Abstract
Background
Ischemic heart disease (IHD) without diabetes is considered an important challenge to human health and is associated with a poor prognosis, as well as a lack of health awareness. We prospectively investigated the relationship between the triglyceride-glucose (TyG) index, a surrogate marker of early insulin resistance, and incident IHD risk in a large cohort of nondiabetic Korean adults using National Health Insurance Service data.
Methods
We assessed 16,455 participants (8426 men and 8029 women) without diabetes using data from a health risk assessment study (HERAS) and Korea Health Insurance Review and Assessment (HIRA) data. The participants were divided into four groups according to TyG index quartiles, calculated as ln [fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2]. We prospectively assessed hazard ratios (HRs) with 95% confidence intervals (CIs) for IHD using multivariate Cox proportional-hazards regression models over a 50-month period that followed the baseline survey.
Results
During the follow-up period, 322 (2.0%) participants developed IHD. HRs of IHD for TyG index quartiles 2–4 were 1.61 (95% CI 1.05–2.48), 1.85 (95% CI 1.21–2.81), and 2.29 (95% CI 1.50–3.51), respectively, after adjusting for age, sex, body mass index, smoking status, alcohol intake, and physical activity.
Conclusions
A higher TyG index precedes and significantly predicts future IHD among nondiabetic Koreans. Accordingly, the TyG index may be a useful measure in assessing cardiovascular risk for nondiabetic adults in the preclinical stage.
Similar content being viewed by others
Background
Ischemic heart disease (IHD) is a source of premature morbidity and mortality among middle-aged and older individuals, and it is an important challenge to human health in both developing and developed countries [1]. The burden of premature IHD in an ageing population cannot be underestimated, as it is a factor that decreases quality of life and increases social burden [2]. Accordingly, assessing and identifying potential risks for IHD in the preclinical stage is worthwhile, facilitating disease prevention and slowing the progression of IHD.
Accumulating evidence suggests that the triglyceride-glucose (TyG) index, a simple and widely accessible measure, is a novel and surrogate marker for early insulin resistance [3, 4]. Epidemiological studies conducted in the Korean population have shown that the TyG index is a better indicator of metabolic syndrome and type 2 diabetes than the homeostasis model assessment of insulin resistance (HOMA-IR) [5, 6]. Koreans comprise a group of East Asians of ethnic homogeneity, with lower overall body mass index (BMI) values and much higher proportions of carbohydrate intake than Westerners [7]. The prevalence of both hypertriglyceridemia (≥ 150 mg/dL) and impaired fasting glucose is reportedly 20–30% among Korean adults, contributing to increased risks of coronary heart disease, according to data from the Korea National Health and Nutrition Examination Survey (KNHANES) [8, 9].
Prospective studies of predictive values of the TyG index for cardiovascular diseases (CVD) have primarily focused on pre-existing coronary arterial disease or diabetes mortality [10, 11], and the TyG index has been shown to be associated with subclinical atherosclerosis symptoms, such as arterial stiffness and preclinical coronary arterial calcification [12,13,14]. Accordingly, since nondiabetic individuals with IHD tend to exhibit poorer prognosis than diabetic patients without IHD [15, 16], we prospectively investigated potential relationships between the TyG index and IHD incidence within a large-scale, community-dwelling, nondiabetic adult cohort using National Health Insurance Service data.
Methods
Study participants
This study is based on a health risk assessment study (HERAS) that aimed to characterize cardiovascular risk factors and to explore surrogate markers of CVD in Korean adults. The study cohort consisted of 20,530 individuals aged ≥ 20 years who voluntarily visited the Health Promotion Center of Gangnam Severance Hospital, Yonsei University College of Medicine for regular health examinations between November 2006 and June 2010. Among 20,530 participants initially assessed, 1,590 (7.7%) participants with a history of IHD or ischemic stroke, a previous diagnosis of type 2 diabetes, or a fasting plasma glucose level ≥ 126 mg/dL [17] were excluded. We also excluded participants who met at least one of the following criteria: age < 30 years, missing data, current use of dyslipidaemia medication or aspirin, or high-sensitivity C-reactive protein (hsCRP) levels ≥ 10 mg/L (N = 2485). After exclusion criteria were applied, 16,455 participants (8426 men and 8029 women) were included in our final analysis (Fig. 1).
Data collection
Each participant completed a lifestyle and medical history questionnaire that included information regarding cigarette smoking, alcohol consumption, and physical activity. Smoking status was defined using the following categories: non-smoker, ex-smoker, and current smoker. Questions regarding alcohol intake included information regarding consumption frequency on a weekly basis. Regular alcohol consumption was defined as alcohol consumption ≥ 140 g per week. Participants were asked about their levels of physical exercise on a weekly basis, and regular exercise was defined as physical activity of moderate intensity ≥ three times per week. Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, in light indoor clothing without shoes. BMI was calculated as an individual’s weight in kilograms divided by the square of his/her height in metres (kg/m2). Systolic blood pressure and diastolic blood pressure were measured on the patient’s right arm using a standard mercury sphygmomanometer in the sitting position after 10 min of rest (Baumanometer, W.A. Baum Co Inc., Copiague, NY, USA). All blood samples were obtained from the antecubital vein after overnight fasting for 12 h. Fasting plasma glucose, total cholesterol, triglyceride, and high-density lipoprotein (HDL) cholesterol levels were measured via enzymatic methods using a Hitachi 7600 automated chemistry analyser (Hitachi Co.; Tokyo, Japan). hsCRP concentrations were measured with a Roche/Hitachi 912 System (Roche Diagnostics, Indianapolis, IN, USA) using a latex-enhanced immunoturbidimetric method with a low limit of detection of 0.09 mg/L. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, a diastolic blood pressure ≥ 90 mmHg, or current use of hypertension medication [18]. Chronic kidney disease (CKD) was defined either as renal tissue damage or reduced renal functioning, as determined by an eGFR value < 60 mL/min/1.73 m2 or proteinuria 1+ or greater [19].
Study outcomes
The primary outcome assessed was IHD, which consisted of angina pectoris (ICD-10 code I20) or acute myocardial infarction (ICD-10 code I21) that occurred after initial study enrolment. To define baseline and post-survey outcomes, we linked a personal, 13-digit identification number that was assigned to each subject with Korea Health Insurance Review and Assessment (HIRA) data, which is a repository of claims data collected in the process of reimbursing healthcare providers, between November 2006 and December 2010. Participants that were found to have had IHD or ischemic stroke (ICD-10 codes I20, I21, and I63) at the time of their initial assessment were excluded before the final analysis.
Statistical analysis
TyG index values were categorised into quartiles as follows: Q1 (≤ 8.08), Q2 (8.09–8.45), Q3 (8.46–8.85), and Q4 (≥ 8.86). All data are presented as means with standard deviations or percentages. The baseline characteristics of the study population according to the TyG index quartiles were compared using an analysis of variance (ANOVA) model for continuous variables and the Pearson’s Chi-squared test for categorical variables. Kaplan–Meier curves were used to assess the cumulative incidence of IHD. The log-rank test was used to determine whether the distributions of cumulative IHD incidence differed among groups. Pairwise comparisons of receiver-operating characteristic (ROC) curves were used to contrast areas under ROC curves (AUC) for IHD incidence based on TyG index, fasting plasma glucose, and serum triglyceride levels. Further, AUC values were used to test the sensitivity and specificity of biomarkers for predicting IHD. In multivariate analysis, after setting the lowest TyG index value quartile as a reference group, hazard ratios (HRs) and 95% confidence intervals (CIs) for incident IHD were calculated using the Cox proportional hazards regression model after adjusting for potential confounding variables [4]. An ex-post power calculation was also performed: for a HR of 1.5, the calculated power was 0.983; for a HR of 2.0, the calculated power was > 0.999. All analyses were performed using SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided, and statistical significance was set at P < 0.05.
Results
Table 1 shows the baseline characteristics of the study population (n = 16,455; 8,426 men and 8,029 women) according to TyG index quartiles. The mean age and BMI of the study population were 46.1 ± 9.5 years and 23.4 ± 3.0 kg/m2, respectively. The mean fasting plasma glucose concentration was 91.4 ± 9.8 mg/dL, the mean triglycerides level was 124.2 ± 84.9 mg/dL, and the mean TyG index value was 8.49 ± 0.56. The prevalences of hypertriglyceridemia and impaired fasting glucose were 18.0% and 25.0%, respectively. Older adults, aged 65 years and older, comprised 4.5% of the study population.
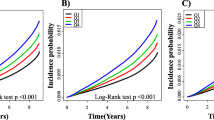
Mean BMI, mean arterial pressure, total cholesterol, and hsCRP values were highest and mean HDL-cholesterol levels were lowest in the highest TyG index quartile group. The greatest proportions of current smokers and alcohol drinkers were members of the fourth TyG index quartile, whereas the proportion of individuals who participated in regular exercise was highest in the first TyG index quartile. The higher TyG index groups had a significantly elevated cumulative incidence of IHD over a 50-month period that followed the baseline survey (log-rank test, P < 0.001) (Fig. 2).
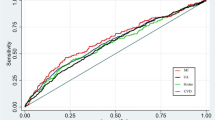
Using a pairwise comparison of ROC analyses of incident IHD, the AUC of TyG index data was significantly higher than that of fasting plasma glucose (P = 0.016) and was marginally significant when compared with the AUC produced using serum triglyceride level data (P = 0.058). The sensitivity, specificity, and AUC of the TyG index for classifying IHD were 80.8%, 37.2%, and 0.613, respectively (Table 2).
Table 3; Fig. 3 show results of the multivariate Cox proportional hazards regression analysis for the prediction of IHD according to TyG index quartile. A total of 322 individuals (2.0%, 322/16,455) developed IHD during the follow-up period. The incidence rate (per 1,000 person years) of IHD increased proportionally as TyG index quartile increased. Compared with the first TyG index quartile, the HRs of incident IHD for the second, third, and fourth quartiles increased in a dose-responsive manner. The HRs of incident IHD were 1.61 (95% CI 1.05–2.48), 1.85 (95% CI 1.21–2.83), and 2.28 (95% CI 1.48–3.51) for the second, third, and fourth TyG index quartiles, respectively, after adjusting for age, sex, BMI, smoking status, alcohol intake, physical activity, mean arterial blood pressure, hsCRP, CKD, and hypertension medication (Model 4).
Discussion
Among community dwelling Korean adults without diabetes, we found that elevated TyG index values were positively and independently associated with IHD incidence in this large-scale, prospective cohort study that included a 50-month follow-up. Our study showed that the association between TyG index and IHD persisted after further adjustment for lifestyle factors, inflammation, and mean arterial blood pressure.
Related studies
Recent meta-analysis of 13 cohort studies reported that higher TyG index values may significantly precede type 2 diabetes [20]. Meanwhile, the TyG index has also been shown to be associated with a higher prevalence of symptomatic coronary artery disease, along with metabolic and behavioral risk factors, and could be used as a marker for atherosclerosis [21]. Other studies have demonstrated significant associations between elevated TyG index values and a higher risk of arterial stiffness and nephric microvascular damage [22], between the TyG index and HOMA-IR [23] and subclinical myocardial injury [24], and between the TyG index and an increased risk of CVD incidence, especially among younger individuals [25].
In a prospective study that considered patients with stable coronary artery disease, Jin et al. showed that the TyG index may be a useful predictive marker of cardiovascular events [11]. Ma et al., in a longitudinal study of 766 patients who underwent percutaneous coronary intervention, reported that subgroups within the top tertile of the TyG index had a 2.17-fold higher risk of adverse cardiovascular outcomes over a median follow-up period of 30 months, compared with the referent first tertile [10]. Another cohort study by Sanchez-Inigo et al. examined the relationship between the TyG index and incident IHD in 5,014 Caucasian men and women with a mean age of 55.51 ± 13.68 years and 53.72 ± 12.84 years, respectively. Their work revealed a positive association between the TyG index and CVD over a median period of 10 years. However, the study performed by Sanchez-Inigo et al. included an older age group, patients with diabetes, and current users of anti-aggregation medications [26]. They were unable to identify a relationship between the TyG index and incident CVD in participants with type 2 diabetes at baseline, which may have been due to effects of medication or the adoption of heathier habits by participants [26].
Meanwhile, some studies have reported that the TyG index is associated with subclinical coronary atherosclerosis in both diabetic patients and the general population [13, 27]. From an epidemiological standpoint, nondiabetic individuals with myocardial infarction have been reported to have a poorer prognosis than diabetic patients without myocardial infarction [15, 16, 28, 29]. To predict future CVD, a health risk assessment over a 5-year period has become as important as that over a 10-year period over the past few decades [30, 31]. For the first time, our study revealed an association between the TyG index and incident IHD among nondiabetic adults in the preclinical stage using an assessment period that did not exceed 5 years in an East Asian population, despite the fact that the incidence of IHD was relatively low.
Possible mechanisms
Some possible explanations for the observed association deserve consideration. The TyG index is considered one of the best indices for identifying individuals with early insulin resistance [3]. In a prospective study of non-obese Chinese adults, Zhang et al. suggested that the TyG index may be valuable for predicting type 2 diabetes [32]. Further, TyG index was determined to be superior to well-known predictive biomarkers of type 2 diabetes, such as HOMA-IR, in a study that assessed 5,354 Korean subjects without diabetes with a mean age of 61.6 years and a mean BMI of 24.2 kg/m2 [5]. Additionally, the TyG index has been suggested to be a useful surrogate marker of overall metabolic health status according to KNHANES, a nationwide survey representing the entire Korean population [33]. Finally, chronic inflammation could contribute to the association between the TyG index and IHD. In the present study, serum hsCRP levels gradually increased with TyG index quartile, which supports the idea that TyG index is closely linked to underlying low-grade inflammation.
Study strengths and limitations
Some strengths and limitations require careful consideration and may affect the interpretation of the results of the present study. A major strength of the work was that we conducted a prospective cohort study using a large number of Korean individuals linked to HIRA data, which are derived from the universal coverage system in Korea. As a result, there was a very low chance that data were missing [34]. This study had some limitations that should also be acknowledged. First, because the study cohort was composed of volunteers that visited a clinic for health promotion screenings conducted at a single hospital, patients appeared to be slightly healthier than most community-based cohorts previously assessed, and some possible confounding variables may not have been measured at baseline. Second, some diabetic individuals may have been included in the study population because glycated haemoglobin A1c and 2-h oral glucose tolerance tests were not performed at the beginning of the study.
Conclusions
In conclusion, an elevated TyG index precedes and significantly predicts future IHD among community dwelling nondiabetic Koreans. Moreover, the TyG index was found to be a more powerful predictive indicator of IHD than fasting glucose or triglyceride levels alone. Accordingly, a high TyG index may be a useful additional measure with which to assess cardiovascular risk for nondiabetic adults in the preclinical stage. Large-scale prospective studies are necessary to elucidate the mechanism for underlying the association between the TyG index and IHD.
Availability of data and materials
The datasets used and/or analysed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- IHD:
-
Ischemic heart disease
- TyG:
-
Triglyceride glucose
- HERAS:
-
Health risk assessment study
- HIRA:
-
Korea Health Insurance Review and Assessment
- HRs:
-
Hazard ratios
- CIs:
-
Confidence intervals
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- BMI:
-
Body mass index
- KNHANES:
-
Korea National Health and Nutrition Examination Survey
- CVD:
-
Cardiovascular diseases
- hsCRP:
-
High sensitivity C-reactive protein
- CKD:
-
Chronic kidney disease
- ANOVA:
-
Analysis of variance
- ROC:
-
Receiver-operating characteristic
- AUC:
-
Area under the receiver-operating characteristic curve
References
Smith SC Jr, Jackson R, Pearson TA, Fuster V, Yusuf S, Faergeman O, et al. Principles for national and regional guidelines on cardiovascular disease prevention: a scientific statement from the World Heart and Stroke Forum. Circulation. 2004;109(25):3112–21.
Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–53.
Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13(1):146.
Park B, Lee HS, Lee YJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean Genome and Epidemiology Study cohort. Transl Res. 2020. https://doi.org/10.1016/j.trsl.2020.08.003.
Lee S-H, Kwon H-S, Park Y-M, Ha H-S, Jeong SH, Yang HK, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS ONE. 2014;9(2):e90430.
Shin K-A. Triglyceride and glucose (TyG) index is a clinical surrogate marker for the diagnosis of metabolic syndrome. Biomed Sci Lett. 2017;23(4):348–54.
Ha K, Kim K, Chun OK, Joung H, Song Y. Differential association of dietary carbohydrate intake with metabolic syndrome in the US and Korean adults: data from the 2007–2012 NHANES and KNHANES. Eur J Clin Nutr. 2018;72(6):848–60.
Kim B-Y, Won JC, Lee JH, Kim H-S, Park JH, Ha KH, et al. Diabetes Fact Sheets in Korea, 2018: an appraisal of current status. Diabetes Metab J. 2019;43(4):487–94.
Roh E, Ko S-H, Kwon H-S, Kim NH, Kim JH, Kim CS, et al. Prevalence and management of dyslipidemia in Korea: Korea National Health and Nutrition Examination Survey during 1998 to 2010. Diabetes Metab J. 2013;37(6):433–49.
Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):31.
Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–46.
Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41.
Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108.
Won KB, Park EJ, Han D, Lee JH, Choi SY, Chun EJ, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19(1):34.
Evans JM, Wang J, Morris AD. Comparison of cardiovascular risk between patients with type 2 diabetes and those who had had a myocardial infarction: cross sectional and cohort studies. Bmj. 2002;324(7343):939–42.
Lee CD, Folsom AR, Pankow JS, Brancati FL. Cardiovascular events in diabetic and nondiabetic adults with or without history of myocardial infarction. Circulation. 2004;109(7):855–60.
Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, et al. 2019 Clinical Practice Guidelines for Type 2 Diabetes Mellitus in Korea. Diabetes Metab J. 2019;43(4):398–406.
Carretero OA, Oparil S. Essential hypertension: part I: definition and etiology. Circulation. 2000;101(3):329–35.
Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–100.
da Silva A, Caldas APS, Rocha DMUP, Bressan J. Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: a systematic review and meta-analysis of cohort studies. Prim Care Diabetes. 2020;9:12.
da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89.
Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro-and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18(1):95.
Aman M, Resnawita D, Rasyid H, Kasim H, Bakri S, Umar H, et al. The concordance of triglyceride glucose index (TyG index) and homeostatic model assessment for insulin resistance (Homa-IR) in non-diabetic subjects of adult Indonesian males. Clin Epidemiol Global Health. 2020;8:777.
Liu Y, Wu M, Xu J, Sha D, Xu B, Kang L. Association between Triglyceride and glycose (TyG) index and subclinical myocardial injury. Nutr Metab Cardiovasc Dis. 2020;9:78.
Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran Lipid and Glucose Study. Cardiovasc Diabetol. 2020;19(1):1–12.
Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97.
Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15(1):155.
Hu FB, Stampfer MJ, Solomon CG, Liu S, Willett WC, Speizer FE, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med. 2001;161(14):1717–23.
Cho E, Rimm EB, Stampfer MJ, Willett WC, Hu FB. The impact of diabetes mellitus and prior myocardial infarction on mortality from all causes and from coronary heart disease in men. J Am Coll Cardiol. 2002;40(5):954–60.
Pylypchuk R, Wells S, Kerr A, Poppe K, Riddell T, Harwood M, et al. Cardiovascular disease risk prediction equations in 400+1000 primary care patients in New Zealand: a derivation and validation study. Lancet. 2018;391(10133):1897–907.
Panagiotakos D, Pitsavos C, Chrysohoou C, Palliou K, Lentzas I, Skoumas I, et al. Dietary patterns and 5-year incidence of cardiovascular disease: a multivariate analysis of the ATTICA study. Nutr Metab Cardiovasc Dis. 2009;19(4):253–63.
Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc Diabetol. 2017;16(1):30.
Lee SH, Han K, Yang HK, Kim MK, Yoon KH, Kwon HS, et al. Identifying subgroups of obesity using the product of triglycerides and glucose: the Korea National Health and Nutrition Examination Survey, 2008–2010. Clin Endocrinol (Oxf). 2015;82(2):213–20.
Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA Data. J Korean Med Sci. 2017;32(5):718–28.
Acknowledgements
The authors would like to thank the Health Insurance Review and Assessment Services for their cooperation.
Funding
This study was supported by a 2010 Grant from the Korean Academy of Medical Sciences.
Author information
Authors and Affiliations
Contributions
BP, YJL, and DHJ designed the study; BP, YJL, and DHJ assisted with data acquisition and interpretation; BP, YJL and HSL performed statistical analyses; BP, YJL, HSL, and DHJ contributed to the discussion; BP and YJL drafted the manuscript; and DHJ revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University College of Medicine, Seoul, Korea.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Park, B., Lee, YJ., Lee, H.S. et al. The triglyceride-glucose index predicts ischemic heart disease risk in Koreans: a prospective study using National Health Insurance Service data. Cardiovasc Diabetol 19, 210 (2020). https://doi.org/10.1186/s12933-020-01186-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-020-01186-2