Abstract
Objective
The triglyceride–glucose (TyG) index has been shown to be a new alternative measure for insulin resistance. However, no study has attempted to investigate the association of the TyG index with incident atrial fibrillation (AF) in the general population without known cardiovascular diseases.
Methods
Individuals without known cardiovascular diseases (heart failure, coronary heart disease, or stroke) from the Atherosclerosis Risk in Communities (ARIC) cohort were recruited. The baseline TyG index was calculated as the Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]. The association between the baseline TyG index and incident AF was examined using Cox regression.
Results
Of 11,851 participants, the mean age was 54.0 years; 6586 (55.6%) were female. During a median follow-up of 24.26 years, 1925 incidents of AF cases (0.78/per 100 person-years) occurred. An increased AF incidence with a graded TyG index was found by Kaplan‒Meier curves (P < 0.001). In multivariable-adjusted analysis, both < 8.80 (adjusted hazard ratio [aHR] = 1.15, 95% confidence interval [CI] 1.02, 1.29) and > 9.20 levels (aHR 1.18, 95% CI 1.03, 1.37) of the TyG index were associated with an increased risk of AF compared with the middle TyG index category (8.80–9.20). The exposure-effect analysis confirmed the U-shaped association between the TyG index and AF incidence (P = 0.041). Further sex-specific analysis showed that a U-shaped association between the TyG index and incident AF still existed in females but not in males.
Conclusions
A U-shaped association between the TyG index and AF incidence is observed in Americans without known cardiovascular diseases. Female sex may be a modifier in the association between the TyG index and AF incidence.
Graphical Abstract

Similar content being viewed by others
Background
Atrial fibrillation (AF) is the most prevalent sustained arrhythmia in clinical practice and is associated with high morbidity and mortality [1]. According to previous statistics, the estimated global prevalence of AF was 46.3 million [2, 3], and by 2050, the number of individuals with AF is estimated to reach 6–16 million in America, which indicates a higher burden of AF-relevant events. There are several risk factors for AF, such as hypertension, obesity, and diabetes. A recent study showed that lower high-density lipoprotein cholesterol, higher blood pressure, abdominal obesity, and higher fasting glucose are positively associated with the risk of AF [4]. As the main feature of type 2 diabetes mellitus (T2DM), insulin resistance (IR) are suspected to be a risk factor for AF. There is evidence that in a nondiabetic individual-based prospective cohort study, a positive association between IR and the risk of AF is found (N = 8175, hazard risk = 1.61) [5]. Individuals with IR are also found to be at higher risk of AF recurrence after catheter ablation [6]. Homeostatic model assessment (HOMA), as the “gold standard” method for the evaluation of insulin sensitivity, is not widely used in clinical practice because of the costly and time-consuming nature of the process11. Recent studies have shown that a novel measure, the triglyceride glucose (TyG) index, is useful for estimating IR, with the advantages of simplicity and wide accessibility [7, 8]. In addition, evidence from epidemiological studies showed that the TyG index may be advantageous for predicting IR risk compared with HOMA-IR [9,10,11].
Previous studies [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] and our recent work [31, 30] have reported a significant association between the TyG index and the incidence of atherosclerosis, myocardial infarction (MI), stroke, coronary artery disease and mortality in the general population regardless of T2DM status. Furthermore, the TyG index is associated with adverse cardiovascular events among diabetic or nondiabetic patients with established cardiovascular diseases (CVDs) [32,33,34,35,36,37,38]. However, no study has focused on the impact of the TyG index on AF incidence in the general population. Accordingly, in this study, by using the dataset from the Atherosclerosis Risk in Communities (ARIC) cohort, we aim to investigate the association between the TyG index and the incidence of AF in individuals without known CVDs.
Methods
Study database
This study is a sub-analysis of the ARIC study [39]. The ARIC study, a prospective cohort aiming to (1) investigate the etiology and natural history of atherosclerosis, (2) investigate the etiology of clinical atherosclerosis diseases, and (3) measure variation in cardiovascular risk factors, medical care, and disease by race, sex, place, and time [39]. The ARIC study sampled 15,792 individuals aged 45 to 64 from 4 US communities. To date, it has finished 7 visits, which include a baseline examination (visit 1), visit 2 (occurring in 1990–1992), visit 3 (occurring in 1993–1995), visit 4 (occurring in 1996–1998), visit 5 (occurring in 2011–2013), visit 6 (occurring in 2016–2017) and visit 7 (occurring in 2018–2019). In this study, we included participants with AF-relevant information at visit 5. The ARIC study complied with the Declaration of Helsinki, and all participants signed informed consent before randomization. The study design, baseline characteristics, and major results have been published previously [39, 40].
Definitions of the TyG index and study outcomes
The baseline TyG index was calculated as the Ln[fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2] [9]. Blood samples were obtained after fasting for 8 h or more and tested in the central clinical chemistry laboratory and central lipid laboratory. Fasting plasma glucose and fasting triglyceride levels were measured via the optimized dart glucose reagent method and enzymatic method, respectively. In ARIC study, the process of sample collection to test was monitored using internal and external quality control.
The interested outcome was AF incidence, which was defined in compliance with the ARIC study: the studied individuals (1) had a fatal AF event; (2) had an incident AF event at visit 2, 3, 4, or 5, which was determined by electrocardiogram (ECG) readings; and (3) had an incident AF event as determined by hospital discharge codes on the cohort eligibility form [41].
Clinical and laboratory measurements
Demographic, clinical, and comorbidity data were recorded. Smoking status was classified as never smoking, past smoking, or current smoking; drinking status was also classified as never drinking, past drinking, or current drinking. Physical activity was assessed by the Baecke Sport Activity Score, which is a sport index of leisure time during the year and ranges from 1 to 5 (a higher score indicates a higher intensity). The Baecke Sport Activity Score was determined by summing the scores from four different items: (1) the sport activity recorded by them (intensity in Mj/h, duration in hours per week, and proportion of the year the activity performed); (2) asking the individuals to rate their physical activity by comparing with others (much less, less, the same, more, much more); (3) how often the participants sweat (never, seldom, sometimes, often, very often); and (4) how often the participants play sports or exercises (never, seldom, sometimes, often, very often) [42, 43]. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Low-density lipoprotein-cholesterol (LDL-C) levels were determined by the Friedewald formula and high-density lipoprotein-cholesterol (HDL-C) levels were determined using standardized enzymatic assays. Cholesterol-lowering medication was determined based on the previous two weeks’ self-reported usage report or medications brought by participants to the visit. Seated blood pressure was recorded by the mean of the last two of three measurements (random-zero sphygmomanometer) after a 5-min seat. Hypertension was defined as systolic blood pressure readings ≥ 140 mmHg, or diastolic blood pressure readings ≥ 90 mmHg, or the use of antihypertensive drugs in the previous two weeks. Peripheral artery disease (PAD) was defined according to the ankle-brachial index (ABI). If ABI04 < 0.90, the participant was diagnosed with PAD. Diabetes was defined as a fasting glucose level ≥ 126 mg/dl or nonfasting glucose level ≥ 200 mg/dl, self-reported physician diagnosis of diabetes, or any use of antidiabetic drugs. Heart failure was defined according to the use of any medication for heart failure or qualifies for the relevant criteria. Coronary heart disease and stroke were defined according to participants’ self-reports or relevant measurements [39, 44, 45]. CVDs included the presence of heart failure, coronary heart disease, or stroke.
Data analyses
The baseline characteristics of patients stratified by the TyG index category were compared. Continuous normally distributed data variables are expressed as the means with standard deviations (SDs), while nonnormally distributed data are expressed as medians with interquartile ranges (IQRs). The normality of the data was analyzed using the Shapiro‒Wilk test. For continuous variables, the differences between the groups were compared using unpaired Student’s t tests between 2 groups and one-way ANOVA among 3 groups (normal distribution) or Mann‒Whitney U tests between 2 groups and Kruskal–Wallis tests among 3 groups (nonnormal distributions). The categorical variables were reported as counts and percentages and compared using χ2 tests. The TyG index was categorized into three groups according to the shape of the exposure‐effect relationship, with the lowest level being regarded as the reference group (8.80–9.20). The Kaplan‒Meier method was used for incidence curve estimation. The association of the TyG index with incident AF was calculated using a univariate or multivariate Cox proportional hazard model, which is expressed by hazard ratios (HRs) and 95% confidence intervals (CIs). The proportional hazards assumption was satisfied by the TyG index (P = 0.26). The adjustments were selected based on a well-established prognostic significance: age, sex, BMI, race, smoking status, drinking status, LDL-C, HDL-C, use of cholesterol-lowering medication, and diseases at baseline, including hypertension, diabetes mellitus, and PAD. The exposure‐effect associations between the TyG index and incident AF were evaluated by using a restricted cubic spline function with 3 knots (10th, 50th, and 90th percentiles) [46]. For sensitivity analysis, we added physical activity and glucose lowering medication to the fully adjusted variables or restricted the study population to a nondiabetic population. SPSS Statistics Version 25.0 (IBM SPSS Statistics, IBM Corporation, Chicago, IL, USA) and the R Programming Language (Version 4.2.0) were used to run the statistical analysis. P ≤ 0.05 was considered to be statistically significant in all analyses.
Results
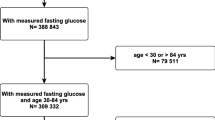
In total, 15,792 subjects were recruited for the ARIC study. After excluding individuals with a race other than African American or Caucasian and the few African American participants from Minneapolis and Washington County (n = 103), with baseline CVD or AF (n = 1887), with unknown prevalent AF status, missing or unreadable ECGs (n = 310), with missing covariates (n = 1058), with no fasting blood sample (n = 484) and missing information on AF during follow-up (n = 99), our study included 11,851 individuals without known CVDs (Fig. 1).
Baseline characteristics of study participants
Baseline characteristics are listed in Table 1. Of 11,851 participants without known CVDs, the mean age was 54.04 (SD: 5.74) years; 6586 (55.6%) were female; 9078 (76.6%) were Caucasian; mean BMI was 27.37 (SD: 5.15) kg/m2; 1059 (8.9%) and 3633 (30.7%) had a history of diabetes and hypertension, respectively; and 286 (2.4%) used cholesterol-lowering medication. Participants were divided into three groups based on the baseline TyG index levels (Group 1: TyG index < 8.80; Group 2: 8.80 ≤ TyG index ≤ 9.20; and Group 3: TyG index > 9.20). The three groups were significantly different in terms of age, sex, BMI, race, smoking status, history of clinical diabetes, hypertension, PAD, cholesterol-lowering medication use, physical activity, and levels of HDL-C and LDL-C.
TyG index and incident AF
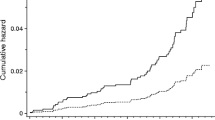
During 246,908.0 person-years of follow-up (median follow-up of 24.3 years), 1,925 incident cases of AF occurred. The crude incidence of AF across the TyG index group is shown in Fig. 2A, which shows an increased AF incidence with the graded TyG index (P < 0.05) in individuals without known CVDs. Similar results were found by the Kaplan‒Meier curves (P for log-rank test < 0.001) in Fig. 3A. Interestingly, the adjusted incidence curve showed the lowest AF incidence in the middle TyG category (8.80–9.20), whereas a similar risk was observed with the first (< 8.80) and third (> 9.20) TyG groups (Fig. 3D). Consistently, in Cox proportional hazard analysis, the unadjusted model showed a graded incidence of AF compared with the middle TyG index. However, both the first TyG index group (HR: 1.15, 95% CI 1.02 to 1.29) and third TyG index group (HR 1.18; 95% CI 1.03 to 1.37) showed an increased incidence compared with the middle TyG index group after adjustment for age, sex, race, body mass index, smoking, drinking, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, use of cholesterol-lowering medication, history of diabetes, hypertension, and PAD (Table 2). The restricted spline model confirmed a U shape between the TyG index and AF incidence in individuals without known CVDs (P = 0.041) (Fig. 4).
A The crude incidence of AF across TyG index group among overall individuals. B The crude incidence of AF across TyG index group basing sex subgroup. TyG-T1 versus TyG-T2 P < 0.001; TyG-T2 versus TyG-T3 P < 0.002; TyG-T1 versus TyG-T3 P < 0.001. TyG-T1: < 8.80, TyG-T2: 8.80–9.20, TyG-T3: > 9.20. TyG index triglyceride–glucose index. AF atrial fibrillation
A Kaplan–Meier analysis for AF among TyG index groups in (A) all population, (B) male, and (C) female. AF-free survival was analyzed by a log-rank test (P < 0.001 in all populations, P = 0.002 in males, P < 0.001 in females). Adjusted incidence curve for AF among TyG index groups in (D) all population, (E) male and (F) female. The adjustments were age, sex, race, body mass index, smoking, drinking, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, use of cholesterol-lowering medication, history of diabetes, hypertension, and peripheral artery disease. AF atrial fibrillation. TyG index triglyceride–glucose index
Multivariable-adjusted hazard ratios for AF based on restricted cubic spines among overall individuals. Each hazard ratio was computed with a TyG index level of 8.59 as the reference. The model was adjusted for age, sex, race, body mass index, smoking, drinking, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, use of cholesterol-lowering medication, history of diabetes, hypertension, and peripheral artery disease. The Red line represents references for HRs, and the area between the dotted line represents 95% CI. Histograms represent the frequency distribution of the baseline TyG index. AF atrial fibrillation. HR hazard ratio, CI confidence interval TyG triglyceride–glucose
Subgroup analysis
The baseline characteristics of the participants stratified by sex are also shown in Additional file 1: Table S1. The females were more likely to be nonsmokers, nondrinkers, and African American and showed significantly lower age, LDL-C, TyG index, glucose, triglycerides, and physical activity while they had higher HDL-C, cholesterol-lowering medication use, and a history of PAD.
We further explored the sex-specific association of the TyG index with AF incidence, which is shown in Fig. 2B. An increased crude AF incidence with TyG index grades was observed, either in males or females. Moreover, the Kaplan‒Meier curves also showed a grade-increased AF incidence with an increased TyG index (P = 0.002 in males, P < 0.001 in females) (Fig. 3B, C). Interestingly, the adjusted incidence curve showed the lowest AF incidence in the middle TyG category (8.80–9.20) in both sexes. However, the AF incidence in the first TyG category (< 8.80) was higher than that in the third TyG category (> 9.20) in males, while the opposite was true in females (Fig. 3E, F). In further exposure‐effect analysis, a U shape of the dose–response curve was seen in females (P = 0.167). For males, the curve increased steeply in the lower TyG index group but was much flatter in the TyG index > 9.20 group (P = 0.063) (Fig. 5A, B).
Multivariable-adjusted hazard ratios for AF based on restricted cubic spines in A male and B female. Each hazard ratio was computed with a TyG index level of A 8.68 and B 8.51 as the reference. Model was adjusted for age, race, body mass index, smoking, drinking, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol, use of cholesterol lowering medication, history of diabetes, hypertension, peripheral artery disease. Red line represents references for HRs, and area between dotted line represent 95% CI. Blue area represents the fraction of the population with different baseline TyG index. HR hazard ratio, CI confidence interval, TyG triglyceride–glucose, AF atrial fibrillation
Sensitivity testing
First, as we previously showed physical activity is associated with a reduced incidence of AF [47]. To account for physical activity, we evaluated the TyG index and incidence of AF after additionally adjusting for physical activity. A U-shaped association of the TyG index with the incidence of AF reminded (Additional file 1: Table S2). Second, with adjustments further applied by glucose-lowering medication, a U-shaped association remained confirmatory (Additional file 1: Table S2). Third, we restricted the study population to nondiabetic individuals. This Cox regression sensitivity analysis also showed a trend of U-shaped association with the TyG index of AF (Additional file 1: Table S3). Finally, considering the positive association between low TyG index (≤ 8.80) and AF incidence after adjustments, we further performed sensitivity analyses between low TyG index and AF incidence stratified by different subgroups, including age, race, BMI, history of hypertension, non-diabetes and LDL-C level. After full adjustments, the positive association between low TyG index and AF incidence still existed (Additional file 1: Fig. S1).
Discussion
Major findings
Based on the longitudinal cohort of ARIC, we found a gradually rising risk of AF in patients without known CVDs with TyG levels after a median follow-up of 24.26 years. However, the full adjusted incidence of AF showed a U-shaped trend with a TyG level, with a nadir at a TyG level of approximately 8.80–9.20. Multivariable-adjusted Cox analysis and exposure-effect analysis further confirmed the U-shaped association. In a further sex-specific analysis, a U-shaped association between the TyG index and AF incidence still existed in females but not in males. Taken together, this study suggests a U-shaped association between the TyG index and AF incidence in people without known CVDs.
HOMA-IR is previously considered as the gold standard for IR. Recently, as an expected new estimating model for IR, the TyG index has been extensively investigated and approved with the strength of convenience compared to HOMA-IR11. Guerrero-Romero et al. found high sensitivity (96.5%) and specificity (85.0%) of the TyG index for recognizing IR [48]. The significant association between the TyG index and an increased risk of CVDs and mortality has been extensively explored [24, 30, 49,50,51,52], regardless of diabetes status. However, no studies have explored the association of the TyG index with AF in the general population.
Hence, it is not surprising that the higher TyG index is associated with an increased risk of AF in the present study. A positive association between the TyG index and AF risk has been observed in certain patients with established CVDs. In patients with hypertrophic obstructive cardiomyopathy undergoing septal myectomy and myocardial infarction receiving percutaneous coronary intervention, the TyG index showed moderate predictive ability for postoperative AF incidence [53, 54]. However, we did not assess the predictive ability of the addition of TyG in the existing risk scores for AF incidence, such as the Framingham Heart Study (FHS) score, the ARIC score, and the Cohorts for Heart and Aging Research in Genomic Epidemiology Atrial Fibrillation (CHARGE-AF) score, Thrombolysis in Myocardial Infarction 48 (TIMI-AF) and C2HEST [55,56,57,58,59], which may be further studied.
We showed that the crude rate of AF incidence increased with the TyG index. However, after full adjustments, we found a U-shaped association of the TyG index with AF incidence. Further sensitivity analyses basing individuals with low TyG index (≤ 8.80) among different subgroups including age, race, BMI, history of hypertension, nondiabetes, and LDL-C level showed the negative association between low TyG index and AF incidence still existed. Several potential mechanisms may be responsible. A strong association between a high TyG index and an increased risk of developing AF may be due to IR. IR in the liver is caused by impaired insulin metabolism influencing glucose metabolism and enhancing insulin-mediated lipogenesis to cause hyperglycemia [60]. Adipo-IR has been associated with β cell dysfunction, which begins in individuals with normal glucose tolerance [61]. Hyperglycemia and β cell impairment are both related to cardiomyocyte metabolism and cardiac function. All the above pathological changes may contribute to the incidence of atrial fibrillation. On the other hand, from the TyG index formula, we know low TyG index could cause by low triglyceride or low glucose levels. The positive association between a low TyG index and AF incidence may be explained by low fasting glucose. A recent systematic evaluation and meta-analysis showed that low fasting plasma glucose (< 4.0 mmol/L) was associated with an increased risk of future all-cause mortality, major cardiovascular events, and all-stroke and ischemic stroke in individuals without baseline cardiovascular disease or diabetes [62]. What’s more, a body of studies also found a low level of glucose showed a higher risk of diseases or events including AF, diabetes, stroke, major cardiovascular event, and all-cause death [63,64,65]. Low glucose may indicate abnormal nutritional and metabolic states which would increase the risk of cardiovascular events [66, 67]. As pancreatic cells are important coordinators in maintaining glucose, amino, and lipid homeostasis, we speculate the positive association between low TyG index and AF incidence may be related to pancreatic cellular dysfunction [68, 69]. Hence, hypoglycemia may be an indicator of cellular dysfunction. For further mechanism, the prolonged QTc interval enhanced adrenergic tone, and cardiac autonomic dysfunction caused by low plasma glucose could increase the risk of arrhythmia [70,71,72].
Sex difference
Sex is a well-known modifier for the incidence and development of AF. Our previous meta-analysis showed that a higher TyG index was associated with coronary artery disease/CVD, even after removing sex-unadjusted studies (HR = 1.59, 95% CI 1.21–2.09) [31]. We showed a different shape of dose–response association trend between the TyG index and AF incidence in different sexes. Similar to our study, a sex difference (P = 0.045) in the relationship between a high TyG index and subclinical atherosclerosis in nondiabetic patients was found: a significantly higher prevalence of subclinical atherosclerosis in the high TyG index group than in the low TyG index group (odds ratio [OR] = 1.51; 95% CI 1.01–2.26) was observed in nondiabetic females but not in nondiabetic males (OR = 0.83; 95% CI 1.56–1.23) [73]. Obesity is a well-known risk factor for AF incidence. Several studies showed metabolically healthy obesity were at increased risk for all-cause mortality and cardiovascular events compared with metabolically healthy normal-weight individuals, although with controversial results [74,75,76,77]. Study by Fauchier et al. found an elevated risk of AF exists in metabolically healthy obese individuals, while female sex may be a modifier, which indicats the possible different role of obesity and metabolism between sex [78]. Despite recent studies attempt to standardize the concept of metabolically healthy obesity, there is no unified definition of metabolically healthy obesity [79, 80]. According to a meta-analysis, insulin sensitivity (eg, euglycemic-hyperinsulinemic clamps, HOMA-IR) was regarded as an important component to define the metabolically healthy obese phenotype [81]. Our analyses of increased AF incidence with high TyG index reinforced the above results. Differences in the location of fat storage in men and women may play a role in the development of IR and diabetes [82, 83]. Women tend to store fat subcutaneously rather than viscerally [84]. As visceral fat is closely linked to insulin resistance, whereas subcutaneous fat may be protective, women may need to gain more weight and experience a greater decline in associated metabolic risk factors to attain the same level of visceral fat storage [85, 86]. This implies that sex differences in metabolic risk factors result from elevated blood glucose levels and diabetes and assessing regional adiposity by imaging to further define both of obesity and metabolically healthy obesity is appropriate [87, 88].Additionally, in rodent models, endogenous estrogens may play a role in higher insulin sensitivity in women [89]. In a large clinical study, menopausal hormone therapy in postmenopausal women improved insulin sensitivity through estrogen receptors in the liver, muscle, and adipose tissue [90, 91]. The protective effect of estrogen is lost when women are exposed to risk factors such as hypertension and hyperlipidemia [92, 93], which may cause a sex difference.
Diabetes is another vital coufounding. Several previous studies have suggested that increased IR is associated with an increased risk of cardiovascular events in patients without diabetes [94, 95]. In our sensitivity analysis in nondiabetic individuals, the shape of the TyG index and AF did not significantly change. Due to the limited sample size of T2DM patients in the present cohort (8.9%), the relationship between the TyG index and AF is not assessed. Further studies are needed to clarify the role of the TyG index on AF in the diabetic population.
Strengths and limitations
Our study has several strengths. To our knowledge, we are the first to investigate the association between the TyG index and the incidence of AF in individuals without known CVDs. Second, our data are from a well-designed longitudinal cohort that included a large sample size and diverse populations with adequate follow-up. Third, we further investigated the sex-specific association of the TyG index with AF incidence and found that female sex may be a modifier. However, our study also had several limitations. First, our analysis is based on an observational cohort study. Several confounding factors, such as nutritional status, may have influenced our results. In our analysis, the TyG index is calculated based on the blood sample at baseline. However, considering the median follow-up of 24.26 years, a fluctuation of TyG index values during follow-up may provide a better insight into the risk of AF incidence. Second, the population in our study is Americans, and the generality in other populations needs to be further verified.
Conclusion
A U-shaped association between the TyG index and the AF incidence is observed in Americans without known CVDs. Female sex may be a modifier in the association between the TyG index and AF incidence.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABI:
-
Ankle brachial index
- AF:
-
Atrial fibrillation
- aHR:
-
Adjusted hazard ratio
- ARIC:
-
Atherosclerosis Risk in Communities
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- ECG:
-
Electrocardiogram
- HDL-C:
-
High-density lipoprotein-cholesterol
- HOMA:
-
Homeostatic model assessment
- IQRs:
-
Interquartile ranges
- IR:
-
Insulin resistance
- LDL-C:
-
Low-density lipoprotein-cholesterol
- OR:
-
Odds ratio
- PAD:
-
Peripheral artery disease
- SDs:
-
Standard deviations
- TyG:
-
Triglyceride–glucose
- T2DM:
-
Type 2 diabetes mellitus
References
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS) the task force for the diagnosis and management of atrial fibrillation of the european society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498.
Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14(3):195–203.
Ahmed AAA, Al-Shami AM, Jamshed S, Zawiah M, Elnaem MH, Mohamed Ibrahim MI. Awareness of the risk factors for heart attack among the general public in Pahang, Malaysia: a cross-sectional study. Risk Manag Healthc Policy. 2020;13:3089–102.
Zheng Y, Xie Z, Li J, Chen C, Cai W, Dong Y, et al. 15Meta-analysis of metabolic syndrome and its individual components with risk of atrial fibrillation in different populations. BMC Cardiovasc Disord. 2021;21(1):90.
Lee Y, Cha SJ, Park JH, Shin JH, Lim YH, Park HC, et al. Association between insulin resistance and risk of atrial fibrillation in non-diabetics. Eur J Prev Cardiol. 2020;27(18):1934–41.
Wang Z, Wang YJ, Liu ZY, Li Q, Kong YW, Chen YW, et al. Effect of insulin resistance on recurrence after radiofrequency catheter ablation in patients with atrial fibrillation. Cardiovasc Drugs Ther. 2022. https://doi.org/10.1007/s10557-022-07317-z.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Tao L-C, Xu J-n, Wang T-t, Hua F, Li J-J. Triglyceride–glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):1–17.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-ZavalaHernández-González MAGSO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Vasques ACJ, Novaes FS, de Oliveira MdS, Souza JRM, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–100.
Cho YK, Lee J, Kim HS, Kim EH, Lee MJ, Yang DH, et al. Triglyceride glucose-waist circumference better predicts coronary calcium progression compared with other indices of insulin resistance: a longitudinal observational study. J Clin Med. 2021;10(1):1–15.
Park GM, Cho YR, Won KB, Yang YJ, Park S, Ann SH, et al. Triglyceride glucose index is a useful marker for predicting subclinical coronary artery disease in the absence of traditional risk factors. Lipids Health Dis. 2020;19(1):7.
Hong You J, Kim JH, Park K, Kim YS, Han DW, Koh B, et al. Association between triglyceride glucose index and coronary artery calcification in Korean adults. Diabetes. 2017;66:A591.
Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–72.
Rojas-Humpire R, Olarte-Durand M, Medina-Ramirez S, Gutierrez-Ajalcrina R, Canaza JF, Huancahuire-Vega S. Insulin resistance indexes as biomarkers of lifetime cardiovascular risk among adults from Peru. J Nutr Metab. 2021;2021:6633700.
Sanchez-Inigo L, Navarro-Gonzalez D, Fernandez-Montero A, Pastrana-Delgado J, Martinez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97.
Shi W, Xing L, Jing L, Tian Y, Yan H, Sun Q, et al. Value of triglyceride–glucose index for the estimation of ischemic stroke risk: Insights from a general population. Nutr Metab Cardiovasc Dis. 2020;30(2):245–53.
Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, et al. Triglyceride–glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2021;20(1):19.
Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med. 2014;62(2):345–9.
Wang A, Wang G, Liu Q, Zuo Y, Chen S, Tao B, et al. Triglyceride–glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol. 2021;20(1):46.
Won KB, Kim YS, Lee BK, Heo R, Han D, Lee JH, et al. The relationship of insulin resistance estimated by triglyceride glucose index and coronary plaque characteristics. Medicine (Baltimore). 2018;97(21): e10726.
Zhao Y, Sun H, Zhang W, Xi Y, Shi X, Yang Y, et al. Elevated triglyceride–glucose index predicts risk of incident ischaemic stroke: the rural Chinese cohort study. Diabetes Metab. 2021;47(4): 101246.
Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride–glucose index on incident cardiovascular events during 16 years of follow-up: Tehran Lipid and Glucose Study. Cardiovasc Diabetol. 2020;19(1):155.
Cho YR, Ann SH, Won KB, Park GM, Kim YG, Yang DH, et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep. 2019;9(1):6129.
Guo W, Zhu W, Wu J, Li X, Lu J, Qin P, et al. Triglyceride glucose index is associated with arterial stiffness and 10-year cardiovascular disease risk in a Chinese population. Front Cardiovasc Med. 2021;8: 585776.
Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361.
Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, et al. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep. 2019;9(1):7320.
Liu Q, Cui H, Ma Y, Han X, Cao Z, Wu Y. Triglyceride–glucose index associated with the risk of cardiovascular disease: the Kailuan study. Endocrine. 2022;75(2):392–9.
Liu F, Ling Q, Xie S, Xu Y, Liu M, Hu Q, et al: Association between triglyceride glucose index and arterial stiffness and coronary artery calcification: a systematic review and exposure-effect meta-analysis. Cardiovasc Diabetol 2023, 22(1):111.
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride–glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124.
Neglia D, Aimo A, Caselli C, Gimelli A. Triglycerides glucose index and high-sensitivity C-reactive protein are predictors of outcome in chronic coronary syndrome. Eur Heart J Cardiovasc Imag. 2021;22(SUPPL 3):ii55.
Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):1–14.
Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, et al. Triglyceride–glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20(1):1–12.
Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride–glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):1–12.
Mao Q, Zhou D, Li Y, Wang Y, Xu S-C, Zhao X-H. The triglyceride–glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-ST-segment elevation acute coronary syndrome. Dis Markers. 2019;2019:11.
Wang L, Cong H-l, Zhang J-x, Hu Y-c, Wei A, Zhang Y-y, et al. Triglyceride–glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):1–11.
Jin J-L, Cao Y-X, Wu L-G, You X-D, Guo Y-L, Wu N-Q, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137.
Aric Investigators. The atherosclerosis risk in communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1898;129(4):687–702.
Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the atherosclerosis risk in communities (ARIC) study. Am Heart J. 2009;158(1):111–7.
Li J, Agarwal SK, Alonso A, Blecker S, Chamberlain AM, London SJ, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129(9):971–80.
Richardson MT, Ainsworth BE, Wu HC, Jacobs DR Jr, Leon AS. Ability of the atherosclerosis risk in communities (ARIC)/Baecke questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24(4):685–93.
Pettee Gabriel K, Griswold ME, Wang W, Conway SH, Windham BG, Palta P, et al. Physical activity trajectories and subsequent fall risk: ARIC study. Prev Med. 2019;121:40–6.
Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101(7):1016–22.
Jones SA, Gottesman RF, Shahar E, Wruck L, Rosamond WD. Validity of hospital discharge diagnosis codes for stroke: the Atherosclerosis Risk in Communities Study. Stroke. 2014;45(11):3219–25.
Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26(20):3735–52.
Wan Q, Zhou Y, Zhu W, Liu X. Sex-specific exposure-effect relationship between physical activity and incident atrial fibrillation in the general population: a dose-response meta-analysis of 16 prospective studies. Front Cardiovasc Med. 2021;8: 710071.
Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Kim J, Shin SJ, Kang HT. The association between triglyceride–glucose index, cardio-cerebrovascular diseases, and death in Korean adults: a retrospective study based on the NHIS-HEALS cohort. PLoS ONE. 2021;16(11): e0259212.
Park B, Lee YJ, Lee HS, Jung DH. The triglyceride–glucose index predicts ischemic heart disease risk in Koreans: a prospective study using National Health Insurance Service data. Cardiovasc Diabetol. 2020;19(1):210.
Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, et al. Triglyceride–glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Triglyceride–glucose index as a surrogate marker of insulin resistance for predicting cardiovascular outcomes in nondiabetic patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. J Atheroscler Thromb. 2021;28(11):1175–94.
Wei Z, Zhu E, Ren C, Dai J, Li J, Lai Y. 6. Triglyceride–glucose index independently predicts new-onset atrial fibrillation after septal myectomy for hypertrophic obstructive cardiomyopathy beyond the traditional risk factors. Front Cardiovasc Med. 2021;8: 692511.
Ling Y, Fu C, Fan Q, Liu J, Jiang L, Tang S. Triglyceride–glucose index and new-onset atrial fibrillation in ST-segment elevation myocardial infarction patients after percutaneous coronary intervention. Front Cardiovasc Med. 2022;9: 838761.
Fanola CL, Giugliano RP, Ruff CT, Trevisan M, Nordio F, Mercuri MF, et al. A novel risk prediction score in atrial fibrillation for a net clinical outcome from the ENGAGE AF-TIMI 48 randomized clinical trial. Eur Heart J. 2017;38(12):888–96.
Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2(2): e000102.
Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2011;107(1):85–91.
Li Y-G, Pastori D, Farcomeni A, Yang P-S, Jang E, Joung B, et al. A Simple Clinical Risk Score (C2HEST) for predicting incident atrial fibrillation in asian subjects: derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest. 2019;155(3):510–8.
Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB Sr, et al. Development of a risk score for atrial fibrillation (Framingham heart study): a community-based cohort study. The Lancet. 2009;373(9665):739–45.
Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87(2):507–20.
Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio metabolism study. Diabetes. 2017;66(4):815–22.
Liao HW, Saver J, Yeh HC, Chen CS, Wu YL, Lee M, et al. Low fasting glucose and future risks of major adverse outcomes in people without baseline diabetes or cardiovascular disease: a systematic review and meta-analysis. BMJ Open. 2019;9(7): e026010.
Humos B, Mahfoud Z, Dargham S, Al Suwaidi J, Jneid H, Abi KC. Hypoglycemia is associated with a higher risk of mortality and arrhythmias in ST-elevation myocardial infarction, irrespective of diabetes. Front Cardiovasc Med. 2022;9: 940035.
Ko SH, Park YM, Yun JS, Cha SA, Choi EK, Han K, et al. Severe hypoglycemia is a risk factor for atrial fibrillation in type 2 diabetes mellitus: Nationwide population-based cohort study. J Diabetes Complicat. 2018;32(2):157–63.
Schulze MB, Fritsche A, Boeing H, Joost HG. Fasting plasma glucose and type 2 diabetes risk: a non-linear relationship. Diabetic Med. 2010;27(4):473–6.
Kim HJ, Ryu J, Ahn SY, Kim S, Lim S, Na KY, et al. Association of insulin resistance with lower glomerular filtration rate and all-cause mortality in the Korean elderly population: a community-based prospective cohort study. Tohoku J Exp Med. 2013;231(4):271–9.
Zhou D, Liu XC, Kenneth L, Huang YQ, Feng YQ. A non-linear association of triglyceride glycemic index with cardiovascular and all-cause mortality among patients with hypertension. Front Cardiovasc Med. 2021;8: 778038.
Gromada J, Chabosseau P, Rutter GA. The α-cell in diabetes mellitus. Nat Rev Endocrinol. 2018;14(12):694–704.
Gilon P. The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes. J Mol Biol. 2020;432(5):1367–94.
Reno CM, Daphna-Iken D, Chen YS, VanderWeele J, Jethi K, Fisher SJ. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes. 2013;62(10):3570–81.
Nordin C. The case for hypoglycaemia as a proarrhythmic event: basic and clinical evidence. Diabetologia. 2010;53(8):1552–61.
Cha SA, Yun JS, Lim TS, Kang YG, Lee KM, Song KH, et al. Baseline-corrected QT (QTc) interval is associated with prolongation of QTc during severe hypoglycemia in patients with type 2 diabetes mellitus. Diabetes Metab J. 2016;40(6):463–72.
Lu YW, Chang CC, Chou RH, Tsai YL, Liu LK, Chen LK, et al. Gender difference in the association between TyG index and subclinical atherosclerosis: results from the I-Lan Longitudinal aging study. Cardiovasc Diabetol. 2021;20(1):206.
Opio J, Croker E, Odongo GS, Attia J, Wynne K, McEvoy M. Metabolically healthy overweight/obesity are associated with increased risk of cardiovascular disease in adults, even in the absence of metabolic risk factors: A systematic review and meta-analysis of prospective cohort studies. Obes Rev. 2020;21(12): e13127.
Yeh TL, Chen HH, Tsai SY, Lin CY, Liu SJ, Chien KL. The relationship between metabolically healthy obesity and the risk of cardiovascular disease: a systematic review and meta-analysis. J Clin Med. 2019;8(8):1228.
Zheng R, Zhou D, Zhu Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70(10):1024–31.
Bo S, Musso G, Gambino R, Villois P, Gentile L, Durazzo M, et al. Prognostic implications for insulin-sensitive and insulin-resistant normal-weight and obese individuals from a population-based cohort. Am J Clin Nutr. 2012;96(5):962–9.
Fauchier G, Bisson A, Bodin A, Herbert J, Semaan C, Angoulvant D, et al. Metabolically healthy obesity and cardiovascular events: a nationwide cohort study. Diabetes Obes Metab. 2021;23(11):2492–501.
Magkos F. Metabolically healthy obesity: what’s in a name? Am J Clin Nutr. 2019;110(3):533–9.
Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41(3):bnaa004.
Rey-López JP, de Rezende LF, Pastor-Valero M, Tess BH. The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obes Rev. 2014;15(10):781–90.
Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(1):60–75.
Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89(6):2569–75.
Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99(5):931–40.
de Mutsert R, Gast K, Widya R, de Koning E, Jazet I, Lamb H, et al. Associations of abdominal subcutaneous and visceral fat with insulin resistance and secretion differ between men and women: the Netherlands epidemiology of obesity study. Metab Syndr Relat Disord. 2018;16(1):54–63.
Kammerlander AA, Lyass A, Mahoney TF, Massaro JM, Long MT, Vasan RS, et al. Sex differences in the associations of visceral adipose tissue and cardiometabolic and cardiovascular disease risk: the Framingham heart study. J Am Heart Assoc. 2021;10(11): e019968.
de Ritter R, de Jong M, Vos RC, van der Kallen CJH, Sep SJS, Woodward M, et al. Sex differences in the risk of vascular disease associated with diabetes. Biol Sex Differ. 2020;11(1):1.
Després JP. Taking a closer look at metabolically healthy obesity. Nat Rev Endocrinol. 2022;18(3):131–2.
Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol. 2012;8(6):342–51.
Duncan AC, Lyall H, Roberts RN, Petrie JR, Perera MJ, Monaghan S, et al. The effect of estradiol and a combined estradiol/progestagen preparation on insulin sensitivity in healthy postmenopausal women. J Clin Endocrinol Metab. 1999;84(7):2402–7.
Mattiasson I, Rendell M, Törnquist C, Jeppsson S, Hulthén UL. Effects of estrogen replacement therapy on abdominal fat compartments as related to glucose and lipid metabolism in early postmenopausal women. Horm Metab Res. 2002;34(10):583–8.
El Khoudary SR, Shields KJ, Janssen I, Hanley C, Budoff MJ, Barinas-Mitchell E, et al. Cardiovascular fat, menopause, and sex hormones in women: the SWAN cardiovascular fat ancillary study. J Clin Endocrinol Metab. 2015;100(9):3304–12.
Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63(3):453–61.
Hedblad B, Nilsson P, Engström G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med. 2002;19(6):470–5.
Robins SJ, Rubins HB, Faas FH, Schaefer EJ, Elam MB, Anderson JW, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the veterans affairs HDL intervention trial (VA-HIT). Diabetes Care. 2003;26(5):1513–7.
Acknowledgements
We acknowledge the grant support from Science and Technology Projects in Guangzhou (202102010007), Guangdong Science and Technology Department (2020B1212060018, 2020B1212030004). We would like to thank Tiangang Song from Nanchang University for the Graphical assistance.
Funding
This work was supported in part by the National Natural Science Foundation of China (J.F-W, 82070237; Y.L-Z, 81870170; X-L, 82100347), Natural Science Foundation of Guangdong Province (Y.L-Z, 2019A1515011682; Y.L-Z, 2021A1515012359), National High Technology Research and Development Program of Guangzhou (J.F-W, 20180304001; J.F-W, 2019GZR110406004); Major Fund for International Cooperation in Guangdong (Y.L-Z, 2021A0505030021); China Postdoctoral Science Foundation (Z.Y-C, 2020M683123; X-L; 2021M703724); Guangdong Basic and Applied Basic Research Foundation (M.X-W, 2019A1515110129); Guangdong Medical Science and Technology Research Foundation (Y-J, A2021006); Guangzhou Science and Technology Plan Project (Y.l-Z, 202102010218). Funding agencies played no role in study design, analysis, or manuscript development.
Author information
Authors and Affiliations
Contributions
All authors have read and approved of the submission of this manuscript. JFW, YLZ, PY and WGZ were responsible for the entire project and revised the draft. PY performed the data extraction, statistical analysis. XL, YJ and AA interpreting the data. AA and XL drafted the first version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the ARIC study was obtained from the Johns Hopkins University, University of Mississippi, University of Minnesota, University of North Carolina at Chapel Hill, University of South Florida, Wake Forrest University. Written informed consent to participate in the ARIC study was obtained from all participants as part of the initial screening that preceded the study interview.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Basic characteristics of participants categorized according to gender. Table S2. Cox proportional hazards analysis evaluating prognostic implication of categorical TyG index for AF. Table S3. Cox proportional hazards analysis evaluating prognostic implication of categorical TyG index for AF among non-diabetic individuals. Figure S1. Multivariable-adjusted hazard ratios for AF based on restricted cubic spines inage≤54 years,age>54 years, African American,Caucasian,BMI<25.0 kg/m²,BMI 25.0-29.9 kg/m²,BMI≥30 kg/m²,nondiabetes,nonhypertension,hypertension,LDL-C<4.1 mmol/L,LDL-C≥4.1 mmol/L. Models were adjusted for age, race, body mass index, smoking, drinking, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol, use of cholesterol lowering medication, history of diabetes, hypertension, peripheral artery disease. Red line represents references for HRs, and area between dotted line represent 95% CI. Blue area represents the fraction of the population with different baseline TyG index. HR: hazard ratio, CI: confidence interval, TyG triglyceride–glucose, AF: atrial fibrillation, BMI: body mass index, LDL-C: low density lipoprotein-cholesterol.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, X., Abudukeremu, A., Jiang, Y. et al. U-shaped association between the triglyceride–glucose index and atrial fibrillation incidence in a general population without known cardiovascular disease. Cardiovasc Diabetol 22, 118 (2023). https://doi.org/10.1186/s12933-023-01777-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01777-9