Abstract
The threat of methicillin-resistant Staphylococcus aureus (MRSA) is increasing worldwide, making it significantly necessary to discover a novel way of dealing with related infections. The quick spread of MRSA isolates among infected individuals has heightened public health concerns and significantly limited treatment options. Vancomycin (VAN) can be applied to treat severe MRSA infections, and the indiscriminate administration of this antimicrobial agent has caused several concerns in medical settings. Owing to several advantageous characteristics, a niosomal drug delivery system may increase the potential of loaded antimicrobial agents. This work aims to examine the antibacterial and anti-biofilm properties of VAN-niosome against MRSA clinical isolates with emphasis on cytotoxicity and stability studies. Furthermore, we aim to suggest an effective approach against MRSA infections by investigating the inhibitory effect of formulated niosome on the expression of the biofilm-associated gene (icaR). The thin-film hydration approach was used to prepare the niosome (Tween 60, Span 60, and cholesterol), and field emission scanning electron microscopy (FE-SEM), an in vitro drug release, dynamic light scattering (DLS), and entrapment efficiency (EE%) were used to investigate the physicochemical properties. The physical stability of VAN-niosome, including hydrodynamic size, polydispersity index (PDI), and EE%, was analyzed for a 30-day storage time at 4 °C and 25 °C. In addition, the human foreskin fibroblast (HFF) cell line was used to evaluate the cytotoxic effect of synthesized niosome. Moreover, minimum inhibitory and bactericidal concentrations (MICs/MBCs) were applied to assess the antibacterial properties of niosomal VAN formulation. Also, the antibiofilm potential of VAN-niosome was investigated by microtiter plate (MTP) and real-time PCR methods. The FE-SEM result revealed that synthesized VAN-niosome had a spherical morphology. The hydrodynamic size and PDI of VAN-niosome reported by the DLS method were 201.2 nm and 0.301, respectively. Also, the surface zeta charge of the prepared niosome was − 35.4 mV, and the EE% ranged between 58.9 and 62.5%. Moreover, in vitro release study revealed a sustained-release profile for synthesized niosomal formulation. Our study showed that VAN-niosome had acceptable stability during a 30-day storage time. Additionally, the VAN-niosome had stronger antibacterial and anti-biofilm properties against MRSA clinical isolates compared with free VAN. In conclusion, the result of our study demonstrated that niosomal VAN could be promising as a successful drug delivery system due to sustained drug release, negligible toxicity, and high encapsulation capacity. Also, the antibacterial and anti-biofilm studies showed the high capacity of VAN-niosome against MRSA clinical isolates. Furthermore, the results of real-time PCR exhibited that VAN-niosome could be proposed as a powerful strategy against MRSA biofilm via down-regulation of icaR gene expression.
Similar content being viewed by others
Introduction
Staphylococcus aureus is a pathogen that poses a substantial risk to human life and presents significant medical issues for the healthcare system [1,2,3]. In hospitalized patients, this pathogen can cause a wide range of illnesses with significant mortality rates, such as necrotizing pneumonia and the infection of the respiratory tract, prosthetic joint, and surgical site [4, 5]. The fast spread of multi-drug resistant (MDR) S. aureus isolates among infected individuals in recent years has raised serious concerns about public health and enormously complicated treatment options against this organism [6, 7]. The high ability of S. aureus to form a biofilm can account for its easy attachment to various biotic and abiotic surfaces in the hospital settings. Moreover, biofilm formation could delay wound healing, causing chronic infections, where 43–88% of S. aureus strains are from diabetic and bedsore ulcers [8]. Indeed, biofilm decreases the effective dosage of antimicrobial agents by inhibiting their diffusion into bacterial space and shielding organisms against unfavorable conditions, including excessive antimicrobial agents and host immunity responses [9, 10]. Conversely, biofilm may offer the best conditions for horizontal gene transfer and spreading of drug resistance genes throughout bacterial populations [11].
Methicillin-resistant S. aureus (MRSA) strains frequently resist many antibiotics, leading to drastic therapeutic challenges in healthcare settings and communities [12, 13]. These isolates are characterized by the mecA gene, which is rapidly transferring among other staphylococcal strains [14]. MRSA isolates are a prominent causative agent in chronic wound infections which have detrimental influences on human quality of life. In this regard, the Centers for Disease Control and Prevention (CDC) reported that, in the United States, the annual (2018) death associated with MRSA was estimated at 20,000, and this mortality rate was greater than other resistant bacterial pathogens [15]. Furthermore, it is proven that MRSA has more ability to form biofilms than methicillin-sensitive S. aureus (MSSA), causing MRSA to be known as a significant pathogenic isolate [16]. The MRSA isolates’ ability to build biofilms causes a delay in the re-epithelialization of infected wounds and soft tissues, lengthening the healing period. Moreover, MRSA biofilm has been linked to chronic wounds such as venous ulcers, pressure sores, and diabetic foot ulcers [17].
Vancomycin (VAN), as a glycopeptide antibiotic, inhibits the synthesis of bacterial cell walls, which plays a crucial role in combating Gram-positive bacterial pathogens. VAN targets the bacterial cell wall by attaching itself to the D-Ala-D-Ala terminus of peptidoglycan precursors, which prevents cell wall cross-linking and ultimately resulting in bacterial cell death [18]. In the 1980s, the prophylaxis and treatment of significant infections related to S. aureus isolates, particularly MRSA, shifted towards administrating VAN [19]. However, the uncontrolled prescription of glycopeptide antibiotics has resulted in the spread of VAN-resistant S. aureus isolates, and an analysis of clinical research published in the literature shows that the susceptibility of MRSA isolates to VAN is rapidly declining globally. This underscores the significant impact of MRSA infections and the urgent need to come up with effective treatment strategies and take prevention measures [20].
Vesicular drug delivery systems were initially introduced by a British scientist, which are composed of concentric bilayer membranes [21, 22]. These systems could be applied to the encapsulation of a wide range of materials and are gaining traction among scientists in biomedical applications [23]. Niosomes are among these vesicular systems that have been presented as powerful drug delivery systems [24]. The bilayer structure of niosomes is composed of amphiphilic molecules, providing several favorable properties. Also, negligible toxicity, biodegradability, non-immunogenicity, bioavailability, structure flexibility, and simple formulation are among the features that improve the pharmaceutical behavior of niosome [25, 26]. However, drug leakage from bilayer membranes is among the main drawbacks of niosomes that could limit their applications in drug delivery [27]. The practical ways to overcome this limitation can include selecting proper non-ionic surfactants, modifying the synthesis methods, and combining the appropriate proportion of ingredients, which considerably impact on niosomal efficiency [28]. Cholesterol is known as a significant membrane additive and could be involved in the niosomal composition, which has a considerable effect on reducing drug leakage from bilayer membrane [29]. In the current research, a nanosized spherical niosome containing VAN at high entrapment capacity was formulated for the gradual drug release from the niosomal system. Furthermore, the physical stability analysis of synthesized nanoparticles could significantly impact the synthesis of the optimized niosome for further biomedical purposes. Several investigations have indicated that niosomal vesicular systems may serve as efficient nano-carriers for medicinal applications, specifically targeting bacterial infections [30]. The antibacterial properties of several niosomal drug delivery systems against different bacterial pathogens, including Staphylococcus epidermidis, Bacillus subtilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, and Escherichia coli were approved [31,32,33]. Also, the results of some experiments exhibited that niosome nanoparticles, as a powerful drug delivery system, hold promise for solving serious challenges related to S. auras [34,35,36]. However, the present research aims to evaluate the antibacterial activity of VAN-niosome against MRSA clinical strains with emphasis on cytotoxicity study on human foreskin fibroblast (HFF) cell line. Furthermore, the inhibitory and eradication effects of VAN-niosome against MRSA biofilms were assessed. Finally, this study aims to suggest a promising approach against MRSA infections by evaluating the anti-biofilm activities of formulated niosome using real-time PCR.
Material and method
Materials
Span 60 (sorbitan monostearate, 98%), cholesterol (≥ 95%), Tween 60 (polyoxyethylene sorbitan monopalmitate, ≥ 98%), chloroform (HPLC grade), methanol (HPLC grade), crystal violet (≥ 90%), antibiotic disks, and all culture media were purchased from Merck, Germany. VAN hydrochloride (≥ 85%), Spectra/ Por® dialysis membrane (MWCO 12–14 KDa), and ultra Amicon tube (cutoff 30 kDa) were also supplied from Sigma-Aldrich, India. The standard MRSA strain, S. aureus ATCC 6538, was also provided by the microbiological collection bank of the Pasteur Institute of Iran.
Bacterial isolation and antibiotic resistance pattern
A total of 36 S. aureus isolates were collected from different inpatient wards of the Loghman-e Hakim Hospital, Tehran, Iran, for three months from November 2021 to January 2022. The strains were taken from 75 clinical wound specimens, including diabetic, bedsore, and burn, using sterile cotton swabs. The collected isolates were diagnosed as S. aureus by routine microbiological analyses [37, 38]. For screening of MRSA, the antibiotic susceptibility profile for oxacillin (1 µg) and cefoxitin (30 µg) disks was carried out [39]. The polymerase chain reaction (PCR) was also performed for final MRSA confirmation by specific primers for the mecA gene [40]. Finally, all MRSA strains were kept in tryptic soy broth (TSB) medium containing 15% glycerol at -20 °C for further analysis.
The disk diffusion method was used to accomplish the antimicrobial susceptibility testing (AST) of S. aureus isolates in compliance with the recommended protocols of the Clinical and Laboratory Standards Institute (CLSI) [41]. The following five antimicrobial classes, including aminoglycosides (gentamycin), macrolides (azithromycin, erythromycin), tetracyclines (doxycycline), fluoroquinolones (ciprofloxacin, levofloxacin), and lincosamides (clindamycin) were tested. Moreover, the MDR pattern was identified as the non-susceptibility to at least one agent in ≥ 3 antimicrobial groups [42].
Screening of biofilm formation
A microtiter plate (MTP) assay was employed to screen the biofilm formation ability of S. aureus isolates [43, 44]. In this method, the overnight cultures of tested isolates in a TSB medium containing 1% glucose were adjusted to match a 0.5 McFarland concentration. Afterward, diluted suspensions were dispensed into a 96-well microtiter plate and overnight incubated at 37 °C. After washing the plate, the biofilms were fixed using absolute methanol and were stained using 200 µl of 1.5% w/v crystal violet solution for 15 min. Subsequently, the unbound dye was aspirated, and each well was rinsed in triplicate. After solubilizing the bound stain with 200 µl of 33% v/v acetic acid solution, the optical densities (ODs) of each well were evaluated using a microtiter plate ELISA reader (BioTek, Germany) at 570 nm. Ultimately, the biofilm patterns were divided into four groups using the following formula: OD ≤ ODc (non-adherent), ODc < OD ≤ 2 × ODc (weak biofilm), 2 × ODc < OD ≤ 4 × ODc (moderate biofilm), and 4 × ODc < OD (strong biofilm). Notably, the biofilm pattern of each isolate was screened in triplicate, and S. aureus ATCC 6538, as a strong biofilm producer, was applied as positive control.
Niosomal formulation
In the current study, the thin-film hydration method was applied to formulate VAN-niosome [45]. The mechanism of niosomal drug formation relies on the self-assembly properties of nonionic surfactants and cholesterol in an aqueous environment. In this research, a defined amount of Tween 60, Span 60, and cholesterol (with a molar ratio of 2:2:1) was dissolved in 20 ml 2:1 v/v of chloroform-methanol and magnetically stirred at 150 rpm for 45 min at 25 °C to obtain a uniform solution. After this step, the organic solution was removed under vacuum conditions at 60 °C for 45 min at 120 rpm using a rotary evaporator (WB Eco Laborota 4000 Model, Heidolph Instruments, Germany). The residual solvent was removed by nitrogen gas, and subsequently, the dried lipid was hydrated in 20 ml of PBS (pH ~ 7.4, 100 mM) containing VAN for 45 min. In the next step, the niosomal formulation was sonicated with a probe sonicator (Hielscher UP50H ultrasonic processor, Germany) for 10 min. Finally, the synthesized suspension was visually observed for turbidity and flocculation, and refrigerated for further experimentations. Notably, the blank niosome was formulated using the same protocol without the addition of VAN. Also, the molar ratio of lipid to VAN was adjusted to 20:1 [46].
Characterization of niosome
Morphology, hydrodynamic size, and surface zeta potential
In our study, field emission scanning electron microscopy (FE-SEM) was used to analyze the niosomal morphology [47]. For FE-SEM micrographs, one droplet of prepared niosome (1:100 diluted with deionized water) was mounted on the FE-SEM sample stub and coated with a 200 nm conductive gold layer. The images were analyzed with ImageJ software (bundled with Java version 1.8.0_172) [48]. Additionally, the niosomal physicochemical characteristics, including hydrodynamic size, surface zeta potential, and polydispersity index (PDI), were assessed with the dynamic light scattering (DLS) method using a Zeta-sizer device (Malvern Instrument Ltd. Malvern, UK) at 633 nm. The DLS method was performed in triplicate, and the synthesized niosome was analyzed at the same conditions of pH, concentration, and temperature (7.4, 0.1 mg ml− 1, and 25 °C).
Entrapment efficiency (EE%)
In the current research, the ultra-centrifugation assay was used to evaluate the EE% of VAN-niosome. Firstly, 2 ml of niosomal formulation was centrifuged at 7,500 g at 25 °C for 25 min in an ultra Amicon tube [49]. Subsequently, the OD of the supernatant was calculated with a UV spectrophotometry (Jasco V-530, Japan) at 281 nm, and the specific amount of free VAN was estimated by a standard curve equation [50]. Finally, the EE% was reported using the following formula: EE% = [(A-B)/A] ×100.
Where A is the specific amount of loaded VAN into niosomal suspension, and B is the specific amount of free VAN in the supernatant.
In vitro release
In this study, the dynamic dialysis method was used to determine the release kinetics of the niosomal VAN formulation [51]. Firstly, the un-entrapped VAN was seperated by ultra-centrifugation at 4 °C at 100,000 g for 45 min [52]. Afterward, the dialysis bag containing 1 ml of VAN-niosome was placed in 30 ml of recipient medium (PBS, 5 mM, pH ~ 7.4) and magnetically stirred for 48 h at 37 °C at 150 rpm. Then, 1 ml of recipient media was sampled at intervals of 1, 2, 4, 8, 24, and 48 h, and the specific amount of released drug was assessed using the standard curve equation. Also, an equivalent volume of fresh PBS (5 mM, pH ~ 7.4, 37 °C) was substituted with the aliquoted samples. Notably, to assess the drug release kinetics from a synthesized niosomal system, the data of release analysis were examined through mathematical methods according to the kinetic models’ equations such as Weibull and Hyperbolic Tangent Function, Korsmeyer–Peppas equation, and zero- to fifth-order polynomials [53].
Fourier-Transform Infrared spectroscopy (FT-IR)
Any possible interaction between loaded VAN and niosome was examined using a FT-IR spectroscopy instrument (Spectrum Two, U.S.A.), which was carried out in the 400–4000 °C temperature range.
Stability studies
To perform the stability analysis, the hydrodynamic size, PDI, and EE% of the synthesized VAN-niosome were assessed for a 30-day storage time at 4 °C and 25 °C.
Cytotoxicity analysis
The MTT (dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide) method was applied to determine the biocompatibility of VAN-niosome against the HFF cell line taken from the Pasteur Institute of Iran, Tehran, Iran [54]. Initially, the HFF cells were cultured in a 96-well microtiter plate at 1 × 104 cells per well, and the plate was overnight incubated with 5% CO2 at 37 °C. Then, the increasing concentrations of samples were added to HFF cells, and after 24 h incubation at 37 °C, each well was filled with 15 µl of 5 mg ml− 1 MTT dye. Subsequently, the plate was incubated for 4 h at 37 °C, and 200 µl of dimethyl sulfoxide (DMSO) was added to each well. Finally, the ODs were measured using a microplate ELISA reader at 570 nm (29), and the cytotoxicity of VAN-niosome was calculated as the following equation: Cell viability (%) = (OD sample / OD negative control) × 100.
Notably, all assays were performed in triplicate, and the well-containing HFF cell without adding samples was considered a negative control. Also, the HFF cell treated with DMSO was considered a positive control.
Antibacterial activity of synthesized niosome
The approved CLSI broth microdilution protocol was used to evaluate the minimum inhibition and bactericidal concentrations (MIC/MBC) of formulated niosomes against MRSA strains [55]. Firstly, Mueller-Hinton broth (MHB) medium containing gradient concentrations of sample was dispensed to a 96-well microtiter plate. In the next step, 0.5 McFarland suspensions of MRSA isolates were inoculated into the wells, and the plate was overnight incubated at 37 °C. The minimum sample concentrations without visible bacterial growth were reported as MICs. The MBC was also considered the well containing the lowest concentration with no-growth (> 99%) on the Mueller-Hinton agar (MHA) medium after overnight incubation at 37 °C. All assays were conducted in triplicate, and microbial strain and uninoculated mediums were positive and negative controls, respectively.
Anti-biofilm activity of synthesized niosome
Biofilm inhibition and eradication
To evaluate the biofilm inhibitory effect of niosomal VAN, 200 µl of selected bacterial suspensions (106 CFU/ml) diluted with TSB were inoculated into a 96-well microtiter sterile plate. Then, the serial dilutions of sample were added into each well, and the plate was overnight incubated at 37 °C. After washing the wells, the formed biofilms were fixed with absolute methanol and subsequently stained with crystal violet. In the final step, the ODs were estimated with a microplate ELISA reader at 570 nm [56,57,58].
The anti-biofilm efficacy of synthesized niosome was also examined using minimal biofilm eradication concentrations (MBEC). Firstly, the MRSA strains were allowed to produce 1- and 3-day-old biofilms. Then, the serial dilutions of sample were added to each well, and the plate was incubated at 37 °C overnight. Subsequently, 10 µl of the well’s content was cultured on an MHA medium. After 48 h incubation, the number of bacterial colonies was enumerated, and the lowest concentration killing all embedded bacteria was determined as MBEC. Notably, S. aureus ATCC 6538 and the uninoculated medium were used as positive and negative controls, respectively [59].
Biofilm gene expression
The impact of niosomal formulation on biofilm gene expression was examined using a real-time PCR assay. At first, the isolates were treated with a sub-MIC concentration of sample. Next, the total RNAs were extracted with an RNX-Plus kit (SinaColon Co, Iran), and reverse-transcribed to cDNA with random hexamer primers. To determine the expression of biofilm-associated gene (icaR), real-time PCR was performed with SYBR green qPCR master mix (SinaColon Co, Iran) as follows: a 10-minute initial denaturation at 95 °C, 40 amplification cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 90 s, extension at 72 °C for 15 s, and a 5-minute final extension at 72 °C. To normalize the expression level of the biofilm gene, the cycle threshold (CT) values of the icaR gene were compared with those of 16 S rRNA, as a housekeeping gene. In the final step, the relative expression of icaR gene was calculated according to the ΔΔCT method [60].
Statistical analysis
The t-test and Chi-squared were applied to compare the investigated parameters with a significance level of less than 0.05. All graphs were created with GraphPad Prism version 9.0.
Results
Bacterial isolation and biofilm formation ability
In current research, a total of 36 S. aureus strains consisting of 12 (33%) MRSA and 24 (66%) MSSA were isolated from 75 clinical samples. Also, the AST results showed that among 36 S. aureus isolates, 20 (55.5%) and 16 (44.4%) strains were MDR and non-MDR, respectively. Furthermore, the MTP method demonstrated that S. aureus strains had a high ability to biofilm formation and 32 of 36 (88.8%) isolates formed a biofilm. Also, among 32 biofilm-forming isolates, 15 isolates (46.8%), 8 isolates (25%), and 9 isolates (28.1%) had strong, moderate, and weak biofilm profiles, respectively. Also, 100% and 70.8% of MRSA and MSSA strains were biofilm formers, respectively. Table 1 presents the drug resistance profile and biofilm pattern of 36 S. aureus strains in this study.
Physicochemical characterization of VAN-niosome
Morphology, hydrodynamic size, PDI, and surface zeta potential
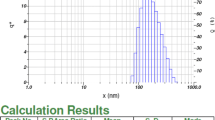
Based on the images taken by FE-SEM, the VAN-niosome had a spherical morphology (Fig. 1). The hydrodynamic size of the VAN-niosome reported by the Zeta-sizer was 201.2 nm. Furthermore, the PDI value was evaluated to be 0.301, representing the acceptable homogenous dispersion for synthesized nanoparticles (Fig. 2). Additionally, the surface zeta charge of the VAN-niosome reported by the DLS method was − 35.4 mV (Table 2).
Size distribution curve of VAN-niosomes obtained by the Stokes-Einstein equation [61]
Niosomal entrapment efficiency (EE%)
In our study, EE% of the niosomal suspension ranged from 58.9 to 62.5%, representing the high potential of niosomes in drug encapsulation. It is proven that incorporation into niosomal formulation could enhance the pharmaceutical activities of loaded drugs, displaying the prominent ability of niosomes to be applied in clinical applications [63].
In vitro release profile
According to statistic evidence, first-order kinetic was the most suitable model for describing the drug release for both free and niosomal formulations. As shown in Fig. 3, approximately 60% of VAN was released from the free formulation in the first 4 h. Whereas, only 30% of incorporated VAN was released from the niosomal suspension during this time. Moreover, within 48 h, around 90% and 48% of drug was released from free and niosomal formulations, respectively. The in vitro drug release study proves that niosomes can be an efficient drug delivery system through preventing burst drug release.
FTIR
FTIR spectrum (Fig. 4) of Span 60, Tween 60, VAN, niosome, and VAN-niosome revealed that the band around 3440 cm− 1 is related to O–H stretching of phenolic elements. The band 2933 cm− 1 is related to C–H stretching of methylene group in aliphatic hydrocarbons and esters, the band around 2300 is assigned C-O and C = C functional groups, the peak around 1700 cm− 1 is related to C = O stretching of fatty acid, esters, ketones, and aldehyde, 1500 cm− 1 is assigned to C = C stretching of aromatic compounds, 1462 cm− 1 is related to C–O–H in-plane bending of fatty acids and others, 1269 cm− 1 is related to C–O stretching of fatty acid and ester, and 1044 cm− 1 is assigned to C–O stretching of alcohols [60, 64,65,66]. The presence of these peaks confirmed that VAN is successfully incorporated in the prepared noisome and was covered by used fatty acid in niosomal composition with functional groups, including ketone, aldehyde, carboxylic acid, and others. Also, the presence of these functional groups presents the stability of the synthesized nanostructure [67].
Physical stability study
In the current research, the stability of the formulated VAN-niosome at 4 °C and 25 °C was analyzed, and its physicochemical properties including hydrodynamic size, PDI, and EE%, were evaluated during a 30-day storage time. As can be seen in Fig. 5, the niosomal VAN formulation kept at 4 °C had less change in hydrodynamic size, PDI, and EE% than the formulation kept at 25 °C, and had more physical stability under these conditions.
Cytotoxicity analysis
The cell viability of VAN-niosome and free VAN in different concentrations was evaluated on HFF cell line (Fig. 6). The MTT assay revealed that the cell viability induced by niosomal formulation was significantly higher than that of free VAN in all investigated concentrations. In addition, the cytotoxicity of bare niosomes was investigated, and any significant toxicity was exhibited on HFF cells.
Antibacterial and antibiofilm analysis
MICs and MBCs
The MICs and MBCs of niosomal VAN formulation against 12 MRSA clinical isolates were analyzed and compared to that of the free VAN (Fig. 7). Our results exhibited that niosoml formulation reduced the MICs of all MRSA isolates by 2-4-fold in comparison to non-niosomal formulation. Also, the VAN-niosome had high bactericidal efficacy, which decreased the MBCs by 2-4-fold against 9 of 12 MRSA isolates. Moreover, the antibacterial ability of the blank niosome was investigated, and no antibacterial activity was found against all MRSA isolates.
Biofilm inhibition
In our research, the inhibitory activity of VAN-niosome against MRSA biofilms was assessed and compared with free VAN. The MTP assay revealed that treatment of MRSA isolates with niosomal VAN formulation caused more inhibition in biofilm formation against all MRSA isolates in comparison to the free VAN (Fig. 8). Notably, the anti-biofilm ability of bare niosomes was analyzed, which had any anti-biofilm effect against all tested isolates.
Biofilm eradication
The anti-biofilm ability of VAN-niosome against MRSA isolates was examined by the MBEC method and compared with free VAN (Table 3). According to our results, the VAN-niosome decreased the 1-day-MBECs against all tested isolates by 2–8-fold in comparison to free VAN. Additionally, the MBEC method exhibited that VAN-niosome eradicated 3-day-old MRSA biofilms at lower concentrations in comparison to non-niosomal formulation. Notably, the anti-biofilm of bare niosomes was investigated, which failed to eradicate any bacterial biofilms at the same concentrations of VAN-niosomal formulation.
Biofilm gene expression
To further confirmation of the anti-biofilm effectiveness of VAN-niosome, after treating MRSA isolates with sub-MIC concentrations of niosomal VAN and free VAN, the mRNA levels of the biofilm-related gene (icaR) were examined using real-time PCR (Fig. 9). Our research revealed that the expression levels of the icaR gene in all MRSA isolates were significantly reduced following treatment with niosomal VAN compared with free drug, suggesting the anti-biofilm potential of synthesized formulation against MRSA biofilms.
Discussion
MRSA is known as one the seriously life-threatening agents with high challenges in hospitalized patients [68, 69]. MRSA has a higher ability to biofilm formation than MSSA isolates, which has a substantial role in emerging drug resistance [16]. In this regard, the present study demonstrated that 100.0% and 70.8% of MRSA and MSSA strains produced biofilm, respectively. The results of our study were in agreement with other studies [60, 70,71,72], where MRSA strains had a superior tendency to produce biofilms compared to MSSA isolates. Biofilm formation is well-known as a prominent factor in developing MDR strains, posing a significant risk of chronic and recurrent infections [44, 73]. Also, our investigation revealed that all MDR isolates were biofilm formers, indicating a strong association between MDR pattern and biofilm production (P-value < 0.001). Our findings were in agreement with the results of Kwon et al. [71] and Neopane et al. [74], where biofilm production was considerably greater in MDR strains. The explanation for the emergence of MDR pattern in biofilm-forming organisms is attributed to intimate cell-to-cell contact inside the biofilm area which promotes the exchange of plasmids carrying drug-resistance genes [74]. Nevertheless, a different published investigation revealed that the adhesion profile and MDR isolates did not significantly correlate [75]. Also, it is proven that additional factors, including the organisms’ genetic make-up, geographic origin, types of specimens, surface adhesion properties, availability of nutrients [76], efflux pumps [77], and toxin systems [78], contributed to developing drug resistance. Nevertheless, further investigations are needed to clarify the exact correlation between biofilm formation and MDR pattern in a larger numbers of S. aureus isolates.
The therapeutic efficacies of a niosomal drug delivery system encapsulating various antibacterial agents rely on designing a formulation with high physical stability [79]. In this study, a stability analysis for a 30-day storage time was performed to investigate the ability of synthesized VAN-niosome to maintain its physicochemical attributes. The physical appearance of prepared niosome was visualized unchanged within 30 days, and neither sedimentation nor flocculation was seen. In addition, the stability analysis revealed that refrigerated niosomes had slower changes in hydrodynamic size, PDI, and EE% in comparison with those kept at room temperature. Moreover, comparing our findings with several studies showed that synthesized niosomes with long-chain non-ionic surfactants (Tween 60 and Span 60) provided greater satisfactory stability than those formulated with Span 40 and Tween 40 [34, 66]. Notably, the findings of in vitro release analysis exhibited that our niosomal formulation had a greater sustained-release pattern compared with other formulations [34, 80]. These superiorities could be discussed that the proper proportion of niosomal ingredients could be a reason for slow-release pattern and better stability of noisome. Additionally, the presence of cholesterol and binary non-ionic surfactants (Tween 60 and Span 60) could reduce the drug leakage from niosomal formulation by increasing bilayer membrane rigidity [81]. Therefore, choosing appropriate surfactants and also luminating the exact amount of niosomal components must be considered as prominent parameters for developing an optimized formulation [82].
Drug resistance in MRSA isolates has been increasing quickly in medical settings in recent years, bringing significant financial challenges to healthcare budgets [83]. Due to some drawbacks, conventional methods have proven difficult to treat MRSA infections effectively [84]; thus, it is critically necessary to devise a novel technique for successfully eliminating the therapeutic obstacles associated with this superbug [27]. Niosome, an influential vesicular drug delivery system, transmits the encapsulated drug into bacterial cells, strengthening the efficacy of anti-MRSA agents [80, 85]. In our study, the anti-MRSA effects of VAN-niosome were investigated, and it was approved that the niosomal delivery system has high potential as an alternative approach against MRSA isolates. Also, the present research showed that encapsulating into niosomal formulation could reduce the MIC and MBC concentrations of free VAN by 2-4-fold (for two comparisons), showing the high ability of niosomal VAN as a powerful antibacterial delivery system. Moreover, in another study by Ghafelehbashi et al. [64] the inhibitory ability of niosomal cephalexin against S. aureus strains was found, where the incorporation into niosome reduced the MIC values of free cephalexin by 4-fold. Also, Jastish et al. [80] exhibited the increased anti-S. aureus behavior of fluoroquinolones-loaded niosome and the niosomal formulation had a lower MIC concentration (at least 4-fold) in comparison with free drugs. In addition, Heidari et al.‘s study [67] proved that the MIC value of tannic acid-loaded niosome was 2-fold lower than the free drug, indicating niosomal formulation was more potent against S. aureus compared with free tannic acid. Additionally, Rezaeiroshan et al. [86] showed the bacteriostatic and bactericidal activities of Trans-ferulic acid-loaded niosome against S. aureus isolates, and both MIC and MBC parameters of free drug were decreased by 2-fold via encapsulating into niosome. The results of mentioned researches were in line with our outcomes, proposing that niosomal encapsulation through enhancing the effective dose of encapsulated drugs can be designed for drug delivery against S. aureus isolates, including MRSA. The improved antibacterial effect of niosomal encapsulation is supported by this hypothesis that pharmaceutics efficacy of loaded agents would be increased by controlled-release profile from niosomal formulation [80, 87]. In addition, it is hypothesized that niosomes could interact with the peptidoglycan bacterial layer, increasing the permeability of adequate drug concentration into sub-cellular space [88]. Also, the sustained drug release from niosomal vesicles can lead to fewer drug intake intervals, and could be an ideal solution for preventing the resistance mechanism in chronic MRSA infections [89].
As biofilm is recognized as a substantial contributor to the increased pathogenesis of S. aureus strains, the early inhibition of bacterial attachment and destruction of formed biofilm on various medical surfaces have become big challenges in healthcare systems [74, 90]. In the present research, the inhibitory activity of VAN-niosome on MRSA biofilms was investigated, and it was demonstrated that entrapment into niosome could significantly decrease the MBEC levels (2-8-fold) of free drug against all MRSA isolates. In agreement with our result, Kashef et al. [91] approved that niosomal ciprofloxacin had a lower MBEC value by 2–4-fold against MRSA isolates in comparison with the free formulation. In another confirmatory study [89], the anti-biofilm ability of niosomal incorporation was confirmed against S. aureus strains, where the bacterial attachment to abiotic surface was considerably prevented via covering with encapsulated drug. In addition, the efficacy of cefazolin-loaded niosome on MRSA biofilm eradication was found by Zafari et al. [63], where niosomal formulation had a greater biofilm elimination rate (4-8-fold) than the free drug. Moreover, Shadvar et al. [92] showed the anti-biofilm efficacy of niosomal amoxicillin against MRSA strains, and it was represented that encapsulating into niosomal system greatly reduced (2-4-fold) the density of produced biofilm at same concentration of free amoxicillin. It could be concluded that niosomes inhibit the biofilm formation and efficiently deliver the incorporated contents into the embedded bacteria through facilitating their diffusion into the biofilm structure. Furthermore, durable accessibility of loaded drugs in biofilm matrix can prevent the resistance mechanisms in biofilm-forming bacteria, which could be improved via sustaining drug release pattern of niosomes [29]. The antibiofilm superiority of niosomal formulation is supported by another hypothesis which niosome, as a physical barrier, competes with biofilm-producing bacteria on surface adhesion and significantly contributes to the inhibition of biofilm formation through the reduction of bacterial attachment [93,94,95]. Furthermore, our investigation demonstrated the down-regulation of biofilm gene (icaR) expression relative to the free formulation, validating the biofilm inhibitory effect of VAN-niosome using real-time PCR. This finding was confirmatory with the Mirzaie et al.‘s study [60], where ciprofloxacin-loaded niosome inhibited MRSA biofilm formation, which could be effective on the biofilm genotypic profile through downregulating the expression of the biofilm-associated gene. Furthermore, Heidari et al. [67] showed that niosomal tannic acid significantly reduced the expression of the S. aureus biofilm gene when compared to free drug. These findings suggest that niosomes may interact with transcription factors involved in the expression of biofilm-associated genes. Furthermore, the generated reactive oxygen species (ROS) may interact with cellular proteins, including translation and transcription factors, through delivering of the incorporated drugs to the embedded bacteria. Therefore, due to significant potential of MRSA strains in biofilm formation, niosomal drug delivery system could be presented for fighting the therapeutic challenges related to this superbug. However, perspective studies should be designed to find the exact mechanisms of niosomes on anti-biofilm activity of encapsulated contents for further development in medical settings.
Conclusion
According to this research, niosomal encapsulation improved the pharmaceutical index of free drug, which can be proposed for delivering antimicrobial agents. In addition, incorporation into niosomal system increased the antibacterial effect of free VAN, which could be developed as an effective drug delivery system against MRSA clinical isolates with negligible cytotoxic effect. Also, the results of real-time PCR exhibited that niosomal drug delivery system enhanced the anti-biofilm ability of free VAN against MRSA clinical isolates and could reduce the biofilm-related challenges in health-care systems. However, in vivo studies should be performed to examine the antibacterial and anti-biofilm roles of niosome, which can provide useful insights for the clinical development of this drug delivery system against bacterial infections.
Data availability
No datasets were generated or analysed during the current study.
References
Kyaw MH, Kern DM, Zhou S, Tunceli O, Jafri HS, Falloon J. Healthcare utilization and costs associated with S. Aureus and P. Aeruginosa pneumonia in the intensive care unit: a retrospective observational cohort study in a US claims database. BMC Health Serv Res. 2015;15(1):241.
Nasaj M, Farmany A, Shokoohizadeh L, Jalilian FA, Mahjoub R, Roshanaei G, Nourian A, Shayesteh OH, Arabestani MR. Development of Chitosan-Assisted Fe 3 O 4@ SiO 2 Magnetic Nanostructures Functionalized with Nisin as a Topical Combating System against Vancomycin-Intermediate Staphylococcus aureus (VISA) Skin Wound Infection in Mice. Journal of Nanomaterials 2022, 2022.
Celik C, Ildiz N, Kaya MZ, Kilic AB, Ocsoy I. Preparation of natural indicator incorporated media and its logical use as a colorimetric biosensor for rapid and sensitive detection of Methicillin-resistant Staphylococcus aureus. Anal Chim Acta. 2020;1128:80–9.
Zhao GHC, Xue Y. In vitro evaluation of chitosan-coated liposome containing both coenzyme Q10 and alpha‐lipoic acid: cytotoxicity, antioxidant activity, and antimicrobial activity. J Cosmet Dermatol. 2018;17(2):258–62.
Durymanov MKT, Lehmann SE, Reineke J. Exploiting passive nanomedicine accumulation at sites of enhanced vascular permeability for non-cancerous applications. J Control Release 2017 Sep, 261:10–22.
Hernández-Aristizábal I, Ocampo-Ibáñez ID. Antimicrobial peptides with antibacterial activity against Vancomycin-resistant Staphylococcus aureus strains: classification, structures, and mechanisms of action. Int J Mol Sci. 2021;22(15):7927.
Ballav S, Jana D, Guchhait KC, Das B, Manna M, Hazra S, Kadokawa J-i, Panda AK, Ghosh C. Efflux Pumps and their role in the development of multi-drug resistant bacterial biofilm. Biofilm Associated Antimicrobial Resistance and its recovery. CRC; 2023. pp. 66–81.
Zhao G, Hochwalt PC, Usui ML, Underwood RA, Singh PK, James GA, Stewart PS, Fleckman P, Olerud JE. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen. 2010;18(5):467–77.
Di Martino P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018;4(2):274.
Jana P, Dey S, Jana D, Raul P, Manna M, Das B, Patra A, Panda AK, Ghosh C. Quorum Sensing Directed Microbial Diversity in infectious Bacteria. Microbial Diversity in the genomic era. Elsevier; 2024. pp. 625–39.
Madsen JS, Burmølle M, Hansen LH, Sørensen SJ. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol. 2012;65(2):183–95.
Algammal AM, Hetta HF, Elkelish A, Alkhalifah DHH, Hozzein WN, Batiha GE-S, El Nahhas N, Mabrok MA. Methicillin-Resistant Staphylococcus aureus (MRSA): one health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect Drug Resist 2020:3255–65.
Ocsoy A, Yusufbeyoglu M, Ildiz S, Ulgen N, Ocsoy A. DNA aptamer-conjugated magnetic graphene oxide for pathogenic bacteria aggregation: selective and enhanced photothermal therapy for effective and rapid killing. ACS Omega. 2021;6(31):20637–43.
Lozano C, Fernández-Fernández R, Ruiz-Ripa L, Gómez P, Zarazaga M, Torres C. Human mecc-carrying MRSA: clinical implications and risk factors. Microorganisms. 2020;8(10):1615.
Tabone M, Bressa C, García-Merino JA, Moreno-Pérez D, Van EC, Castelli FA, Fenaille F, Larrosa M. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci Rep. 2021;11(1):3558.
Hosseini M, Shapouri Moghaddam A, Derakhshan S, Hashemipour SMA, Hadadi-Fishani M, Pirouzi A, Khaledi A. Correlation between biofilm formation and antibiotic resistance in MRSA and MSSA isolated from clinical samples in Iran: a systematic review and meta-analysis. Microb Drug Resist. 2020;26(9):1071–80.
Simonetti O, Marasca S, Candelora M, Rizzetto G, Radi G, Molinelli E, Brescini L, Cirioni O, Offidani A. Methicillin-resistant Staphylococcus aureus as a cause of chronic wound infections: alternative strategies for management. AIMS Microbiol. 2022;8(2):125.
Liu F, Rajabi S, Shi C, Afifirad G, Omidi N, Kouhsari E, Khoshnood S, Azizian K. Antibacterial activity of recently approved antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) strains: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2022;21(1):37.
Rani NNIM, Hussein ZM, Mustapa F, Azhari H, Sekar M, Chen XY, Amin MCIM. Exploring the possible targeting strategies of liposomes against methicillin-resistant Staphylococcus aureus (MRSA). Eur J Pharm Biopharm. 2021;165:84–105.
Liu W-T, Chen E-Z, Yang L, Peng C, Wang Q, Xu Z, Chen D-Q. Emerging resistance mechanisms for 4 types of common anti-MRSA antibiotics in Staphylococcus aureus: a comprehensive review. Microb Pathog. 2021;156:104915.
Abdul Rasool BKAMN, Alburaimi N, Abdallah F, Shamma AS. A narrative review of the potential roles of lipid-based vesicles (vesiculosomes) in burn management. Sci Pharm. 2022;90(3):39.
Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–IN227.
Jain S, Jain V, Mahajan S. Lipid based vesicular drug delivery systems. Adv Pharmacol Sci. 2014;2014:1–12.
Nwabuife JC, Pant AM, Govender T. Liposomal delivery systems and their applications against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Adv Drug Deliv Rev. 2021;178:113861.
Daubioul C, Rousseau N, Demeure R, Gallez B, Taper H, Declerck B, Delzenne N. Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J Nutr. 2002;132(5):967–73.
Azeem A, Anwer MK, Talegaonkar S. Niosomes in sustained and targeted drug delivery: some recent advances. J Drug Target. 2009;17(9):671–89.
Hemmati J, Chegini Z, Arabestani MR. Niosomal-based drug delivery platforms: a promising therapeutic approach to fight Staphylococcus aureus drug resistance. J Nanomater 2023, 2023.
Yasamineh S, Yasamineh P, Kalajahi HG, Gholizadeh O, Yekanipour Z, Afkhami H, Eslami M, Kheirkhah AH, Taghizadeh M, Yazdani Y. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int J Pharm. 2022;624:121878.
Mehrarya M, Gharehchelou B, Haghighi Poodeh S, Jamshidifar E, Karimifard S, Farasati Far B, Akbarzadeh I, Seifalian A. Niosomal formulation for antibacterial applications. J Drug Target. 2022;30(5):476–93.
Yeo PL, Lim CL, Chye SM, Ling APK, Koh RY. Niosomes: a review of their structure, properties, methods of preparation, and medical applications. Asian Biomed. 2017;11(4):301–14.
Awate PS, Pimple TP, Pananchery JF, Jain AS. Formulation and evaluation of berberine hcl as niosomal drug delivery system. Asian J Pharm Res. 2020;10(3):149–59.
Raeiszadeh M, Pardakhty A, Sharififar F, Farsinejad A, Mehrabani M, Hosseini-Nave H, Mehrabani M. Development, physicochemical characterization, and antimicrobial evaluation of niosomal myrtle essential oil. Res Pharm Sci. 2018;13(3):250–61.
Pooprommin P, Manaspon C, Dwivedi A, Mazumder A, Sangkaew S, Wanmasae S, Tangpong J, Ongtanasup T, Eawsakul K. Alginate/pectin dressing with niosomal mangosteen extract for enhanced wound healing: evaluating skin irritation by structure-activity relationship. Heliyon. 2022;8(12):e12032.
Barakat HS, Kassem MA, El-Khordagui LK, Khalafallah NM. Vancomycin-eluting niosomes: a new approach to the inhibition of staphylococcal biofilm on abiotic surfaces. AAPS PharmSciTech. 2014;15:1263–74.
Allam A, El-Mokhtar MA, Elsabahy M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J Pharm Pharmacol. 2019;71(8):1209–21.
Osman N, Omolo CA, Gafar MA, Devnarain N, Rambharose S, Ibrahim UH, Fasiku VO, Govender T. Niosomes modified with a novel pH-responsive coating (mPEG-OA) enhance the antibacterial and anti-biofilm activity of Vancomycin against methicillin-resistant Staphylococcus aureus. Nano Express. 2024;5(1):015008.
Amini C, Rahmani Z, Hosseini SS, Bagheri P, Goudarzi M. First Report of the emergence of mecC Gene and CC8/ST239 tigecycline-resistant Staphylococcus aureus Clonal Lineage isolated from Chronic Suppurative Otitis Media. Arch Clin Infect Dis 2023, 18(5).
Karmakar A, Dua P, Ghosh C. Biochemical and molecular analysis of Staphylococcus aureus clinical isolates from hospitalized patients. Can J Infect Dis Med Microbiol. 2016;2016(1):9041636.
Hasan R, Acharjee M, Noor R. Prevalence of Vancomycin resistant Staphylococcus aureus (VRSA) in methicillin resistant S. Aureus (MRSA) strains isolated from burn wound infections. Tzu Chi Med J. 2016;28(2):49–53.
Khan R, Irchhaiya R. Niosomes: a potential tool for novel drug delivery. J Pharm Investig. 2016;46:195–204.
Clinical and Laboratory Standards Institutes (CLSI). Clinical and Laboratory Standards for Antimicrobial Susceptibility Testing. 2019. pp. 1–319.
Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas M, Giske CG, Harbarth S, Hindler J, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81.
Stepanović S, Vuković D, Hola V, Bonaventura GD, Djukić S, Ćirković I, Ruzicka F. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis. 2007;115(8):891–9.
Stepanović S, Vuković D, Dakić I, Savić B, Švabić-Vlahović M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175–9.
Moazeni E, Gilani K, Sotoudegan F, Pardakhty A, Najafabadi AR, Ghalandari R, Fazeli MR, Jamalifar H. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J Microencapsul. 2010;27(7):618–27.
Shamkani F, Barzi SM, Badmasti F, Chiani M, Zafari M, Shafiei M. Enhanced anti-biofilm activity of the minocycline-and-gallium-nitrate using niosome wrapping against Acinetobacter baumannii in C57/BL6 mouse pneumonia model. Int Immunopharmacol. 2023;115:109551.
Jacob S, Nair AB, Al-Dhubiab BE. Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition. J Liposome Res. 2017;27(4):283–92.
WS R. Imagej, us national institutes of health, bethesda, maryland, Usa. https://imagejnihgov/ij/2011.
Chasanah U, Mahmintari N, Hidayah F, El Maghfiroh F, Rahmasari D, Nugraheni RW. Thin-layer hydration method to prepare a green tea extract niosomal gel and its antioxidant performance. Eur Pharm J. 2021;68(1):125–35.
Pande S, Parikh JR. Development and validation of UV-Spectrophotometric method for estimation of Vancomycin Hydrochloride. J Drug Deliv Ther. 2019;9(3–s):116–8.
Blainski A, Lopes GC, De Mello JCP. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18(6):6852–65.
Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, Carr B, Redman CW, Harris AL, Dobson PJ. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomed: Nanotechnol Biol Med. 2011;7(6):780–8.
Heredia NS, Vizuete K, Flores-Calero M, Pazmiño VK, Pilaquinga F, Kumar B, Debut A. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly (lactic-co-glycolic acid). PLoS ONE. 2022;17(3):e0264825.
Ali H, Shirode AB, Sylvester PW, Nazzal S. Preparation, characterization, and anticancer effects of simvastatin–tocotrienol lipid nanoparticles. Int J Pharm. 2010;389(1–2):223–31.
MA W. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. Clsi (Nccls). 2006;26:M7–A7.
Bardbari AM, Arabestani MR, Karami M, Keramat F, Aghazadeh H, Alikhani MY, Bagheri KP. Highly synergistic activity of melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis. 2018;37:443–54.
Eales M. The visualisation of interactions between Acinetobacter baumannii and the antimicrobial peptides: colistin sulphate, bicarinalin and BP100. University of Lincoln; 2015.
Gopal R, Kim YG, Lee JH, Lee SK, Chae JD, Son BK, Seo CH, Park Y. Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant Acinetobacter baumannii strains. Antimicrob Agents Chemother. 2014;58(3):1622–9.
Mirzaei R, Alikhani MY, Arciola CR, Sedighi I, Yousefimashouf R, Bagheri KP. Prevention, inhibition, and degradation effects of melittin alone and in combination with Vancomycin and rifampin against strong biofilm producer strains of methicillin-resistant Staphylococcus epidermidis. Biomed Pharmacother. 2022;147:112670.
Mirzaie A, Peirovi N, Akbarzadeh I, Moghtaderi M, Heidari F, Yeganeh FE, Noorbazargan H, Mirzazadeh S, Bakhtiari R. Preparation and optimization of ciprofloxacin encapsulated niosomes: a new approach for enhanced antibacterial activity, biofilm inhibition and reduced antibiotic resistance in ciprofloxacin-resistant methicillin-resistance Staphylococcus aureus. Bioorg Chem. 2020;103:104231.
Guha P, Roy B, Karmakar G, Nahak P, Koirala S, Sapkota M, Misono T, Torigoe K, Panda AK. Ion-pair amphiphile: a neoteric substitute that modulates the physicochemical properties of biomimetic membranes. J Phys Chem B. 2015;119(11):4251–62.
Janic B, Liu F, Bobbitt K, Brown S, Chetty I, Mao G, Movsas B, Wen N. Cellular uptake and radio-sensitization effect of small gold nanoparticles in MCF-7 breast cancer cells. J Nanomed Nanotechnol. 2018;9(2):1–13.
Zafari M, Adibi M, Chiani M, Bolourchi N, Barzi SM, Nosrati MSS, Bahari Z, Shirvani P, Noghabi KA, Ebadi M. Effects of cefazolin-containing niosome nanoparticles against methicillin-resistant Staphylococcus aureus biofilm formed on chronic wounds. Biomed Mater. 2021;16(3):035001.
Ghafelehbashi R, Akbarzadeh I, Yaraki MT, Lajevardi A, Fatemizadeh M, Saremi LH. Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int J Pharm. 2019;569:118580.
Nouruzi E, Hosseini SM, Asghari B, Mahjoub R, Zare EN, Shahbazi M-A, Kalhori F, Arabestani MR. Effect of poly (lactic-co-glycolic acid) polymer nanoparticles loaded with Vancomycin against Staphylococcus aureus biofilm. BMC Biotechnol. 2023;23(1):39.
Rezaeiroshan A, Saeedi M, Morteza-Semnani K, Akbari J, Gahsemi M, Nokhodchi A. Development of trans-ferulic acid niosome: an optimization and an in-vivo study. Int J Drug Deliv Technol. 2020;59:101854.
Heidari F, Akbarzadeh I, Nourouzian D, Mirzaie A, Bakhshandeh H. Optimization and characterization of tannic acid loaded niosomes for enhanced antibacterial and anti-biofilm activities. Adv Powder Technol. 2020;31(12):4768–81.
Celik C, Ildiz N, Sagiroglu P, Atalay MA, Yazici C, Ocsoy I. Preparation of nature inspired indicator based agar for detection and identification of MRSA and MRSE. Talanta. 2020;219:121292.
Turek D, Van Simaeys D, Johnson J, Ocsoy I, Tan W. Molecular recognition of live methicillin-resistant Staphylococcus aureus cells using DNA aptamers. World J Transl Med. 2013;2(3):67–74.
Piri-Gharaghie T, Jegargoshe-Shirin N, Saremi-Nouri S, Khademhosseini S-h, Hoseinnezhad-Lazarjani E, Mousavi A, Kabiri H, Rajaei N, Riahi A, Farhadi-Biregani A. Effects of Imipenem-containing niosome nanoparticles against high prevalence methicillin-resistant Staphylococcus Epidermidis biofilm formed. Sci Rep. 2022;12(1):5140.
Kwon AS, Park GC, Ryu SY, Lim DH, Lim DY, Choi CH, Park Y, Lim Y. Higher biofilm formation in multidrug-resistant clinical isolates of Staphylococcus aureus. Int J Antimicrob Agents. 2008;32(1):68–72.
Haddadian A, Robattorki FF, Dibah H, Soheili A, Ghanbarzadeh E, Sartipnia N, Hajrasouliha S, Pasban K, Andalibi R, Ch MH. Niosomes-loaded selenium nanoparticles as a new approach for enhanced antibacterial, anti-biofilm, and anticancer activities. Sci Rep. 2022;12(1):21938.
Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33(26):5967–82.
Neopane P, Nepal HP, Shrestha R, Uehara O, Abiko Y. In vitro biofilm formation by Staphylococcus aureus isolated from wounds of hospital-admitted patients and their association with antimicrobial resistance. Int J Gen Med 2018:25–32.
Eyoh AB, Toukam M, Atashili J, Fokunang C, Gonsu H, Lyonga EE, Mandi H, Ikomey G, Mukwele B, Mesembe M. Relationship between multiple drug resistance and biofilm formation in Staphylococcus aureus isolated from medical and non-medical personnel in Yaounde, Cameroon. Pan Afr Med J 2014, 17.
Kokare C, Chakraborty S, Khopade A, Mahadik KR. Biofilm: importance and applications. Indian J Biotechnol. 2009;8:159–68.
de Morais Oliveira-Tintino CD, Muniz DF, dos Santos Barbosa CR, Silva Pereira RL, Begnini IM, Rebelo RA, da Silva LE, Mireski SL, Nasato MC. Lacowicz Krautler MI: NorA, Tet (K), MepA, and MsrA efflux pumps in Staphylococcus aureus, their inhibitors and 1, 8-naphthyridine sulfonamides. Curr Pharm Des. 2023;29(5):323–55.
Kato F, Yamaguchi Y, Inouye K, Matsuo K, Ishida Y, Inouye M. A novel gyrase inhibitor from toxin–antitoxin system expressed by Staphylococcus aureus. FEBS J. 2023;290(6):1502–18.
Akbarzadeh I, Rezaei N, Bazzazan S, Mezajin MN, Mansouri A, Karbalaeiheidar H, Ashkezari S, Moghaddam ZS, Lalami ZA, Mostafavi E. In silico and in vitro studies of GENT-EDTA encapsulated niosomes: a novel approach to enhance the antibacterial activity and biofilm inhibition in drug-resistant Klebsiella pneumoniae. Biomater Adv. 2023;149:213384.
Satish J, Amusa AS, Gopalakrishna P. In vitro activities of fluoroquinolones entrapped in non-ionic surfactant vesicles against ciprofloxacin-resistant bacteria strains. J Pharm Technol Drug Res. 2012;1(1):1–11.
Alomrani AH, Al-Agamy MH, Badran MM. In vitro skin penetration and antimycotic activity of itraconazole loaded niosomes: various non-ionic surfactants. J Drug Deliv Sci Technol. 2015;28:37–45.
Agarwal R, Katare O, Vyas S. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 2001;228(1–2):43–52.
Fan KC, Lin J, Yannuzzi NA, Al-Khersan H, Patel NA, Maestre-Mesa J, Zaidi M, Miller D, Flynn HW. In vitro susceptibilities of methicillin-susceptible and resistant staphylococci to traditional antibiotics compared to a novel fluoroquinolone. J Ophthalmic Inflamm Infect. 2020;10:1–5.
Taz K, Jobayer M, Shamsuzzaman S. Nasal Colonization of Methicillin Resistant Staphylococcus aureus among Healthcare Providers in a Tertiary Care Hospital, Bangladesh. Mymensingh Med Journal: MMJ. 2019;28(3):627–33.
Huang C-M, Chen C-H, Pornpattananangkul D, Zhang L, Chan M, Hsieh M-F, Zhang L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials. 2011;32(1):214–21.
Rezaeiroshan A, Saeedi M, Morteza-Semnani K, Akbari J, Hedayatizadeh-Omran A, Goli H, Nokhodchi A. Vesicular formation of trans-ferulic acid: an efficient approach to improve the radical scavenging and antimicrobial properties. J Pharm Innov 2022:1–10.
Kopermsub P, Mayen V, Warin C. Potential use of niosomes for encapsulation of nisin and EDTA and their antibacterial activity enhancement. Food Res Int. 2011;44(2):605–12.
Furneri PM, Fresta M, Puglisi G, Tempera G. Ofloxacin-loaded liposomes: in vitro activity and drug accumulation in bacteria. Antimicrob Agents Chemother. 2000;44(9):2458–64.
Dwivedi A, Mazumder A, Nasongkla N. Layer-by-layer nanocoating of antibacterial niosome on orthopedic implant. Int J Pharm. 2018;547(1–2):235–43.
Huang X, Wang P, Li T, Tian X, Guo W, Xu B, Huang G, Cai D, Zhou F, Zhang H. Self-assemblies based on traditional medicine berberine and cinnamic acid for adhesion-induced inhibition multidrug-resistant Staphylococcus aureus. ACS Appl Mater Interfaces. 2019;12(1):227–37.
Kashef MT, Saleh NM, Assar NH, Ramadan MA. The antimicrobial activity of ciprofloxacin-loaded niosomes against ciprofloxacin-resistant and biofilm-forming Staphylococcus aureus. Infect Drug Resist 2020:1619–29.
Shadvar P, Mirzaie A, Yazdani S. Fabrication and optimization of Amoxicillin-loaded niosomes: an appropriate strategy to increase antimicrobial and anti-biofilm effects against multidrug-resistant Staphylococcus aureus strains. Drug Dev Ind Pharm. 2021;47(10):1568–77.
Nejadnik MR, van der Mei HC, Norde W, Busscher HJ. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials. 2008;29(30):4117–21.
Banerjee I, Pangule RC, Kane RS. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv Mater. 2011;23(6):690–718.
Francolini I, Donelli G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol Med Microbiol. 2010;59(3):227–38.
Acknowledgements
The authors of this paper would like to express their gratitude to Hamadan University of Medical Sciences for their financial support, which was provided through grant number 140106084787.
Funding
This work was supported by the Research Center of Hamadan University of Medical Sciences on grant number 140106084787.
Author information
Authors and Affiliations
Contributions
M.S., M.C. and M.R.A. supervised all stages of designing and conducting the study. J.H. and M.C. performed experiments. J.H. and G.R. analyzed the data. J.H., B.A., S.S., M.S., and M.R.A. prepared the manuscript draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The present study received ethical approval from the Institutional Review Board (IRB) of Hamadan University of Medical Sciences (IR.UMSHA.REC.1401.496). All experiments conducted as part of this study were carried out in compliance with the relevant guidelines and regulations, or in alignment with the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all participants, including patients and the parents or legal guardians of any children involved in the study.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hemmati, J., Chiani, M., Asghari, B. et al. Antibacterial and antibiofilm potentials of vancomycin-loaded niosomal drug delivery system against methicillin-resistant Staphylococcus aureus (MRSA) infections. BMC Biotechnol 24, 47 (2024). https://doi.org/10.1186/s12896-024-00874-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12896-024-00874-1