Abstract
Gender and biological sex impact the pathogenesis of numerous diseases, including metabolic disorders such as diabetes. In most parts of the world, diabetes is more prevalent in men than in women, especially in middle-aged populations. In line with this, considering almost all animal models, males are more likely to develop obesity, insulin resistance and hyperglycaemia than females in response to nutritional challenges. As summarised in this review, it is now obvious that many aspects of energy balance and glucose metabolism are regulated differently in males and females and influence their predisposition to type 2 diabetes. During their reproductive life, women exhibit specificities in energy partitioning as compared with men, with carbohydrate and lipid utilisation as fuel sources that favour energy storage in subcutaneous adipose tissues and preserve them from visceral and ectopic fat accumulation. Insulin sensitivity is higher in women, who are also characterised by higher capacities for insulin secretion and incretin responses than men; although, these sex advantages all disappear when glucose tolerance deteriorates towards diabetes. Clinical and experimental observations evidence the protective actions of endogenous oestrogens, mainly through oestrogen receptor α activation in various tissues, including the brain, the liver, skeletal muscle, adipose tissue and pancreatic beta cells. However, beside sex steroids, underlying mechanisms need to be further investigated, especially the role of sex chromosomes, fetal/neonatal programming and epigenetic modifications. On the path to precision medicine, further deciphering sex-specific traits in energy balance and glucose homeostasis is indeed a priority topic to optimise individual approaches in type 2 diabetes prevention and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few years, addressing gender and sex differences has emerged as a priority topic in several medical areas, including metabolic diseases [1]. While gender mainly refers to the socially constructed identities of individuals, sex dimorphism relies on the fundamental biological disparities that differently influence physiological or pathophysiological processes in males and females. Although not fully understood, underlying mechanisms largely involve sex steroid hormones and sex chromosomes but also include sex specificities in fetal/neonatal programming and epigenetic modifications. Recent guidelines, thus, emphasise the need to consider such sex differences during preclinical (cellular and animal models) to clinical research efforts, avoiding the traditional male predominance when using these approaches [2].
It is now obvious that sex has a significant impact on the pathogenesis of metabolic disorders, such as type 2 diabetes. The first dimorphic aspect concerns diabetes prevalence, with a male predominance reported in humans and also in most animal models, with females being generally protected from diet-induced metabolic disorders [3]. Therefore, the present review aims to discuss how sex differences in energy balance and metabolic homeostasis influence susceptibility to diabetes, with a specific focus on the protective actions of endogenous oestrogens.
Diabetes is more prevalent in men: epidemiological evidence
Except in some parts of the world, such as the Middle East and North Africa, diabetes is more prevalent in men than in women, especially in middle-aged populations. Analysing 751 population-based studies (4.4 million adults from 146 countries), the NCD Risk Factor Collaboration first showed that age-standardised prevalence rates more markedly increased in men (4% to 9%) than in women (5% to 8%) between 1980 and 2014, despite some substantial disparities across geographical areas [4]. Similarly, the US National Health and Nutrition Examination Survey recently reported a higher prevalence of diabetes among men compared with women (13% vs 11% for the 2013–2016 period, in adults aged 20–79 years) [5]. The last global estimates published by the International Diabetes Federation also indicate sex differences in worldwide diabetes prevalence in adult populations (9.1% in men vs 8.4% in women), suggesting that about 12.3 million more men than women worldwide were living with diabetes in 2017. The peak in diabetes prevalence occurs earlier in men (65–69 years of age) than in women (70–79 years of age) and male predominance is, therefore, specifically observed in middle-aged populations (35–69 years of age) [6].
Studies offering systematic screening procedures in large populations confirmed a male predominance when diagnosis of diabetes was based on fasting plasma glucose (FPG) and/or HbA1c measurements, but not when considering 2 h plasma glucose after an OGTT (2hPG-OGTT). Among the 7680 men and 9251 women included in the European Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe (DECODE) study, undiagnosed diabetes and impaired fasting glucose, both defined by isolated FPG, were more prevalent in men in the 30–69 years age range. However, the prevalence of impaired glucose tolerance, was higher in women in all age groups [7]. In 13,016 inhabitants (aged 30–60 years) of the Copenhagen county (Denmark) who participated in the Inter99 study, diagnosis of dysglycaemia was reported in 49.6% (95% CI 43.4%, 55.6%) of men and 34.6% (95% CI 28.6%, 41.0%) of women by the age of 60 years. The risk of diabetes (OR 1.7 [95% CI 1.3, 2.1]) and impaired fasting glucose (OR 3.0 [95% CI 2.4, 3.7]), but not of impaired glucose tolerance (OR 1.0 [95% CI 0.9, 1.2]), appeared to be higher in men than in women [8]. In individuals with normal glucose tolerance, women generally exhibit lower FPG and HbA1c levels but increased 2hPG-OGTT levels, as compared with men [9, 10]. However, these differences could be the consequence of challenging all individuals with the same amount of glucose, regardless of sex-dependent characteristics, such as body size, muscle mass or physical fitness [9], but they could also be owing to delayed gut glucose absorption in women as compared with men [11]. These later observations perfectly illustrate the need for considering both sexes, as well as their phenotypic and biological specificities, in all studies devoted to metabolic regulation.
A critical role for sex steroid hormones in diabetes susceptibility
Both clinical and experimental studies indicate that post-pubertal sex steroid hormones largely contribute to sex differences in diabetes susceptibility. The protective role of endogenous oestrogens in women is evidenced by the deleterious impact of the menopause on body composition and glucose homeostasis, leading to an increased incidence of metabolic disorders vs premenopausal women [12]. Early menopause and premature ovarian insufficiency are associated with an increased risk of type 2 diabetes as compared with premenopausal women, while a 21–35% reduction in diabetes incidence has been reported in menopausal women receiving oestrogen-based hormonal therapy vs placebo [13,14,15]. Further demonstrating the contribution of the oestrogen pathway to diabetes susceptibility in humans, rare loss-of-function mutations in the gene encoding either aromatase (the enzyme that converts androgens into oestrogens) or oestrogen receptor α (ERα) result in dysmetabolic phenotypes in individuals of both sexes [16]. Similarly, deletion of aromatase or ERα in transgenic mice also leads to obesity, insulin resistance and impaired glucose tolerance [17, 18]. Moreover, in all animal models, oestrogen-associated protection of females from high-fat-diet (HFD)-induced obesity and hyperglycaemia is totally abolished by bilateral ovariectomy, but restored by oestrogen administration [18, 19].
Androgens are also associated with metabolic risks, but mainly in pathophysiological situations leading to unbalanced androgen/oestrogen ratio. In men with hypogonadism, low testosterone plasma concentrations are correlated with an increased risk of type 2 diabetes and vascular diseases, while testosterone supplementation clearly improves glucose and lipid homeostasis [20]. However, besides the direct activation of androgen receptors, part of the metabolic actions of testosterone can also result from indirect mechanisms, through its aromatisation into oestrogens. Conversely, androgen excess can lead to significant metabolic alterations in women. High testosterone plasma levels are thought to favour insulin resistance and diabetes in women with polycystic ovary syndrome (PCOS), but the most direct demonstration comes from the development of metabolic disorders in transsexual people on high-dose androgens [3]. Furthermore, dihydrotestosterone administration was recently reported to predispose female mice to diabetes by promoting insulin resistance and beta cell failure through androgen receptor activation in neurons and beta cells, respectively [21]. Overall, it is now clear that androgens play a complex role in the pathogenesis of obesity and type 2 diabetes in both males and females, as recently reviewed [21, 22].
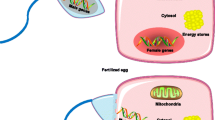
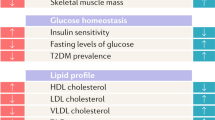
In summary, although the androgen/oestrogen ratio undoubtedly has an impact on metabolic regulation, both human and animal studies demonstrate that endogenous oestrogens protect females from type 2 diabetes, at least during their reproductive life. As detailed below, oestrogens largely contribute to sexual dimorphisms in energy balance and metabolic homeostasis, which are the main determinants of sex differences in type 2 diabetes susceptibility. The main sexually dimorphic body composition and metabolic traits in humans, and the tissue-specific actions of oestrogens (as reported in animal models) are summarised in Figs 1 and 2, respectively.
Main sex dimorphisms in body composition and metabolic homeostasis in humans (premenopausal women vs age-matched men). This figure is available as part of a downloadable slideset
Tissue-specific actions of oestrogens on energy balance and metabolic regulation in rodent models. FGF21, fibroblast growth factor 21. This figure is available as part of a downloadable slideset
Biological sex as a determinant of energy balance and body composition
Sex influences energy partitioning
The sexual dimorphism regarding energy partitioning is classically viewed as an evolutionary adaptation allowing females to better withstand periods of undernutrition, with the ultimate aim of preserving their reproductive functions. Energy storage is generally favoured in females, whereas males predominantly mobilise energy stores to enable sustained physical activity [3]. Sex differences in adipose tissue distribution respond to these physiological considerations, with a predominance of subcutaneous tissue in women, which is better adapted for large and long-term storage. Further supporting functional differences in adipose tissue, sex-specific gene expression signatures were recently found in human abdominal and gluteal subcutaneous depots [23].
Sex also influences the utilisation of carbohydrates and lipids as fuel sources. At rest and during the post-absorptive state, women are more likely to incorporate NEFAs into triacylglycerols, thus promoting fat storage, whereas men are more prone to produce energy through plasma NEFA oxidation. Metabolic adaptation during exercise also differs between the sexes since women preferentially oxidise lipids while men use carbohydrates as the predominant fuel source [3].
Known to play a critical role in the regulation of energy storage and metabolic fluxes, in a functional perspective, the liver is undoubtedly one of the most sexually dimorphic organs [24]. Indeed, in order to regulate fertility in relation to nutrient availability, activation of ERα in hepatocytes adapts hepatic metabolism in female mice to control lipid synthesis, lipoprotein production and IGF-1 expression [24]. Moreover, while male mice restrain lipogenic and gluconeogenic pathways to preserve amino acid reserves in conditions of short-term fasting, female mice maintain hepatic lipid synthesis using amino acids as a fuel source, clearly illustrating sex differences in liver-associated metabolic adaptations [25].
Sex specificities in energy expenditure
Contrasting with observations in rodent models, clear differences have not been demonstrated in energy expenditure between women and men when adjusted for lean mass [26]. The relative contribution of fat mass to resting metabolic rate is higher in women than in men, even after adjustment for plasma sex steroid concentrations, body fat distribution and insulin sensitivity [27]. This female trait correlates with higher expression of genes involved in mitochondrial function in subcutaneous adipose tissues, including UCP1 [27]. Accordingly, upon the recent study of sex differences in cardio-metabolic traits in a large panel of inbred mouse strains, males were found to have reduced mitochondrial function in adipose tissues, which was associated with an increased susceptibility to obesity and metabolic disorders. These sex differences correlated with the expression of a cluster of genes involved in adipose tissue ‘beiging’ and mitochondrial functions in adipose tissues [28]. In line with this observation, oestrogens have recently been demonstrated to enhance thermogenesis in brown adipose tissue and to promote beiging of white adipocytes. Indeed, in vitro and in vivo approaches have demonstrated that selective activation of ERα induces adipose tissue beiging through induction of AMP-activated protein kinase (AMPK) and adipose triglyceride lipase (ATGL)-mediated lipolysis, resulting in NEFA generation and uncoupling protein 1 (UCP-1) activation [29]. Finally, as revealed by 18F-fluoro-2-deoxy-d-glucose (18F-FDG) positron-emission tomography–computed tomography (PET–CT) scanning, brown adipose tissue is better preserved and more metabolically active in women than in men, thus contributing to enhanced energy expenditure in the former [30, 31].
Besides their influence on adipose tissues, oestrogens contribute to sexual dimorphism in energy balance through direct effects on the central nervous system (CNS). In rodent models, ERα activation in hypothalamic pro-opiomelanocortin (POMC) and steroidogenic factor-1 (SF-1) neurons controls food intake and energy expenditure, respectively [32]. More specifically, ERα signalling induces AMPK inhibition in the ventromedial nucleus, leading to enhanced thermogenesis in brown adipose tissue through the sympathetic nervous system [33]. Oestrogens also enhance leptin sensitivity within the brain, reinforcing their impact on food intake [34]. In addition, as compared with males, female mice are less prone to HFD-induced hypothalamic inflammation and lipotoxicity; in the CNS they have lower concentrations of saturated fatty acids and sphingolipids but higher amounts of polyunsaturated fatty acids [35].
In contrast, oestrogen-induced peripheral signals are also able to regulate energy expenditure. For instance, ERα activation in hepatocytes indirectly promotes energy expenditure in female mice by enhancing fibroblast growth factor 21 (FGF21) synthesis, thus conferring protection against adipose tissue accumulation [36].
Consequences on body composition and ectopic fat
As compared with age-matched men, healthy premenopausal women exhibit higher global fat mass and reduced fat-free mass due to lower skeletal muscle mass. As previously mentioned, women are characterised by an increased propensity to store adipose tissue in subcutaneous sites, especially in gluteofemoral locations, as compared with preferential deposition in visceral area in men. This leads to significant sex differences in body composition [3, 26]. Of note, women are also less susceptible to ectopic fat deposition in most tissues, such as the liver or the myocardium. Women are, thus, protected from non-alcoholic fatty liver disease (NAFLD) before menopause, with the protective role of oestrogens having been evidenced experimentally [37]. Consistent with this, lower dietary fatty acid oxidation and a sustained increase in de novo lipogenesis in the liver have been reported in healthy men, as compared with women [38]. Conversely, women have a higher propensity to accumulate intramyocellular lipids in leg skeletal muscles, but without deleterious consequences on insulin sensitivity [39]. This probably explains why, despite a female predominance in the worldwide prevalence of obesity, diabetes is more prevalent in men [3]. Interestingly, at least in middle-aged populations of European origin, women have a higher BMI than men at diagnosis of type 2 diabetes [40].
Although ageing induces body composition changes in both sexes, menopause triggers the progressive accumulation of visceral fat that contributes to the increased risk of metabolic disorders [12]. Sex steroids influence body composition in both sexes and post-menopausal changes, thus, illustrates the beneficial role of oestrogens in women. Recent data from mouse models also reveal that oestrogen signalling in adipocytes protects mice from adipose tissue inflammation and fibrosis and, thus, contributes to the prevention of obesity [41]. However, sex steroids are not the only contributors to the sexual dimorphism in body composition. Indeed, new mouse models that allow us to dissociate the specific contribution of sex chromosomes from the influence of gonadal hormones have recently provided evidence that the number of X chromosomes is positively associated with weight gain and adiposity, whereas the Y chromosome exerts deleterious effects on glucose metabolism [42].
Finally, it is obvious that such sex-specific biological traits interfere with environmental determinants to modulate individual susceptibility to obesity and type 2 diabetes in humans. For instance, gender-specific patterns in dietary behaviour, mainly influenced by cultural and social factors, are likely to have an impact on the incidence of metabolic disorders in both sexes [43].
Sex-dimorphic traits in the regulation of glucose homeostasis
Females are more insulin sensitive than males
In a large population of individuals with normal blood glucose levels, insulin sensitivity was assessed with the oral glucose insulin sensitivity index and found to be higher in women than in men, even after adjustment for age and BMI [10]. However, this sex advantage disappears when glucose tolerance deteriorates towards type 2 diabetes, with a similar extent of insulin resistance observed in both sexes [44]. Hyperinsulinaemic−euglycaemic clamp studies confirm that healthy women are more sensitive to insulin than men when matched for physical fitness (41% increase in whole body insulin sensitivity). This is due to enhanced glucose uptake by skeletal muscle in women [45, 46]. In agreement with this, sex differences have been reported in muscle characteristics, with a higher proportion of type I fibres and capillary density in women, which both favour enhanced insulin action [46].
The observation that women are less prone to insulin resistance than men is rather unexpected considering their increased fat mass, circulating NEFA levels and lipid content in myocytes, as well as a lower skeletal muscle mass. As a plausible explanation, experimental data indicate that women are protected from NEFA-induced insulin resistance and, thus, more resistant to lipotoxicity, especially in skeletal muscles [47]. Oestrogens confer protection against insulin resistance through activation of the ERα pathway in insulin-sensitive tissues, as demonstrated in mouse models [18, 19]. For example, in mice with specific myocyte ERα deletion, muscle-associated oxidative metabolism was altered and hyperglycaemia developed, indicating that oestrogens preserve mitochondrial function and insulin sensitivity [48]. The liver is also involved in this phenomenon, since ERα signalling in hepatocytes mediates protective effects against steatosis and insulin resistance in HFD-fed female mice [49].
Sex also has an impact on pancreatic endocrine function
In normoglycaemic individuals, estimations of beta cell function following an OGTT or a standardised meal suggest that women exhibit a greater insulin secretion capacity than men [10]. Insulin secretion is more markedly increased in obese men, as a way to compensate for a higher level of insulin resistance. However, type 2 diabetes is characterised by similar impairments in beta cell function in both sexes [44]. Besides functional differences, analysis of pancreatic biopsies from human donors recently estimated that islets from women contain 6% more beta cells than those from men [50].
Using human islets or rodent models, experimental studies demonstrate that sex hormones act as key regulators of islet biology in a sex-specific manner. More specifically, endogenous oestrogens stimulate insulin synthesis and secretion and exert protective effects on islets from females, preserving beta cell function and preventing apoptosis induced by metabolic injuries, such as oxidative stress and lipotoxicity [51]. Interestingly, the beneficial actions of oestrogens on beta cells could explain why the male predominance in diabetes prevalence is not restricted to type 2 diabetes but also applies to insulin-deficient forms of diabetes, such as type 1 diabetes, that are diagnosed post puberty [52]. Indeed, type 1 diabetes incidence is characterised by a sex ratio close to 1 in children, but a significant male excess (sex ratio ~1.7) is reported in the 15–40 year age range, mainly in populations of European origin [53].
Finally, it has been proposed that enhanced insulin secretion in women could also reflect sex differences in glucose-dependent glucagon-like peptide-1 (GLP-1) secretion. Normoglycaemic women were, indeed, characterised by a 20% increase in serum GLP-1 concentrations following an OGTT as compared with men, even after adjustment for BMI. This sex difference was no longer observed in individuals with prediabetes or type 2 diabetes, irrespective of age or weight [54]. Further supporting the enhancing effect of oestrogens on incretin response, oestradiol was demonstrated to positively regulate proglucagon-derived peptide secretion in mouse and human alpha and L cells [55].
Future directions: how far are we from a sex-specific medicine in diabetes?
Alongside the critical roles of oestrogens (as described in this review), the complex mechanisms responsible for sex-dimorphic metabolic regulation need to be further characterised. It is suggested that analysis of sex steroid balance in males and females cannot be restricted to circulating hormones levels but should also integrate their molecular regulation at the tissue level. For example, aromatisation should be further considered, especially in well-recognised sites of oestrogen biosynthesis, such as the brain or adipose tissues. In addition, it is also important to consider the regulation of local steroid activity resulting from sulfonation and desulfation processes, which lead to hormonally deactivated or activated molecules, respectively. In mouse models, both steroid sulfatase and oestrogen sulfotransferase (EST) have been demonstrated to interfere with the pathogenesis of type 2 diabetes in a sex-specific manner [56]. For instance, inactivation of EST, the enzyme responsible for oestrogen deactivation, increases energy expenditure, improves insulin sensitivity, and reduces hepatic gluconeogenesis and lipogenesis in different mouse models of obesity-related metabolic disorders, but only in females [57]. Although not easy to address (and largely underestimated until now), such fine regulation of the paracrine and intracrine actions of sex steroids could be crucial for local metabolic regulation in both sexes.
Interesting new insights have been provided into the contribution of sex chromosomes to dimorphic gene expression in metabolic tissues [58]. New fields are also currently being explored, such as the role of the gut microbiome in sex-biased susceptibility to metabolic disorders [59]. Finally, in addition to genetic differences, sex-specific epigenetic modifications in responses to various physiological or pathophysiological situations, including exposure to hyperglycaemia and environmental factors, undoubtedly represent an additional layer for integration into the determinants of sex differences in metabolism [3, 60]. Therefore, the study of sexual dimorphism in metabolism should no longer be limited to the period of reproductive life alone, but considered from the preconception period and during the entire life.
Deciphering sex-specific traits in energy balance and glucose homeostasis is certainly of major interest to optimise individual approaches to diabetes prevention and treatment. Sex has already been reported to influence therapeutic responses in type 2 diabetes. For instance, insulin-naive women initiating a basal insulin regimen showed a smaller improvement in HbA1c associated with an increased rate of hypoglycaemia vs men [61]. This may be related to the fact that women exhibit reduced counter-regulatory hormonal response (glucagon and adrenaline [epinephrine]), together with lower rates of endogenous glucose production compared with men [62]. More widely, to definitely consider sex as a pillar of precision medicine, sex dimorphisms in metabolic pathways still need to be better characterised in humans, considering the effect of age, ethnic origin and pathophysiological status, such as the different phenotypical clusters of diabetes.
Finally, studying sex differences in metabolism could also lead to the development of new therapeutic approaches targeting sex-dimorphic metabolic pathways or sex hormone receptors. Countering the deleterious metabolic effects of menopause in women at risk of type 2 diabetes is obviously a priority objective in terms of public health. Beyond lifestyle adaptations, hormone replacement therapy has been associated with reduced type 2 diabetes incidence in clinical trials, as previously mentioned [14, 15], but the uncertainties regarding its benefit–risk balance do not allow for its extended use in this context. Menopausal women could, thus, particularly benefit from new selective oestrogen receptor modulators that are able to mediate the protective actions of oestrogens on body composition and glucose metabolism with limited side effects on reproductive tissues [63]. Tissue-specific targeting could also be a relevant strategy, as illustrated by the protection conferred by a GLP-1–oestrogen conjugate against diet-induced obesity and glucose intolerance in mice via selective ERα activation in the CNS and the pancreas [64].
Conclusion
It is now clear that many aspects of energy and glucose homeostasis are regulated differently in males and females, influencing their predisposition to diabetes and associated metabolic disorders. Moreover, sex biases have also been described in the occurrence and the progression of diabetic complications, reinforcing the need for sex-specific approaches in diabetes management [65]. As in almost all diseases, personalised management of diabetes should take into account the sex of the patient.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- CNS:
-
Central nervous system
- ERα:
-
Oestrogen receptor α
- EST:
-
Oestrogen sulfotransferase
- FPG:
-
Fasting plasma glucose
- GLP-1:
-
Glucagon-like peptide-1
- HFD:
-
High-fat-diet
- 2hPG-OGTT:
-
2 h plasma glucose after an OGTT
References
Rich-Edwards JW, Kaiser UB, Chen GL, Manson JE, Goldstein JM (2018) Sex and gender differences research design for basic, clinical, and population studies: essentials for investigators. Endocr Rev 39(4):424–439. https://doi.org/10.1210/er.2017-00246
Mauvais-Jarvis F, Arnold AP, Reue K (2017) A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab 25(6):1216–1230. https://doi.org/10.1016/j.cmet.2017.04.033
Kautzky-Willer A, Harreiter J, Pacini G (2016) Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 37(3):278–316. https://doi.org/10.1210/er.2015-1137
NCD Risk Factor Collaboration (NCD-RisC) (2016) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387(10027):1513–1530. https://doi.org/10.1016/S0140-6736(16)00618-8
Peters SAE, Muntner P, Woodward M (2019) Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation 139(8):1025–1035. https://doi.org/10.1161/CIRCULATIONAHA.118.035550
Cho NH, Shaw JE, Karuranga S et al (2018) IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281. https://doi.org/10.1016/j.diabres.2018.02.023
The DECODE Study Group (2003) Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 26(1):61–69. https://doi.org/10.2337/diacare.26.1.61
Glumer C, Jorgensen T, Borch-Johnsen K (2003) Prevalences of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care 26(8):2335–2340. https://doi.org/10.2337/diacare.26.8.2335
Faerch K, Borch-Johnsen K, Vaag A, Jorgensen T, Witte DR (2010) Sex differences in glucose levels: a consequence of physiology or methodological convenience? The Inter99 study. Diabetologia 53(5):858–865. https://doi.org/10.1007/s00125-010-1673-4
Kautzky-Willer A, Brazzale AR, Moro E et al (2012) Influence of increasing BMI on insulin sensitivity and secretion in normotolerant men and women of a wide age span. Obesity 20(10):1966–1973. https://doi.org/10.1038/oby.2011.384
Anderwald C, Gastaldelli A, Tura A et al (2011) Mechanism and effects of glucose absorption during an oral glucose tolerance test among females and males. J Clin Endocrinol Metab 96(2):515–524. https://doi.org/10.1210/jc.2010-1398
Mauvais-Jarvis F, Clegg DJ, Hevener AL (2013) The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34(3):309–338. https://doi.org/10.1210/er.2012-1055
Anagnostis P, Christou K, Artzouchaltzi AM et al (2019) Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Endocrinol 180(1):41–50. https://doi.org/10.1530/EJE-18-0602
Kanaya AM, Herrington D, Vittinghoff E et al (2003) Glycemic effects of postmenopausal hormone therapy: the heart and estrogen/progestin replacement study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 138(1):1–9. https://doi.org/10.7326/0003-4819-138-1-200301070-00005
Margolis KL, Bonds DE, Rodabough RJ et al (2004) Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47(7):1175–1187. https://doi.org/10.1007/s00125-004-1448-x
Grumbach MM, Auchus RJ (1999) Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab 84(12):4677–4694. https://doi.org/10.1210/jcem.84.12.6290
Jones ME, Thorburn AW, Britt KL et al (2000) Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A 97(23):12735–12740. https://doi.org/10.1073/pnas.97.23.12735
Handgraaf S, Riant E, Fabre A et al (2013) Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas ERαAF-1 is dispensable. Diabetes 62(12):4098–4108. https://doi.org/10.2337/db13-0282
Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P (2009) Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 150(5):2109–2117. https://doi.org/10.1210/en.2008-0971
Yassin A, Haider A, Haider KS et al (2019) Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: eight-year data from a registry study. Diabetes Care 42(6):1104–1111. https://doi.org/10.2337/dc18-2388
Navarro G, Allard C, Morford JJ et al (2018) Androgen excess in pancreatic beta cells and neurons predisposes female mice to type 2 diabetes. JCI Insight 3(12). https://doi.org/10.1172/jci.insight.98607
Hammes SR, Levin ER (2019) Impact of estrogens in males and androgens in females. J Clin Invest 129(5):1818–1826. https://doi.org/10.1172/JCI125755
Karastergiou K, Fried SK, Xie H et al (2013) Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab 98(1):362–371. https://doi.org/10.1210/jc.2012-2953
Maggi A, Della Torre S (2018) Sex, metabolism and health. Mol Metab 15:3–7. https://doi.org/10.1016/j.molmet.2018.02.012
Della Torre S, Mitro N, Meda C et al (2018) Short-term fasting reveals amino acid metabolism as a major sex-discriminating factor in the liver. Cell Metab 28(2):256–267. https://doi.org/10.1016/j.cmet.2018.05.021
Karastergiou K, Smith SR, Greenberg AS, Fried SK (2012) Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ 3(1):13. https://doi.org/10.1186/2042-6410-3-13
Nookaew I, Svensson PA, Jacobson P et al (2013) Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J Clin Endocrinol Metab 98(2):E370–E378. https://doi.org/10.1210/jc.2012-2764
Norheim F, Hasin-Brumshtein Y, Vergnes L et al (2019) Gene-by-sex interactions in mitochondrial functions and cardio-metabolic traits. Cell Metab 29(4):932–949 e934. https://doi.org/10.1016/j.cmet.2018.12.013
Santos RS, Frank AP, Fatima LA, Palmer BF, Oz OK, Clegg DJ (2018) Activation of estrogen receptor alpha induces beiging of adipocytes. Mol Metab 18:51–59. https://doi.org/10.1016/j.molmet.2018.09.002
Cypess AM, Lehman S, Williams G et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360(15):1509–1517. https://doi.org/10.1056/NEJMoa0810780
Virtanen KA, Lidell ME, Orava J et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360(15):1518–1525. https://doi.org/10.1056/NEJMoa0808949
Xu Y, Nedungadi TP, Zhu L et al (2011) Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14(4):453–465. https://doi.org/10.1016/j.cmet.2011.08.009
de Morentin PBM, Gonzalez-Garcia I, Martins L et al (2014) Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 20(1):41–53. https://doi.org/10.1016/j.cmet.2014.03.031
Clegg DJ, Brown LM, Woods SC, Benoit SC (2006) Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55(4):978–987. https://doi.org/10.2337/diabetes.55.04.06.db05-1339
Morselli E, Frank AP, Palmer BF, Rodriguez-Navas C, Criollo A, Clegg DJ (2016) A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int J Obes 40(2):206–209. https://doi.org/10.1038/ijo.2015.114
Allard C, Bonnet F, Xu B et al (2019) Activation of hepatic estrogen receptor-alpha increases energy expenditure by stimulating the production of fibroblast growth factor 21 in female mice. Mol Metab 22:62–70. https://doi.org/10.1016/j.molmet.2019.02.002
Lonardo A, Nascimbeni F, Ballestri S et al (2019) Sex differences in NAFLD: state of the art and identification of research gaps. Hepatology. 70(4):1457–1469. https://doi.org/10.1002/hep.30626
Pramfalk C, Pavlides M, Banerjee R et al (2015) Sex-specific differences in hepatic fat oxidation and synthesis may explain the higher propensity for NAFLD in men. J Clin Endocrinol Metab 100(12):4425–4433. https://doi.org/10.1210/jc.2015-2649
Moro C, Galgani JE, Luu L et al (2009) Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J Clin Endocrinol Metab 94(9):3440–3447. https://doi.org/10.1210/jc.2009-0053
Logue J, Walker JJ, Colhoun HM et al (2011) Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 54(12):3003–3006. https://doi.org/10.1007/s00125-011-2313-3
Davis KE, Neinast MD, Sun K et al (2013) The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2(3):227–242. https://doi.org/10.1016/j.molmet.2013.05.006
Chen X, McClusky R, Itoh Y, Reue K, Arnold AP (2013) X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology 154(3):1092–1104. https://doi.org/10.1210/en.2012-2098
Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G (2017) Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res 120:34–42. https://doi.org/10.1016/j.phrs.2017.03.008
Tura A, Pacini G, Moro E, Vrbikova J, Bendlova B, Kautzky-Willer A (2018) Sex- and age-related differences of metabolic parameters in impaired glucose metabolism and type 2 diabetes compared to normal glucose tolerance. Diabetes Res Clin Pract 146:67–75. https://doi.org/10.1016/j.diabres.2018.09.019
Nuutila P, Knuuti MJ, Maki M et al (1995) Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes 44(1):31–36. https://doi.org/10.2337/diab.44.1.31
Lundsgaard AM, Kiens B (2014) Gender differences in skeletal muscle substrate metabolism - molecular mechanisms and insulin sensitivity. Front Endocrinol 5:195. https://doi.org/10.3389/fendo.2014.00195
Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT (2001) Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes 50(6):1344–1350. https://doi.org/10.2337/diabetes.50.6.1344
Ribas V, Drew BG, Zhou Z et al (2016) Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med 8(334):334ra354. https://doi.org/10.1126/scitranslmed.aad3815
Zhu L, Brown WC, Cai Q et al (2013) Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62(2):424–434. https://doi.org/10.2337/db11-1718
Marchese E, Rodeghier C, Monson RS et al (2015) Enumerating β-cells in whole human islets: sex differences and associations with clinical outcomes after islet transplantation. Diabetes Care 38(11):e176–e177. https://doi.org/10.2337/dc15-0723
Mauvais-Jarvis F (2016) Role of sex steroids in β cell function, growth, and survival. Trends Endocrinol Metab 27(12):844–855. https://doi.org/10.1016/j.tem.2016.08.008
Gale EA, Gillespie KM (2001) Diabetes and gender. Diabetologia 44(1):3–15. https://doi.org/10.1007/s001250051573
Ostman J, Lonnberg G, Arnqvist HJ et al (2008) Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983-2002. J Intern Med 263(4):386–394. https://doi.org/10.1111/j.1365-2796.2007.01896.x
Faerch K, Torekov SS, Vistisen D et al (2015) GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes 64(7):2513–2525. https://doi.org/10.2337/db14-1751
Handgraaf S, Dusaulcy R, Visentin F, Philippe J, Gosmain Y (2018) 17-β Estradiol regulates proglucagon-derived peptide secretion in mouse and human α- and L cells. JCI Insight 3(7):e98569. https://doi.org/10.1172/jci.insight.98569
Garbacz WG, Jiang M, Xie W (2017) Sex-dependent role of estrogen sulfotransferase and steroid sulfatase in metabolic homeostasis. Adv Exp Med Biol 1043:455–469. https://doi.org/10.1007/978-3-319-70178-3_21
Gao J, He J, Shi X et al (2012) Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes. Diabetes 61(6):1543–1551. https://doi.org/10.2337/db11-1152
Zore T, Palafox M, Reue K (2018) Sex differences in obesity, lipid metabolism, and inflammation—a role for the sex chromosomes? Mol Metab 15:35–44. https://doi.org/10.1016/j.molmet.2018.04.003
Weger BD, Gobet C, Yeung J et al (2019) The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metab 29(2):362–382. https://doi.org/10.1016/j.cmet.2018.09.023
Dearden L, Bouret SG, Ozanne SE (2018) Sex and gender differences in developmental programming of metabolism. Mol Metab 15:8–19. https://doi.org/10.1016/j.molmet.2018.04.007
Kautzky-Willer A, Kosi L, Lin J, Mihaljevic R (2015) Gender-based differences in glycaemic control and hypoglycaemia prevalence in patients with type 2 diabetes: results from patient-level pooled data of six randomized controlled trials. Diabetes Obes Metab 17(6):533–540. https://doi.org/10.1111/dom.12449
Amiel SA, Maran A, Powrie JK, Umpleby AM, Macdonald IA (1993) Gender differences in counterregulation to hypoglycaemia. Diabetologia 36(5):460–464. https://doi.org/10.1007/bf00402284
Gourdy P, Guillaume M, Fontaine C et al (2018) Estrogen receptor subcellular localization and cardiometabolism. Mol Metab 15:56–69. https://doi.org/10.1016/j.molmet.2018.05.009
Finan B, Yang B, Ottaway N et al (2012) Targeted estrogen delivery reverses the metabolic syndrome. Nat Med 18(12):1847–1856. https://doi.org/10.1038/nm.3009
Maric-Bilkan C (2017) Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin Sci 131(9):833–846. https://doi.org/10.1042/CS20160998
Funding
Work in the authors’ laboratory is supported by grants from the Agence Nationale de la Recherche (ANR-18-CE14-0002-01), the Société Francophone du Diabète (SFD), the Société Française de Nutrition (SFN) and the Région Occitanie (Région/FEDER - HEPATOMICS).
Author information
Authors and Affiliations
Contributions
BT and PG were responsible for drafting the article. All authors revised it critically and provided important intellectual content. All authors approved the version to be published.
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Slideset of figures
(PPTX 278 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tramunt, B., Smati, S., Grandgeorge, N. et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 63, 453–461 (2020). https://doi.org/10.1007/s00125-019-05040-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-019-05040-3