Abstract
Background
The efficacies of artemisinin based combinations have been excellent in Africa, but also comprehensive evidence regarding their safety would be important. The aim of this review was to synthesize available evidence on the safety of dihydroartemisinin-piperaquine (DHA-PQ) compared to artemether-lumefantrine (AL) for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa.
Methods
A systematic literature search was done to identify relevant articles from online databases PubMed/ MEDLINE, Embase, and Cochrane Center for Clinical Trial database (CENTRAL) for retrieving randomized control trials comparing safety of DHA-PQ and AL for treatment of uncomplicated P. falciparum malaria among children in Africa. The search was performed from August 2020 to 30 April 2021. Using Rev-Man software (V5.4.1), the extracted data from eligible studies were pooled as risk ratio (RR) with 95% confidence interval (CI).
Results
In this review, 18 studies were included, which involved 10,498 participants were included. Compared to AL, DHA-PQ was associated with a slightly higher frequency of early vomiting (RR 2.26, 95% CI 1.46 to 3.50; participants = 7796; studies = 10; I2 = 0%, high quality of evidence), cough (RR 1.06, 95% CI 1.01 to 1.11; participants = 8013; studies = 13; I2 = 0%, high quality of evidence), and diarrhoea (RR 1.16, 95% CI 1.03 to 1.31; participants = 6841; studies = 11; I2 = 8%, high quality of evidence) were more frequent in DHA-PQ treatment arm.
Conclusion
From this review, it can be concluded that early vomiting, diarrhoea, and cough were common were significantly more frequent in patients who were treated with the DHA-PQ than that of AL, and both drugs are well tolerated. More studies comparing AL with DHA-PQ are needed to determine the comparative safety of these drugs.
Similar content being viewed by others
Background
Malaria is the major cause for vast majority of deaths among children under the age of five [1,2,3]. In 2019, an estimated 229 million cases were reported globally from 87 malaria endemic countries [3], of which 215 million cases were reported in the World Health Organization (WHO) African Region [3]. The risk of malaria infections among children aged under five years was higher in 2018, and Plasmodium falciparum parasite were responsible for an estimated 24 million malaria cases in African children [1].
All African counties, where P. falciparum malaria is endemic, have introduced the recommended artemisinin-based combination therapy (ACT) in the confirmed cases of P. falciparum malaria since 2004 [1]. The artemisinin component is active against the asexual stage of the parasite responsible for the disease, but also the sexual stages of the parasite involved in the transmission to mosquitoes. The partner drug with a longer half-life eliminates the residual parasite over several weeks post treatment [4]. Artemisinin and partner drugs protect each other to prevent resistance development [5,6,7,8].
The efficacies of artemisinin-based combinations have been excellent in Africa [9, 10]. Artemether-lumefantrine (AL) is one of the most commonly used combinations in sub-Saharan Africa. It is the first-line treatment for uncomplicated malaria in several countries [11, 12]. AL showed good safety and tolerability profile [10, 13, 14]. Hence, previous reviews reported mild or moderate severity adverse event of gastrointestinal and nervous systems in patients who were treated with AL [15] and prolongation of the QTc interval; pyrexia, early vomiting, and diarrhoea were common in patients treated with DHA-PQ [16].
In the majority of African countries, the first-line treatment for uncomplicated malaria is generally AL or AS/AQ, with DHA-PQ as a second-line treatment in many countries [11, 12]. Most of the previous studies have compared the efficacies of AL and other artemisinin-based combinations [17, 18], but also comprehensive evidence regarding their safety would be important. Given the wide range of ACT available for treatment the malaria and their potential adverse events (AEs), it is vital to compare their safety profiles. This systematic review and meta-analysis was, therefore, to synthesize available evidence on the safety of dihydroartemisinin-piperaquine compared to artemether-lumefantrine for the treatment of uncomplicated P. falciparum malaria among children in Africa.
Methods
This protocol has been registered at the International Prospective Register of Systematic Reviews (PROSPERO) database, ID: CRD42020200337 [19]. The methods and findings of the review have been reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) [20].
Eligibility criteria
The PICOS format was used to identify eligible studies [21].
Participants
Children having uncomplicated falciparum malaria residing in Africa, regardless of gender, were included.
Interventions
A target dose (range) of 4 (2–10) mg/kg bw per day dihydroartemisinin and 18 (16–27) mg/kg bw per day piperaquine given once a day for 3 days for children weighing ≥ 25 kg. The target doses and ranges for children weighing < 25 kg are 4 (2.5–10) mg/kg bw per day dihydroartemisinin and 24 (20–32) mg/kg bw per day piperaquine once a day for 3 days.
Comparator
The 1:6 fixed dose combination tablet consisting artemether (20 mg) and lumefantrine (120 mg).
The body weight-adjusted dosages used have been: 25–35 kg, 3 tablets per dose: 15 to 25 kg, 2 tablets per dose; and < 15 kg, 1 tablet.
The medication administered twice a day for three days (total six doses). The first two doses taken eight hours apart; the third dose is taken after 24 h the first and then every 12 h on days 2 and 3.
Outcome measures
Adverse events including serious adverse events were also assessed. An adverse event (AE) was defined as any unfavourable, unintended sign, symptom, syndrome, or disease that develops or worsens with the use of a medicinal product, regardless of whether it is related to the actual medicinal product. A serious AE was defined as any untoward medical occurrence that at any dose; resulted in death; was life threatening; requiring hospitalization or prolongation of hospitalization; resulted in a persistent or significant disability or incapacity; or caused a congenital anomaly or birth defect [22].
Studies
Randomized controlled trials conducted in Africa which compared the safety of DHA-PQ versus AL for the treatment of uncomplicated falciparum malaria in children, written in English, and published between 2004 to April 2021 were included.
Electronic searches
A systematic literature search was done to identify relevant articles from online databases PubMed/ MEDLINE, Embase, and Cochrane Center for Clinical Trial database (CENTRAL). The search was limited to human trials, randomized control trials, and published between 2004 and April 2021. The search was done according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions [21]. Additionally, to search and assess ongoing or unpublished trials, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform, and the US Food and Drug Administration (FDA) were searched.
The search strategies in PubMed for the MeSH terms and text words was "Child"[Mesh]) AND "Plasmodium falciparum"[Mesh]) OR "Acute malaria" [Supplementary Concept]) OR "Artemether, Lumefantrine Drug Combination/therapeutic use"[Mesh]) OR "Lumefantrine"[Mesh]) OR "dihydroartemisinin" [Supplementary Concept]) OR "piperaquine" [Supplementary Concept]) OR ("Randomized Controlled Trial" [Publication Type] OR "Randomized Controlled Trials as Topic"[Mesh] OR "Controlled Clinical Trial" [Publication Type])) AND ("Drug Therapy"[Mesh] OR "Drug Therapy, Combination"[Mesh] OR "drug therapy" [Subheading])) AND ("Africa"[Mesh] OR "Africa South of the Sahara"[Mesh] OR "Africa, Western"[Mesh] OR "Africa, Southern"[Mesh] OR "Africa, Northern"[Mesh] OR "Africa, Eastern"[Mesh] OR "Africa, Central"[Mesh]. The searching strategies for Cochrane Center for Clinical Trial database (CENTRAL) and Embase are found in Additional file 1.
Study selection, data collection, and data analysis
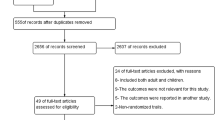
The Cochrane Handbook for Systematic Reviews of Interventions [23] was followed. Furthermore, the software package provided by Cochrane (RevMan 5.4.1) was used. To import the research articles from the electronic databases and remove duplicates, ENDNOTE software version X7 was used. Two authors independently review the results of the literature search and obtained full-text copies of all potentially relevant trials. Disagreements were resolved through discussion. When clarification was necessary, the trial authors were contacted for further information. The screening and selection process was reported in a PRISMA flow chart (Fig. 1).
Data extraction and management
The title and abstract was produced from the electronic search, and was independently screened by two authors based on RCTs that were assessed human P. falciparum malaria. The information collected were trial characteristics including methods, participants, interventions, and outcomes as well as data on dose and drug ratios of the combinations. Also, relevant information such as title, journal, year of publication, publication status, study design, study setting, malaria transmission intensity, follow-up period, sample size, funding of the trial or sources of support, baseline characteristics of study subjects and adverse events including serious AEs were extracted from each article using the well-prepared extraction format in the form of a table adapted from Cochrane and modified to make suitable for this study.
Furthermore, the number of participants randomized, and the number analysed in each treatment group for each outcome were also collected. One author independently extracted data and information collected was cross-checked by another investigator. The number of participants experiencing the event and the number of participants in each treatment group were documented.
Assessment of risk of bias in included studies
The risk of bias for each trial was evaluated by two review authors independently using the Cochrane Collaboration's tool for assessing the 'Risk of bias' [21]. To decrease the risk of bias amongst six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias, this guidance were used. The risks were classified as high risk, unclear risk, and low risk.
Measures of treatment effect
The main outcomes in this review were total of patients who experienced one or more adverse events. A number of patients with AEs from the studies were combined and presented using risk rations accompanied by 95% CIs.
Assessment of heterogeneity
Heterogeneity among the included trials was assessed by inspecting the forest plots and the Cochrane Q and I2 statistic used to measure heterogeneity among the trials in each analysis, the Chi2 test with a P < 0.10 to indicate statistical significance was used, and the results were interpreted following Cochrane Handbook for Systematic Reviews of Interventions Version 6.0, Chapter 10: Analyzing data and undertaking meta-analyses [24].
Assessment of reporting bias
To assess the possibility of publication bias, funnel plots for asymmetry (Egger’s test P < 0.05) were used [25].
Data synthesis
The meta-analyses was done consistent with the recommendations of Cochrane [23]. To aid interpretation, identity codes were given to included trials together with the first author, year of publication, and three first letter of the country where the trial being conducted. Trials were shown in forest plots in chronological order of the year the trials were published. A random-effects model was used, as trials were done by different researchers, operating independently, and it could be implausible that all the trials had functionally equivalence, with a common effect estimate.
Sensitivity analysis
To investigate the strength of the methodology used in the primary analysis and to restore the integrity of the randomization process, a series of sensitivity analyses were conducted using following steps were used: adding and excluding trials which were classified as high risk for bias back into the analysis in a stepwise fashion, and to assess the influence of small-study effects on the results of our meta-analysis, fixed-effect and random-effects estimates of the intervention effect were compared.
Quality of evidence
Quality of evidence was assessed using GRADE criteria and the GRADE pro software [26]. The results were presented in a ‘Summary of Findings’ table. Randomized trials are initially categorized as high quality but downgraded after assessment of five criteria [27]. The levels of evidence were defined as 'high', 'moderate', 'low', or 'very low'. The recommendations of Section 8.5 and Chapter 13 of the Cochrane Handbook for Systematic Reviews of Interventions was followed [28]. The imprecision was judged based on the optimal information size criteria and CI [29].
Results
A total of 3211 studies through the databases were searched, of which 49 full-text trials for eligibility were assessed and 18 of them fulfilled the inclusion criteria for meta-analysis and for qualitative analysis (see Fig. 1).
Characteristics of included studies
In this review, 18 studies were included, which enrolled 10,498 participants with uncomplicated P. falciparum malaria were included Table 1.
Characteristics of excluded studies
Thirty one studies were excluded with reason, Additional file 2.
Methodological quality and risk of bias
The 'Risk of bias' assessments were summarized in Fig. 2.
Adverse events
Gastrointestinal adverse events
Early vomiting
The relative risk of early vomiting in patients treated with the DHA-PQ was higher than AL (RR 2.26, 95% CI 1.46 to 3.50; participants = 7796; studies = 10; I2 = 0%, high quality of evidence, Fig. 3).
Publication bias
The funnel plot showed that all studies lay symmetrically around the pooled effect estimate implying that there was no publication bias (P = 0.5, Additional file 3).
Diarrhoea
Similarly, the relative risk of early vomiting in patients treated with the DHA-PQ was higher than AL (RR 1.16, 95% CI 1.03 to 1.31; participants = 6841; studies = 11; I2 = 8%, high quality of evidence, Fig. 3).
Publication bias
The funnel plot showed that all studies lay symmetrically around the pooled effect estimate implying that there was no publication bias (P = 0.9, Additional file 4).
Other gastrointestinal adverse events
The risk of vomiting did not have significant difference between the two treatment groups (RR 1.02, 95% CI 0.87 to 1.19; participants = 8789; studies = 13; I2 = 20%, high quality of evidence, Fig. 4). Similarly, there was no significant difference between the two treatment groups on the relative risk of anorexia (RR 0.95, 95% CI 0.84 to 1.07; participants = 6841; studies = 11; I2 = 0%, high quality of evidence), abdominal pain (RR 0.80, 95% CI 0.57 to 1.11; participants = 2732; studies = 8; I2 = 53%, high quality of evidence, Fig. 4), gastroenteritis (RR 0.57, 95% CI 0.19 to 1.68; participants = 469, and loss of appetite (RR 2.06, 95% CI 0.52 to 8.14; participants = 469; studies = 1, [40]).
Cardio-respiratory adverse events
Cough
Cough was the most common cardio-respiratory adverse event, and significantly higher number of participants from DHA-PQ treatment group experienced cough (RR 1.06, 95% CI 1.01 to 1.11; participants = 8013; studies = 13; I2 = 0%, high quality of evidence, Fig. 5).
Publication bias
The funnel plot shows that all studies lie symmetrically around the pooled effect estimate implying that there was no publication bias (P = 0.84, Additional file 5).
Other cardiorespiratory and hematological adverse events
The relative risk of developing coryza did not have significant difference between the two treatment groups (RR 1.00, 95% CI 0.92 to 1.10; participants = 832; studies = 2; I2 = 0%, Fig. 5). In addition, the relative risk of respiratory adverse events such as rhinorrhea, respiratory tract infection, rhinitis, and pallor was not significantly different between the two treatment groups (RR 1.59, 95% CI 0.89 to 2.83; participants = 442; studies = 1, [45]), (RR 1.23, 95% CI 0.59 to 2.57; participants = 299; studies = 1, [37]), (RR 3.35, 95% CI 1.11 to 10.12; participants = 469; studies = 1, [40]), 95% CI 0.91 to 1.92; participants = 1548; studies = 1, [34]). Similarly, the relative risk of cardiac adverse events like QTc interval prolongation (Fridericia’s correction and Bazett’s correction) was not significantly different between the two treatment groups (RR 0.98, 95% CI 0.51 to 1.90; participants = 1548; studies = 1, [34] and (RR 0.98, 95% CI 0.09 to 10.81 and RR 1.32, 95% CI 0.91 to 1.92, participants = 1548, studies = 1, [34]).
Neuropsychiatry adverse event
Weakness/malaise
The relative risk of developing weakness or malaise was not significantly different between the two treatment groups (RR 0.88, 95% CI 0.74 to 1.03; participants = 3407; studies = 8; I2 = 0%, high quality of evidence, Fig. 6). Also, the relative risk of headache was not significantly different between the two treatment groups (RR 0.81, 95% CI 0.47 to 1.38; participants = 598; studies = 3; I2 = 72%, Fig. 6).
Musculoskeletal/dermatological adverse events
Pruritus was the most common dermatological adverse event, and the relative risk of developing pruritus was not significantly different between the two treatment groups (RR 1.00, 95% CI 0.56 to 1.78; participants = 1952; studies = 5; I2 = 49%, moderate quality of evidence, Fig. 7). Also, the relative risk of developing skin rash was not significantly different between the two treatment groups (RR 1.40, 95% CI 0.99 to 1.96; participants = 1720; studies = 3; I2 = 0%, Fig. 7).
Other musculoskeletal/dermatological adverse events
The relative risk of musculoskeletal or dermatological adverse events such as: skin and subcutaneous disorder, urticarial, hypersensitivity, pyoderma, conjunctivitis, joint pain, tinea-capitis, itchiness, frunculosis was not significantly different between the two treatment groups (RR 1.19, 95% CI 0.78 to 1.80; participants = 1548; studies = 1, [34]), (RR 0.25, 95% CI 0.02 to 2.70; participants = 1548; studies = 1, [34]), (RR 0.98, 95% CI 0.09 to 10.81; participants = 1548; studies = 1, [33]), (RR 1.00, 95% CI 0.33 to 3.05; participants = 442; studies = 1, [45]), (RR 0.47, 95% CI 0.19 to 1.12; participants = 442; studies = 1, [45]), (RR 0.49, 95% CI 0.07 to 3.46; participants = 418; studies = 1, [43]), (RR 1.24, 95% CI 0.54 to 2.81; participants = 469; studies = 1, [40]), (RR 0.34, 95% CI 0.01 to 8.22; participants = 703; studies = 1 [47],) and (RR 3.03, 95% CI 0.12 to 74.02; participants = 703; studies = 1, [47]), respectively.
Other adverse events
Pyrexia
The relative risk of pyrexia was the same in both treatment groups (RR 0.94, 95% CI 0.85 to 1.04; participants = 4620; studies = 6; I2 = 0%, Fig. 8). Similarly, the relative risk of otitis media was the same in both treatment groups (RR 0.66, 95% CI 0.23 to 1.91; participants = 1157; studies = 2; I2 = 0%, Fig. 8).
Serious adverse event
Fourteen studies reported 59 serious adverse events in the DHA-PQ and 35 in the AL treatment groups. However, the distributions of serious adverse events were not significantly different in the two treatment groups (RR 1.27, 95% CI 0.83 to 1.96; participants = 9558; studies = 14; I2 = 0%, high quality of evidence, Fig. 9). Eight deaths were reported from two multi-center trials, and the cause of death for seven of them was sepsis, severe malaria, and severe diarrhoea. But, the causal relationship of the study drug and death of one participant didn’t rule out. All serious adverse events were likely a consequence of malaria and judged to be unrelated to study medications.
Publication bias
The funnel plot showed that all studies lay symmetrically around the pooled effect estimate implying that there was no publication bias (P = 0.50, Additional file 6).
Quality of the evidence
The quality of the evidence in this review assessed using the GRADE approach and presented the evidence in three summary of findings tables for safety (Summary of findings for the main comparison; Additional file 7). The quality of evidence on comparative adverse effects and serious adverse events; early vomiting, diarrhoea, and cough were slightly more frequent in the DHA-PQ arm (high quality of evidence). Generally, the quality of evidence of safety of the two treatments was high quality.
Discussion
In this study both drugs were well tolerated by children. There were comparable occurrences of adverse events in both treatment arms. But, early vomiting, diarrhoea, and cough were common were significantly more frequent in patients who were treated with the DHA-PQ than that of AL (high quality of evidence). All serious adverse events were not related to study medications. Eight deaths have occurred in all studies. But, all serious adverse events were consistent with malaria symptoms and judged to be unrelated to study medication.
As also seen in one study from Papua New Guinea, the overall frequency of adverse events were slightly higher in DHA-PQ treatment arm than that of AL [48]. However, cough was more frequent in patients who were treated with AL, but headache and runny nose were common in DHA-PQ treatment group [48]. A recent review on the efficacy and safety of the two ACT’s also reported that cough, anorexia, diarrhoea, and vomiting were the most common adverse events. In this review more patients from DHA-PQ treatment arm had cough than that of AL [49] and similarly, gastrointestinal adverse events were more frequent in patients who were treated with DHA-PQ in another study done in South East Asia and Africa [50,51,52,53]. Studies from the Thailand-Myanmar border [54, 55] and elsewhere in Africa [56,57,58] have reported that DHA-PQ cause drug induced electrocardiographic QT prolongation, but a recent study also reported that the QT prolongation caused by piperaquine is not associated with an increased risk of sudden death [59]. In breastfeeding infants DHA–PQ has previously been linked to an increased risk of vomiting [60]. The mechanism accountable for the increased risk of early vomiting among breastfeeding participants treated with DHA–PQ is not known.
However, the temporal relationship suggests that the susceptibility of gastric mucosa of breastfed infants could be related to the pro-emetic effect of piperaquine than that in weaned infants [60]. To determine whether the co-administered milk may also affect this interaction further assessment might be needed [60]. However, the absence of effect with AL implies that the mechanism is given to DHA–PQ, most likely piperaquine [17]. Regardless of the treatment groups, most of these adverse events are associated with age (≤ 18 years), efavirenz-based ART [52], efavirenz-based ART [53], and administration of DHA-PQ with food could increase piperaquine exposure and it needs to be administered in fasting state [53, 54, 61].
Most of the RCTs reported AEs rather than adverse reactions of the antimalarial drugs. This made it difficult to determine the causal relationship between the antimalarial drugs and the AEs. It was, therefore, difficult to determine whether an adverse event is symptomatic of the disease or drug related. In some other studies, safety reporting was either selective or inadequate, with some authors failing to indicate the severity of AEs. Some of these limitations have been identified in studies evaluating the quality of safety reporting in RCTs.
Conclusion
From this review, it can be concluded that early vomiting, diarrhoea, and cough were common were significantly more frequent in patients who were treated with the DHA-PQ than that of AL, and both drugs are well tolerated. More studies comparing AL with DHA-PQ are needed to determine the comparative safety of these drugs.
Availability of data and materials
All relevant data are within the manuscript and its supporting information files.
Abbreviations
- AE:
-
Adverse event
- ACT:
-
Artemisinin-based combination therapy
- AL:
-
Artemether-lumefantrine
- ART:
-
Antiretroviral therapy
- BW:
-
Body weight
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CI:
-
Confidence interval
- DHA-PQ:
-
Dihydroartemisinin-piperaquine
- GADE:
-
Grading of recommendations assessment development and evaluations
- PICO:
-
Population intervention comparison and outcome
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RCTs:
-
Randomized control trials
- RR:
-
Risk ratio
- WHO:
-
World Health Organization
References
WHO . World Malaria Report 2019. Geneva: World Health Organization; 2019.
WHO. Guidelines for treatment of malaria. 3rd ed. Geneva: World Health Organization; 2015.
World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization; 2020.
Bretscher MT, Griffin JT, Hugo P, Baker M, Ghani A, Okell L. A comparison of the duration of post-treatment protection of artemether-lumefantrine, dihydroartemisinin-piperaquine and artesunate-amodiaquine for the treatment of uncomplicated malaria. Malar J. 2014;13:19.
Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev. 2009;2009:CD007483.
WHO. World Malaria Report 2014. Geneva: World Health Organization; 2014.
Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D. Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev. 2014;2014:CD010927.
Fairhurst RM, Dondorp AM. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol Spectr. 2016;4(10):1128.
Plucinski MM, Dimbu PR, Macaia AP, Ferreira CM, Samutondo C, Quivinja J, et al. Efficacy of artemether–lumefantrine, artesunate–amodiaquine, and dihydroartemisinin–piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in Angola, 2015. Malar J. 2017;16:62.
Davlantes E, Dimbu PR, Ferreira CM, Florinda Joao M, Pode D, Félix J, et al. Efficacy and safety of artemether–lumefantrine, artesunate–amodiaquine, and dihydroartemisinin–piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in three provinces in Angola, 2017. Malar J. 2018;17:144.
World Health Organization. World Malaria Report 2017. Geneva: World Health Organization; 2017.
Ministry of Health, Uganda. Uganda Clinical Guidelines. National Guidelines for Management of Common Conditions. Kampala: Ministry of Health; 2016. p. 195–9.
Makanga M, Bassat Q, Falade CO, Premji ZG, Krudsood S, Hunt P, et al. Efficacy and Safety of artemether-lumefantrine in the treatment of acute, uncomplicated Plasmodium falciparum malaria: a pooled analysis. Am J Trop Med Hyg. 2011;85:793–804.
Ebstie YA, Zeynudin A, Belachew T, Desalegn Z, Suleman S. Assessment of therapeutic efficacy and safety of artemether-lumefantrine (Coartem®) in the treatment of uncomplicated Plasmodium falciparum malaria patients in Bahir Dar district, Northwest Ethiopia: an observational cohort study. Malar J. 2015;14:236.
Falade C, Manyando C. Safety profile of Coartem®: the evidence base. Malar J. 2009;8:S6.
European Medicines Agency. Eurartesim (dihydroartemisinin/piperaquine) 20 mg/160 mg and 40 mg/320 mg film-coated tablets: EU summary of product characteristics [online]. 2017.
Assefa DG, Yismaw G, Makonnen E. Efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa: a systematic review and meta-analysis of randomized control trials. Malar J. 2021;20:340.
Assefa DG, Yismaw G, Makonnen E. Comparative effect of dihydroartemisinin-piperaquine and artemether-lumefantrine on gametocyte clearance and haemoglobin recovery in children with uncomplicated Plasmodium falciparum malaria in Africa: a systematic review and meta-analysis of randomized control trials. Int J Infect Dis. 2021;113:136–47.
Assefa DG, Zeleke ED, Molla W, Mengistu N, Sefa A, Mebratu A, et al. Systematic review and meta-analysis of the efficacy and safety of Dihydroartemisinin Piperaquine versus Artemether-Lumefantrine for the treatment of uncomplicated falciparum malaria in African children. PROSPERO. 2020. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020200337.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, 2011. www.cochrane-handbook.org.
WHO. Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organization; 2009.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). 2021. www.training.cochrane.org/handbook.
Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., Eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). 2021. www.training.cochrane.org/handbook.
Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74:785–94.
McMaster University GRADEproGDT. McMasterUniversity (developed by Evidence Prime), 2020. https://gradepro.org/.
Schünemann, Brożek, Guyatt, Oxman. Introduction to GRADE Handbook (Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach), 2013. https://gradepro.org.
Higgins JPT, SavovicJ, PageMJ, ElbersRG, Sterne JAC . Chapter 8: Assessing risk of bias in a randomized trial. Cochrane Handbook for Systematic Reviews ofInterventions version 6.0 (updated July 2019). 2019. www.training.cochrane.org/handbook.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64:1283–93.
Kamya MR, Yeka A, Bukirwa H, Lugemwa M, Rwakimari JB, Staedke SG, et al. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin Trials. 2007;2:e20.
Zongo I, Dorsey G, Rouamba N, Dokomajilar C, Sere Y, Rosenthal PJ, et al. Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin Infect Dis. 2007;45:1453–61.
Mens PF, Sawa P, van Amsterdam SM, Versteeg I, Omar SA, Schallig HD, et al. A randomized trial to monitor the efficacy and effectiveness by QT-NASBA of artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment and transmission control of uncomplicated Plasmodium falciparum malaria in western Kenya. Malar J. 2008;7:237.
Yeka A, Dorsey G, Kamya MR, Talisuna A, Lugemwa M, Rwakimari JB, et al. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda. PLoS ONE. 2008;3:e2390.
Bassat Q, Mulenga M, Tinto H, Piola P, Borrmann S, Menendez C, et al. Dihydroartemisinin-piperaquine and artemether-lumefantrine for treating uncomplicated malaria in African children. A randomised, non-inferiority trial. PLoS ONE. 2009;4:e7871.
Arinaitwe E, Sandison TG, Wanzira H, Kakuru A, Homsy J, Kalamya J, et al. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clin Infect Dis. 2009;49:1629–37.
Borrmann S, Sasi P, Mwai L, Bashraheil M, Abdallah A, Muriithi S, et al. Declining responsiveness of Plasmodium falciparum infections to artemisinin-based combination treatments on the Kenyan coast. PLoS ONE. 2011;6:e26005.
Nambozi M, Van Geertruyden JP, Hachizovu S, Chaponda M, Mukwamataba D, Mulenga M, et al. Safety and efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Zambian children. Malar J. 2011;10:50.
Four Artemisinin-Based Combinations Study Group. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med. 2011;8:e1001119.
Agarwal A, McMorrow M, Onyango P, Otieno K, Odero C, Williamson J, et al. A randomized trial of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated malaria among children in western Kenya. Malar J. 2013;12:254.
Ogutu BR, Onyango KO, Koskei N, Omondi EK, Ongecha JM, Otieno GA, et al. Efficacy and safety of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children aged less than five years: results of an open-label, randomized, single-centre study. Malar J. 2014;13:33.
Onyamboko MA, Fanello CI, Wongsaen K, Tarning J, Cheah PY, Tshefu KA, et al. Randomized comparison of the efficacies and tolerabilities of three artemisinin-based combination treatments for children with acute Plasmodium falciparum malaria in the Democratic Republic of the Congo. Antimicrob Agents Chemother. 2014;58:5528–36.
Kakuru A, Achan J, Muhindo MK, Ikilezi G, Arinaitwe E, Mwangwa F, et al. Artemisinin-based combination therapies are efficacious and safe for treatment of uncomplicated malaria in HIV-infected Ugandan children. Clin Infect Dis. 2014;59:446–53.
Nji AM, Ali IM, Moyeh MN, Ngongang EO, Ekollo AM, Chedjou JP, et al. Randomized non-inferiority and safety trial of dihydroartemisin-piperaquine and artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroonian children. Malar J. 2015;14:27.
Ursing J, Rombo L, Rodrigues A, Kofoed PE. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in children aged less than 15 years in Guinea-Bissau - an open-label non-inferiority randomised clinical trial. PLoS ONE. 2016;11:e0161495.
Grandesso F, Guindo O, WoiMesse L, Makarimi R, Traore A, Dama S, et al. Efficacy of artesunate-amodiaquine, dihydroartemisinin-piperaquine and artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Maradi. Niger Malar J. 2018;17:52.
Yeka A, Wallender E, Mulebeke R, Kibuuka A, Kigozi R, Bosco A, et al. Comparative efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated malaria in Ugandan children. J Infect Dis. 2019;219:1112–20.
Gansané A, Moriarty LF, Ménard D, Yerbanga I, Ouedraogo E, Sondo P, et al. Anti-malarial efficacy and resistance monitoring of artemether-lumefantrine and dihydroartemisinin-piperaquine shows inadequate efficacy in children in Burkina Faso, 2017–2018. Malar J. 2021;20:48.
Tavul L, Hetzel MW, Teliki A, Walsh D, Kiniboro B, Rare L, et al. Efficacy of artemether–lumefantrine and dihydroartemisinin–piperaquine for the treatment of uncomplicated malaria in Papua New Guinea. Malar J. 2018;17:350.
Assefa DG, Zeleke ED, Bekele D, Tesfahunei HA, Getachew E, Joseph M, et al. Efficacy and safety of dihydroartemisinin–piperaquine versus artemether–lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Ugandan children: a systematic review and meta-analysis of randomized control trials. Malar J. 2021;20:174.
Myint HY, Ashley EA, Day NP, Nosten F, White NJ. Efficacy and safety of dihydroartemisinin-piperaquine. Trans R Soc Trop Med Hyg. 2007;101:858–66.
Kakolwa MA, Mahende MK, Ishengoma DS, Mandara CI, Ngasala B, Kamugisha E, et al. Efficacy and safety of artemisinin-based combination therapy, and molecular markers for artemisinin and piperaquine resistance in Mainland Tanzania. Malar J. 2018;17:369.
Creek D, Bigira V, Arinaitwe E, Wanzira H, Kakuru A, Tappero J, et al. Increased risk of early vomiting among infants and young children treated with dihydroartemisinin-piperaquine compared with artemether-lumefantrine for uncomplicated malaria. Am J Trop Med Hyg. 2010;83:873–5.
Yavo W, Faye B, Kuete T, Djohan V, Oga SA, Kassi RR, et al. Multicentric assessment of the efficacy and tolerability of dihydroartemisinin-piperaquine compared to artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Malar J. 2011;10:198.
Saito M, Yotyingaphiram W, Cargill Z, Gilder ME, Min AM, Phyo AP, et al. Electrocardiographic effects of four antimalarials for pregnant women with uncomplicated malaria on the Thailand-Myanmar border: a randomised controlled trial. Antimicrob Agents Chemother. 2021;65:e02473-e2520.
Manning J, Vanachayangkul P, Lon C, Spring M, So M, Sea D, et al. Randomized, double-blind, placebo-controlled clinical trial of a two-day regimen of dihydroartemisinin-piperaquine for malaria prevention halted for concern over prolonged corrected QT interval. Antimicrob Agents Chemother. 2014;58:6056–67.
Oduro AR, Owusu-Agyei S, Gyapong M, Osei I, Adjei A, Yawson A, et al. Post-licensure safety evaluation of dihydroartemisinin piperaquine in the three major ecological zones across Ghana. PLoS ONE. 2017;12:e0174503.
Kabanywanyi AM, Baiden R, Ali AM, Mahende MK, Ogutu BR, Oduro A, et al. Multi-country evaluation of safety of dihydroartemisinin/piperaquine post-licensure in African public hospitals with electrocardiograms. PLoS ONE. 2016;11:e0164851.
Mhamilawa LE, Wikström S, Mmbando BP, Ngasala B, Mårtensson A. Electrocardiographic safety evaluation of extended artemether-lumefantrine treatment in patients with uncomplicated Plasmodium falciparum malaria in Bagamoyo District. Tanzania Malar J. 2020;19:250.
Chan XHS, Win YN, Mawer LJ, Tan JY, Brugada J, White NJ. Risk of sudden unexplained death after use of dihydroartemisinin–piperaquine for malaria: a systematic review and Bayesian meta-analysis. Lancet Infect Dis. 2018;18:913–23.
Sevene E, Banda CG, Mukaka M, Maculuve S, Macuacua S, Vala A, et al. Efficacy and safety of dihydroartemisinin–piperaquine for treatment of Plasmodium falciparum uncomplicated malaria in adult patients on antiretroviral therapy in Malawi and Mozambique: an open label non-randomized interventional trial. Malar J. 2019;8:277.
Funck-Brentano C, Bacchieri A, Valentini G, Pace S, Tommasini S, Voiriot P, et al. Effects of dihydroartemisinin-piperaquine phosphate and artemether-lumefantrine on QTc interval prolongation. Sci Rep. 2019;9:777.
Acknowledgements
We would like to express our gratitude to the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University, for funding the study.
Funding
This review was funded by Center for Innovative Drugs and Therapeutic Trial for Africa (CDT-Africa), Addis Ababa University.
Author information
Authors and Affiliations
Contributions
DGA developed the protocol as used in [7]. For this review, DGA reviewed the reference list, extracted data, and entered it into Review Manager (Rev-Man 5.4.1). DGA, EDZ, WM, NM, AS, AM, AFB, and EB conducted the analyses, constructed summary of findings tables, and evaluated the quality of evidence using the GRADE approach. EM and GY were responsible for the quality assessment and review of the study. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
We declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Detailed search strategy.
Additional file 2.
Characteristics of excluded studies.
Additional file 3.
Funnel plot of comparison: dihydroartemisinin-piperaquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria among African children, outcome: Gastrointestinal adverse events (early vomiting).
Additional file 4.
Funnel plot of comparison: dihydroartemisinin-piperaquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria among African children, outcome: Gastrointestinal adverse events (diarrhoea).
Additional file 5.
Funnel plot of comparison: dihydroartemisinin-piperaquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria among African children, outcome: Cough.
Additional file 6
. Funnel plot of comparison: dihydroartemisinin-piperaquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria among African children, outcome: Serious adverse event (including death).
Additional file 7.
GRADE summary of findings table on adverse events and serious adverse events.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Assefa, D.G., Zeleke, E.D., Molla, W. et al. Safety of dihydroartemisinin-piperaquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa: a systematic review and meta-analysis of randomized control trials. Malar J 21, 4 (2022). https://doi.org/10.1186/s12936-021-04032-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-021-04032-2