it was running (n = 6), or the head of household forgot to tie the collection bag, potentially allowing for escaped anophelines (n = 4). A univariate negative binomial generalized linear mixed-effects model was performed with number of An. gibbinsi per trap as the outcome for all variables of interest using the glmer.nb function from the MASS package in R. For each model, a household-level random intercept term was included to account for the repeated household visits. Multivariate models were also performed using the glmer.nb function from MASS with a household-level random intercept term. The best model was selected using Akaike information criterion (AIC).
Results
Anopheles gibbinsi distribution
Each of the enrolled 24 households was visited 9 times, resulting in 216 trap nights. Of the 3091 female anophelines collected in this study, An. funestus was collected at the highest abundance (n = 2177, 70.4%), followed by An. gibbinsi (n = 453, 14.6%). Anopheles gibbinsi were collected from 15 of 24 (62.5%) study households, and made up 33.7% (n = 267) animal pen traps, 18.3% (n = 110) traps outdoors where humans gather, and 4.5% (n = 76) indoor sitting room traps (Table 1). Among animal traps, 88% (n = 235) were captured in goat pens (Additional file 1: Table S2), and the majority of An. gibbinsi were collected in inland households (n = 447, 98.7%) (Fig. 1).
Molecular confirmation
Three samples that had all identifying features (i.e., no missing tarsi, all wing scales present, wings, and proboscis intact) were morphologically confirmed as An. gibbinsi at the BSPH and through photographs sent to University of the Witwatersrand (Additional file 1: Figure S1). COI and ITS2 PCRs were performed on these samples and sequenced. The product size (including primers) from the ITS2 PCR for all three samples was 507 base pairs, and the COI product size was 709 base pairs. All three ITS2 sequences were 100% identical to each other, and COI sequences were 98.9% identical to each other. The NCBI BLAST result for all ITS2 and COI sequences was “An. sp. 6” with > 99% identity for every specimen (Additional file 1: Table S3). These three samples were used as An. gibbinsi reference samples for the remaining study (OM459761-OM459763, OM56804-OM56806).
Of the 17 samples sent to the University of the Witwatersrand, seven were morphologically confirmed as An. gibbinsi. Two samples were identified as Anopheles marshallii complex, one was identified as An. funestus, and six were unable to be confirmed due to loss of legs. ITS2 PCR and sequencing was successful on 5 of 7 that were confirmed as An. gibbinsi, and all matched the reference samples with 100% identity.
Morphological accuracy
Four hundred and fifty-three An. gibbinsi were morphologically identified. An ITS2 PCR was performed on all 453 samples, and 443 (97.8%) produced a 507 bp band on electrophoretic analysis, confirming the An. gibbinsi identification. Five (1.1%) samples were molecularly identified as An. funestus s.s., two (0.4%) samples had an ITS2 PCR amplicon size of 439 bp and remain unidentified to species level after sequencing, and three (0.7%) samples failed amplification after three attempts. Twenty-four An. gibbinsi samples (5.4%) representing all three trap types were selected to undergo ITS2 and COI fragment sequencing to confirm species identity. All ITS2 sequences matched the reference samples with 100% identity, and COI sequences matched reference samples with > 99% identity. All specimens had a NCBI BLAST result of “An. sp. 6” with > 99% identity for both COI and ITS2 fragments (Additional file 1: Table S3).
Host preference and parasite detection
A PCR assay to detect human, goat, cow, dog, pig, and chicken DNA was performed on 417/443 (94.1%) molecularly confirmed An. gibbinsi samples to assess host preference. Twenty-six samples were excluded because they did not have an intact abdomen. Host DNA was detected in 83 (19.9%) samples. Sixty three of 417 (15.1%) samples were recorded as visually blood fed during morphology, and host DNA was detected in 54 (85.7%) of those specimens (Additional file 1: Table S4). Additionally, host DNA was detected in 29/354 (8.2%) that were not recorded as visually blooded (Additional file 1: Table S4). Of the An. gibbinsi samples positive for host DNA, goat DNA was detected in 71/83 (85.5%) (Table 2). Other hosts detected were pig (n = 7, 8.2%), human (n = 4, 4.8%), and dog (n = 1, 1.2%). The trap type with the highest proportion of specimens with host DNA were from traps placed near animal pens (76/246, 30.8%), followed by traps placed indoors (5/63, 7.9%), and lastly from traps placed near gathering locations (2/103, 1.9%) (Table 2). CSP ELISAs were performed on all 443 samples, but none were positive for sporozoites.
Risk factor analysis
In the univariate analysis, household level risk factors associated with a higher abundance of An. gibbinsi were inland households, animal pens, using an open well or stream/pond for a water source, households with natural wall materials, and not receiving IRS in the previous campaign (October 2018) (Table 3). Increased distance from large streams and increased number of rooms in a household were associated with decreased abundance of An. gibbinsi (Table 3). Many of these variables were correlated. For example, inland households were associated with decreased distance to streams, smaller households, and using a stream or pond as a water source, so only one of these variables was included in the final multivariate model. The multivariate model with the lowest AIC included only household location and trap location, and revealed inland households and animal pen traps remained associated with increased counts of An. gibbinsi (Table 3).
Discussion
Prior to this study, An. gibbinsi had not been reported in Zambia, very little was known about this species’ foraging behaviours, and no genetic data were associated with this taxon. Perhaps the most unexpected finding is that the ITS2 and COI fragments sequenced from morphologically confirmed An. gibbinsi matched with 100% identity to “An. sp. 6” data in GenBank from other published work [8, 18]. Identifying An. sp. 6 as An. gibbinsi provides more context for this understudied taxon, as findings from the existing literature can be linked to create a composite understanding of its behaviour and vector potential. For example, An. sp. 6 was molecularly identified in Nchelenge District in a study from 2016, but morphology did not identify it as An. gibbinsi, due to damage to the specimen [18]. In Kenya, specimens morphologically identified as both An. gambiae and An. funestus were only molecularly identified as An. sp. 6 [34], illustrating the challenges of identifying these uncommon and often unknown taxa [8]. Generating reference sequences for morphologically confirmed specimens will be invaluable as Anopheles species identification continues to incorporate high-throughput molecular techniques and genomic data.
The most common species misidentification was with An. funestus (1.1%) followed by an unidentified species (0.4%). This is similar to published reports that found 16/25 (64%) of An. sp. 6 morphologically identified as An. funestus, and 9/25 (36%) identified as An. gambiae [34]. Misidentification of anophelines is a common problem when using only morphology, especially when samples are damaged from trapping methods. This underscores the need for more training in morphological identification, and for the development of reliable genetic references for comparison across anopheline taxa.
In this study, 60% of An. gibbinsi specimens were caught near animal pens, and goat DNA was detected in 85.5% of all An. gibbinsi specimens with detectable host DNA. Additionally, traps near animal pens had the highest proportion of An. gibbinsi with host blood compared to trap placements near humans or sheltered/indoor locations, suggesting that this species is largely zoophilic and exophilic. However, 16.3% (n = 73) of specimens were caught indoors, and 5.4% (n = 4) of those were found with a human blood meal. This is similar to findings from the Kenyan highlands where 2/11 (18.%) of An. sp. 6 harboured human blood meals [34]. While this species appears to be largely zoophilic and exophilic, opportunistic feeding on humans and occasionally indoors may not be unusual, as similarly reported for other secondary and understudied malaria vectors, including Anopheles coustani, Anopheles rufipes, and Anopheles squamosus [4, 6, 10, 11].
Given the high proportion of goat blood meals in the samples, it is unsurprising that animal pens were associated with a higher abundance of An. gibbinsi than traps placed indoors or near where people gather outdoors in both the univariate and multivariate analysis. Inland households, increased proximity to streams, and using an open well/pond were also associated with higher counts of An. gibbinsi. These suggest that An. gibbinsi may be using slow moving streams and rivers as breeding sites in Nchelenge District during the dry season. However, considering that inland households were specifically selected because of their proximity to potential breeding sites, it is possible there is another explanation for these associations that was not captured in this study. Additionally, walls made from natural materials were also associated with higher An. gibbinsi counts. Natural wall materials and using a stream or pond as a water source compared to a bore hole may indicative of lower socioeconomic status or temporary housing, which may also impact mosquito densities.
This study did not detect any An. gibbinsi specimens positive for P. falciparum sporozoites, but parasite-positive An. sp. 6 (2/27, 7.4%) were reported from the Kenyan highlands found using a multiplexed qPCR method [34]. Additionally, another study from the Kenyan highlands found 7.7% of morphologically-identified An. gibbinsi positive for sporozoites by CSP ELISA [2]. Given the high transmission in Nchelenge District and potential for An. gibbinsi to serve as a vector, it is important that this species be included in ongoing and future malaria surveillance. Future studies should also assess vector competence with live field-captured mosquitoes to more fully understand the capacity for this species to transmit malaria parasites.
Conclusion
This study documented An. gibbinsi as an anopheline species present in the dry season of 2019 in Nchelenge District, Zambia: the first report of this anopheline species from Zambia. Comparison of COI and ITS2 sequences to NCBI’s GenBank database revealed > 99% identity to An. sp. 6, which has been implicated in malaria transmission in Kenya [2, 34]. Most specimens were captured near animal pens, and host DNA detection revealed a propensity for goats. Although this finding may be skewed by the collection method, composite data suggest that this species is largely zoophilic and exophilic. However, 5% of specimens with detected host DNA had fed on humans, indicating that this potential vector species is likely to ingest human malaria parasites. The vector competence of An. gibbinsi, as supported by reports from Kenya, suggest that this species should continue to be monitored in Nchelenge District. Importantly, this study also provides genetic references for An. gibbinsi, which will help inform future studies as molecular identification and verification become more common in malaria entomology.
Availability of data and materials
The datasets used in the present study are not publicly available to protect the confidentiality and privacy of study participants, but are available from the corresponding author upon appropriate reasonable request and approval from the corresponding national research and ethics committee.
Abbreviations
- IRS:
-
Indoor residual spray
- ITN:
-
Insecticide treated net
- CSP:
-
Circumsporozoite protein
- CDC LT:
-
Center for disease control light trap
- DRC:
-
Democratic Republic of Congo
- BSPH:
-
Johns Hopkins Bloomberg School of Public Health
- ITS2:
-
Internal transcribed spacer 2
- COI:
-
Cytochrome oxidase I
- PCR:
-
Polymerase chain reaction
- JHMI:
-
Johns Hopkins Medical Institute
- MR4:
-
Malaria Research and Reference Reagent Resource Center
- ELISA:
-
Enzyme-linked immunoabsorbant assay
- AIC:
-
Akaike information criterion
- JHMRI:
-
Johns Hopkins Malaria Research Institute
- NCBI:
-
National Center for Biotechnology Information
References
WHO. World malaria report. 20 years of global progress and challenges. Geneva: World Health Organization; 2020. p. 2020.
Cooke MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, Mabuka D, et al. ‘A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015;14:259.
Stevenson JC, Simubali L, Mbambara S, Musonda M, Mweetwa S, Mudenda T, et al. Detection of Plasmodium falciparum infection in Anopheles squamosus (Diptera: Culicidae) in an area targeted for malaria elimination. Southern Zambia J Med Entomol. 2016;53:1482–7.
Antonio-Nkondjio C, Kerah CH, Simard F, Awono-Ambene P, Chouaibou M, Tchuinkam T, et al. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol. 2006;43:1215–21.
Mustapha AM, Musembi S, Nyamache AK, Machani MG, Kosgei J, Wamuyu L, et al. Secondary malaria vectors in western Kenya include novel species with unexpectedly high densities and parasite infection rates. Parasit Vectors. 2021;14:252.
Fornadel CM, Norris LC, Franco V, Norris DE. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.I. and Anopheles squamosus in Macha Zambia. Vector Borne Zoonotic Dis. 2011;11(1173):9.
Burke A, Dandalo L, Munhenga G, Dahan-Moss Y, Mbokazi F, Ngxongo S, et al. A new malaria vector mosquito in South Africa. Sci Rep. 2017;7:43779.
St. Laurent B, Cooke M, Krishnankutty SM, Asih P, Mueller JD, Kahindi S, et al. Molecular characterization reveals diverse and unknown malaria vectors in the western Kenyan highlands. Am J Trop Med Hyg. 2016;94:327–35.
Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330.
Chaccour C, Killeen GF. Mind the gap: residual malaria transmission, veterinary endectocides and livestock as targets for malaria vector control. Malar J. 2016;15:2.
Burke A, Dahan-Moss Y, Duncan F, Qwabe B, Coetzee M, Koekemoer L, et al. Anopheles parensis contributes to residual malaria transmission in South Africa. Malar J. 2019;18:7.
Gebhardt ME, Searle KM, Kobayashi T, Shields T, Hamapumbu H, Simubali L, et al. Understudied anophelines contribute to malaria transmission in a low-transmission setting in Choma District, Southern Province Zambia. Am J Trop Med Hyg. 2022;106:1406.
Das S, Muleba M, Stevenson JC, Pringle JC, Norris DE. Beyond the entomological inoculation rate: characterizing multiple blood feeding behavior and Plasmodium falciparum multiplicity of infection in Anopheles mosquitoes in northern Zambia. Parasit Vectors. 2017;10:45.
Das S, Muleba M, Stevenson JC, Norris DE. Habitat partitioning of malaria vectors in Nchelenge District Zambia. Am J Trop Med Hyg. 2016;94:1234–44.
Stevenson JC, Pinchoff J, Muleba M, Lupiya J, Chilusu H, Mwelwa I, et al. Spatio-temporal heterogeneity of malaria vectors in northern Zambia: implications for vector control. Parasit Vectors. 2016;9:510.
Hast MA, Chaponda M, Muleba M, Kabuya JB, Lupiya J, Kobayashi T, et al. The impact of three years of targeted IRS with pirimiphos-methyl on malaria parasite prevalence in a high-transmission area of northern Zambia. Am J Epidemiol. 2019;188:2120–30.
Hast MA, Stevenson JC, Muleba M, Chaponda M, Kabuya JB, Mulenga M, et al. Risk factors for household vector abundance using indoor CDC light traps in a high malaria transmission area of Northern Zambia. Am J Trop Med Hyg. 2019;101:126–36.
Jones C, Ciubotariu I, Muleba M, Lupiya J, Mbewe D, Simubali L, et al. Multiple novel clades of anopheline mosquitoes caught outdoors in Northern Zambia. Front Trop Dis. 2021. https://doi.org/10.3389/fitd.2021.780664.
Okara RM, Sinka ME, Minakawa N, Mbogo CM, Hay SI, Snow RW. Distribution of the main malaria vectors in Kenya. Malar J. 2010;9:69.
Bafort JM. Anopheles marshalli s. I. a secondary vector of malaria in Africa. Trans R Soc Trop Med Hyg. 1985;79:566–7.
Evans AM. Notes on Anophelines. Ann Trop Med Parasitol. 1935;29:469–73.
Steyn JJ. The effect of cultivation of swamps on the anopheline fauna in Kigezi district. Uganda J Entomol Soc Southern Africa. 1948;11:76–82.
Gillies MT, Meillon BD. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). Johannesburg: South African Institute for Medical Research; 1968.
Latin Square Designs. The Concise encyclopedia of statistics. New York: Springer New York; 2008.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). Johannesburg: South African Institute for Medical Research; 1987.
Dorman J, Ciubotariu I, Levy M, Fola A, Carpi G. High-throughput gDNA extraction of mosquito tissues using QIAcube HT Protcols.io2020 updated July 1, 2020. https://www.protocols.io/view/high-throughput-gdna-extraction-of-mosquito-tissue-bhgcj3sw. Accessed Jan 26 2022.
Lobo NF, Laurent BS, Sikaala CH, Hamainza B, Chanda J, Chinula D, et al. Unexpected diversity of Anopheles species in Eastern Zambia: implications for evaluating vector behavior and interventions using molecular tools. Sci Rep. 2015;5:17952.
Ciubotariu II, Jones CM, Kobayashi T, Bobanga T, Muleba M, Pringle JC, et al. Genetic diversity of Anopheles coustani (Diptera: Culicidae) in malaria transmission foci in Southern and Central Africa. J Med Entomol. 2020;57:1782–92.
Hoffman JE, Ciubotariu II, Simubali L, Mudenda T, Moss WJ, Carpi G, et al. Phylogenetic complexity of morphologically identified Anopheles squamosus in Southern Zambia. Insects. 2021;12:146.
Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed Polymerase Chain Reaction targeting Cytochrome B. Am J Trop Med Hyg. 2005;73:336–42.
Izadpanah M, Mohebali N, Elyasi Gorji Z, Farzaneh P, Vakhshiteh F, Shahzadeh Fazeli SA. Simple and fast multiplex PCR method for detection of species origin in meat products. J Food Sci Technol. 2018;55:698–703.
Kumar A, Kumar RR, Sharma BD, Mendiratta SK, Gokulakrishnan P, Kumar D, et al. Authentication of goat (Capra hircus) meat using PCR amplification of mitochondrial cytochrome b gene. Small Rumin Res. 2015;131:17–20.
Jones CM, Lee Y, Kitchen A, Collier T, Pringle JC, Muleba M, et al. Complete Anopheles funestus mitogenomes reveal an ancient history of mitochondrial lineages and their distribution in southern and central Africa. Sci Rep. 2018;8:9054.
Zhong D, Hemming-Schroeder E, Wang X, Kibret S, Zhou G, Atieli H, et al. Extensive new Anopheles cryptic species involved in human malaria transmission in western Kenya. Sci Rep. 2020;10:16139.
Acknowledgements
The authors gratefully acknowledge the Southern Africa ICEMR field teams in Nchelenge and the Tropical Diseases Research Centre (TDRC) for their logistical support and participation in field collections. The authors are also very grateful to the communities in Nchelenge District, Zambia, for their participation with this study. The authors would also like to thank Jack Dorman and Dr. Giovanna Carpi at Purdue University for performing the abdominal DNA extractions. The reagents for the CSP ELISAs were obtained through BEI Resources, National Institute of Allergy and Infectious Diseases, National Institutes of Health. The Plasmodium falciparum Sporozoite ELISA Reagent Kit, MRA-890, was contributed by Robert A. Wirtz. The authors are grateful to Zandile Langa (from the Wits Research Institute for Malaria, Faculty of Health Sciences, University of the Witwatersrand, and the Centre for Emerging Zoonotic & Parasitic Diseases, Vector Control Reference Laboratory, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa) for her assistance with the ITS2 PCR.
Funding
This work was supported in part by funding from the National Institutes of Health International Centers of Excellence for Malaria Research (U19AI089680), Bloomberg Philanthropies, and the Johns Hopkins Malaria Research Institute (JHMRI), and a JHMRI pre-doctoral fellowship to MEG. LLK is supported by a DST/NRF South African Research Chairs Initiative Grant (UID 64763).
Author information
Authors and Affiliations
Consortia
Contributions
MEG, DEN, and JCS conceived and designed the study. MEG and MM coordinated and supervised field collections performed by JSL, DM, and MEG. RSK and MEG completed all laboratory assays, and MEG performed the statistical analysis. MC morphologically identified samples sent to University of the Witwatersrand, and LLK and YMD prepared and sequenced those samples. MEG, DEN, WJM, RSK, MC, and LLK drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance and approval were obtained from the Institutional Review Boards of the Tropical Diseases Research Center in Zambia and the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland. Informed consent for household participation in the study was given by the head of household at the time of enrollment.
Animal ethics declaration
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Identifying features (Coetzee 2020) of an An. gibbinsi sample that was molecularly confirmed as An. species 6. Table S1. Primers included in Host DNA PCR. Table S2. Sequenced An. gibbinsi samples. Table S3. Host DNA detection by visually blooded status.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gebhardt, M.E., Krizek, R.S., Coetzee, M. et al. Expanded geographic distribution and host preference of Anopheles gibbinsi (Anopheles species 6) in northern Zambia. Malar J 21, 211 (2022). https://doi.org/10.1186/s12936-022-04231-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04231-5