Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) infections are considered an important public health problem, and treatment options are limited. Accordingly, in this meta-analysis, we analyzed published studies to survey in vitro activity of recently approved antibiotics against MRSA isolates.
Methods
We searched electronic databases; PubMed, Scopus, and Web of Science to identify relevant studies (until November 30, 2020) that have focused on the in vitro activity of telavancin, dalbavancin, oritavancin, and tedizolid against MRSA isolates. Statistical analyses were conducted using STATA software (version 14.0).
Results
Thirty-eight studies were included in this meta-analysis. Overall in vitro activity of tedizolid on 12,204 MRSA isolates was 0.250 and 0.5 µg/mL for MIC50 and MIC90, (minimum inhibitory concentration at which 50% and 90% of isolates were inhibited, respectively), respectively. The overall antibacterial activity of dalbavancin on 28539 MRSA isolates was 0.060 and 0.120 µg/mL for MIC50 and MIC90, respectively. The overall antibacterial activity of oritavancin on 420 MRSA isolates was 0.045 and 0.120 µg/mL for MIC50 and MIC90, respectively. The overall antibacterial activity of telavancin on 7353 MRSA isolates was 0.032 and 0.060 µg/mL for MIC50 and MIC90, respectively. The pooled prevalence of tedizolid, telavancin, and dalbavancin susceptibility was 100% (95% CI: 100–100).
Conclusion
Telavancin, dalbavancin, oritavancin, and tedizolid had potent in vitro activity against MRSA isolates. The low MICs and high susceptibility rates of these antibiotics recommend a hopeful direction to introduce useful antibiotics in treating MRSA infections in the future.
Similar content being viewed by others
Introduction
Staphylococcus aureus (S. aureus) is a prominent cause of hospital-acquired and community-acquired infections ranging from superficial skin and soft tissue infections to endocarditis [1, 2].
For two reasons, A) methicillin-resistant Staphylococcus aureus (MRSA) is a well-recognized public health problem worldwide [3], and B) Antibiotic-resistance pattern of MRSA. Currently, World Health Organization (WHO) considers S. aureus, especially MRSA, as one of the fundamental clinical challenges throughout the world. [4]. There are limited therapeutic options for the treatment of MRSA infections. Vancomycin is introduced as a drug of choice for treating serious infections due to MRSA. However, overuse of vancomycin leads to the emergence of non-susceptible strain [5,6,7]. For example, vancomycin-resistance S. aureus (VRSA) strains have been reported from many countries, including the USA, India, Iran, and Pakistan [5,6,7].
Furthermore, linezolid and clindamycin are other favorable antibiotics against MRSA infections [8]. Despite different mechanisms of action, the emergence of resistant strains to these antibiotics is rising [8,9,10,11,12]. Increased antibiotic resistance in MRSA isolates is one of this century's most globally significant problems [4]. Several new agents such as telavancin, dalbavancin, oritavancin, and tedizolid have recently been licensed for the treatment of infections caused by MRSA.
Following the emergence of strains with reduced susceptibility to vancomycin (first generation of glycopeptide), the second generation of semisynthetic lipoglycopeptides has been developed as alternatives for treating MRSA infections. Telavancin, dalbavancin, and oritavancin have been introduced as critical lipoglycopeptide antibiotics recently approved by the Food and Drug Administration (FDA). Telavancin was the first approved lipoglycopeptide by the FDA in 2009 [13]. Furthermore, dalbavancin and oritavancin were first approved by the FDA in 2014 [14, 15]. Lipoglycopeptides are semisynthetic derivatives characterized by adding a lipophilic side chain, which prolongs their half-lives and increases their activities against Gram-positive cocci [16]. Lipoglycopeptides inhibit cell wall synthesis by binding to C-terminal D-alanyl-D-alanine (D-Ala-D-Ala) of cell wall precursor units [17, 18]. The N-alkyl-p-chlorophenylbenzyl substituent in oritavancin confers significantly enhanced activity against vancomycin-intermediate and-resistant staphylococci [17]. In addition, lipoglycopeptides can interfere with cellular membrane functions [17, 19].
Linezolid, the first oxazolidinone antibacterial agent, was approved in the United States in early 2000. The following approved oxazolidinone was tedizolid. Tedizolid is a second-generation oxazolidinone class approved in 2014 by the FDA. This antibiotic is a bacteriostatic compound against gram-positive bacteria [20]. Similar to linezolid, the mechanical action of tedizolid is inhibiting protein synthesis by binding to the 23S ribosomal RNA of the 50S subunit [21]. Tedizolid is an oxazolidinone but differs from other oxazolidinones by possessing a modified side chain at the C-5 position of the oxazolidinone nucleus that improves potency through additional binding site interactions [22]. Not many in-depth studies are available that directly compare the susceptibilities of telavancin, dalbavancin, oritavancin, and tedizolid to different MRSA strains. Therefore, this systematic meta-analysis was conducted to survey in vitro activity of recently approved antibiotics against MRSA isolates by analyzing the related published studies.
Methods
Guidelines
This review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) [23].
Search strategy
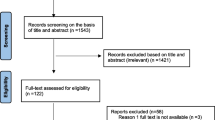
A systematic search was conducted to evaluate the antibacterial activity of recently approved antibiotics against MRSA strains. The electronic databases: Medline, Embase, and Web of Science were searched to identify relevant articles until November 30, 2020. The search strategy was based on keywords derived from our research questions. The keywords used in the search were: "tedizolid", "dalbavancin", "oritavancin", "telavancin", "delafloxacin", "Methicillin-Resistant Staphylococcus aureus", and "minimum inhibitory concentration". The Boolean operators were used to combine all descriptors. The search strategy was adapted to the features of each database. If possible, we searched for synonyms or used the search option for similar terms before every keyword. No limitation was applied during the searching procedure of databases, but the inclusion of the study in our full analysis required at least the English abstract to be available. The records found through database searching were merged, and the duplicates were removed using EndNote X7 (Thomson Reuters, New York, NY, USA). Reference lists of all eligible articles were also reviewed to find any additional potentially relevant studies. The flow chart of the selected articles is shown in Fig. 1.
Eligibility criteria
Identified studies that were consistent with the criteria included original articles published in English concerning the antibacterial activity of recently approved antibiotics against MRSA strains. After screening, duplicate studies, non-original articles (reviews, short communications, case studies, abstracts without full text, and book chapters), and studies that lack information regarding the minimum inhibitory concentration (MIC) were excluded.
One reviewer performed the searches; then, initial screening was done by two independent reviewers for potentially relevant records matching the inclusion/exclusion criteria based on title and abstracts. Full articles were obtained from these records and were assessed for relevance by two independent reviewers. Any discrepancies with the third reviewer were resolved by consulting. Whereas the initial study was not available, requests were made to the authors.
Data extraction and quality assessment
Two reviewers coded and extracted the data independently. This process was also overseen by the third author again. All studies were consistent with the following inclusion criteria: (1) antibacterial activity was determined using one of the standard methods, including broth microdilution, agar dilution, and epsilometer (E)-test, (2) MIC50 and MIC90 (minimum inhibitory concentration at which 50% and 90% of isolates were inhibited, respectively) and their ranges were available, also (3) original studies that were performed on clinically derived isolates. Meanwhile, exclusion criteria were (1) studies that have not reported the MIC or have not used the standard susceptibility testing methods, (2) studies with a sample size < 10 isolates, and (3) studies performed on samples with animals or environment origin. Neither reviews nor systematic review articles, case reports, and articles available only in the abstract that lacks necessary information were included. Moreover, the quality of included studies was critically appraised using the Newcastle–Ottawa Scale [24]. The pre-defined review protocol was registered at the PROSPERO international prospective register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO, registration number CRD11111).
Statistical analysis
The meta-analysis was performed by computing the pooled using a random-effects model with Stata/SE software, v.17 (StataCorp, College Station, TX) on studies presenting raw data on antibacterial activity of tedizolid, dalbavancin, oritavancin, telavancin, and delafloxacin against MRSA strains. The inconsistency across studies was examined by the forest plot as well as the I2 statistic. Values of I2 (25%, 50%, 75%) were interpreted as the presence of low, medium, or high heterogeneity, respectively. So, the DerSimonian and Laird random effects models were used [25]. Publication bias was assessed using Egger's test. All statistical interpretations were reported on a 95% confidence interval (CI) basis.
Study outcomes
The primary outcome of interest was the pooled prevalence susceptibility of tedizolid, dalbavancin, and telavancin against MRSA isolates. The secondary outcomes of interest were the MIC50 and MIC90 of tedizolid, dalbavancin, and telavancin against MRSA isolates.
Results
Systematic literature search
A total of 540 records were identified in the initial search. Among these, 357 articles were excluded after an initial screening of the title and abstract due to their irrelevance and duplication. The full texts of the remaining 183 articles were reviewed (Fig. 1). Out of 183 articles, 145 were excluded for the following reasons: meta-analysis, review, conference abstract, and article without full text (n = 70), non-relevant data, or no MIC data (n = 75). Finally, the detailed characteristics of 38 included studies in this meta-analysis are indicated in Table 1.
Characteristics of included studies
All included studies had a cross-sectional design. All included studies in this meta-analysis were high-quality (Additional file 2: Table) [24]. However, most reports have been from America (n = 19), Asia (n = 8), Europe (n = 8), and multiple continents (n = 7). In the current study, to determine the effective concentration of tedizolid, dalbavancin, oritavancin, and telavancin against MRSA isolates, the mode of MIC50, MIC90, and MIC ranges was estimated (Table 2). To analyze the trends for changes in the tedizolid, dalbavancin, oritavancin, and telavancin susceptibility in recent years, we performed a subgroup analysis for two periods (2010–2015 and 2016–2020) (Tables 3, 5, Additional file 1: Figure). No significant difference was observed in the pooled prevalence of tedizolid, dalbavancin, oritavancin, and telavancin susceptibilities against MRSA isolate for two periods (2010–2015 and 2016–2020) (Tables 3, 4, 5).
Antibacterial activity of tedizolid
The prevalence of tedizolid susceptibility is available in 21 studies. The overall antibacterial activity of tedizolid in 12,204 MRSA isolates was at 0.250 and 0.500 µg/mL for MIC50 and MIC90, respectively. Out of 21 studies, the pooled prevalence of tedizolid susceptibility was 100% (95% CI: 100–100) (Table 6). There was no substantial heterogeneity among the 21 studies (p = 0.99; I2 = 0%).
Antibacterial activity of dalbavancin
The prevalence of dalbavancin susceptibility is available in 11 studies. The overall antibacterial activity of tedizolid was 0.060, and 0.120 µg/mL for MIC50 and MIC90 in 28539 MRSA isolates, respectively. Out of 11 studies, the pooled prevalence of dalbavancin susceptibility was 100% (95% CI: 100–100) (Table 6). There was no substantial heterogeneity among the 11 studies (p = 0.61; I2 = 0%).
Antibacterial activity of oritavancin
The prevalence of oritavancin susceptibility was available in 2 studies. The overall antibacterial activity of oritavancin was 0.045, and 0.120 µg/mL for MIC50 and MIC90 in 420 MRSA isolates, respectively.
Antibacterial activity of telavancin
The prevalence of telavancin susceptibility was available in 8 studies. The overall antibacterial activity of telavancin was 0.032, and 0.060 µg/mL for MIC50 and MIC90 in 7353 MRSA isolates, respectively. From 8 studies, the pooled prevalence of telavancin susceptibility was 100% (95% CI: 100–100) (Table 6). There was no substantial heterogeneity among the eight studies (p = 0.86; I2 = 0%).
Discussion
MRSA is considered one of the most critical human health problems worldwide [26]. Empirical therapies by vancomycin and linezolid were reliable options for treating MRSA infections [27]. However, reports on decreasing susceptibility to vancomycin and linezolid are worrying [28]. It is critical to introduce and characterize new effective and safe antibiotics to prevent and control the infections related to MRSA strains [29]. The findings from a systematic review demonstrated that the prevalence of VRSA increased in recent years around the world [30]. It also was shown that different continents and countries are struggling with VRSA strains [30].
Compared with the classic glycopeptides, our meta-analysis shows a higher antibacterial activity of a new class of lipoglycopeptides (telavancin and dalbavancin susceptibilities were 100%). Moreover, the estimated MIC values of three lipoglycopeptides (MIC50/MIC90, 0.060/0.120 µg/mL for dalbavancin, MIC50/MIC90, 0.032/0.060 µg/mL for telavancin, MIC50/MIC90, 0.045/0.120 µg/mL for oritavancin) against MRSA strains are much lower than the MIC value of vancomycin for MRSA in the literature [31]. Moreover, against both vancomycin-resistant Enterococcus (VRE) and vancomycin-susceptible Enterococcus (VSE), the MIC value of lipoglycopeptides is much lower than the MIC value of vancomycin [16].
MIC50/90 values of dalbavancin (0.06/0.12 µg/mL) are very similar to another systematic review published by Sadr in 2017 [32]. Moreover, compared to vancomycin, previous studies indicated that dalbavancin showed potent activity against biofilm-forming bacteria [33, 34]. However, a network meta-analysis showed no significant differences between dalbavancin and vancomycin in treating acute bacterial skin and soft-tissue infections (SSTIs) [35]. Dalbavancin susceptibility was more than 99% in the published systematic review in 2017, as our results [32].
In our study, the MIC50 value of oritavancin against MRSA strains is similar to a systematic review by Mendes et al. in 2015 [36]. Solo clinical trials show that oritavancin is more effective than vancomycin against MRSA infections [37]. Mendes et al. [36] evaluated the activity in vitro of oritavancin and comparators against Gram-positive pathogens causing SSTIs in European and US hospitals. They showed that oritavancin susceptibility in Gram-positive clinical isolates from the United States and Europe were 98.4% and 98.9%, respectively [36]. However, our meta-analysis studied worldwide data, and oritavancin susceptibility was 100%.
A previous systematic review and meta-analysis published in 2019 reported that the MIC50 and MIC90 of tedizolid were 0.250 and 0.500 µg/mL, respectively [38]. These MIC values are lower than the MIC values of vancomycin against MRSA strains [39, 40]. It was also shown that the MIC of tedizolid is much lower than the MIC of vancomycin against VISA strains [41]. In addition, tedizolid demonstrated greater in vitro potency than linezolid against MRSA strains, but further research is required for a treatment recommendation. However, published studies showed that some adverse events are related to the simultaneous administration of telavancin and tedizolid [42, 43]. Moreover, in our meta-analysis, the MIC values and susceptibility rates for all four antibiotics were investigated in two periods (2010–2015 and 2016–2020), and findings were very similar between the two periods. The limited use of these antibiotics and their specific action mechanisms help explain this lack of change.
In conclusion, our results demonstrated that dalbavancin, oritavancin, telavancin, and tedizolid have antibacterial activity in vitro against MRSA isolates. However, future preclinical and clinical research are necessitated to support our findings.
Availability of data and materials
All the data in this review are included in the manuscript.
Abbreviations
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MIC50 :
-
Minimum inhibitory concentration at which 50% of isolates were inhibited
- MIC90 :
-
Minimum inhibitory concentration at which 90% of isolates were inhibited
- S. aureus:
-
Staphylococcus aureus
- VRSA:
-
Vancomycin-resistance S. aureus
- FDA:
-
Food and drug administration
- D-Ala-D-Ala:
-
D-alanyl-D-alanine
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses guidelines
- MIC:
-
Minimum inhibitory concentration
- CI:
-
Confidence interval
- VRE:
-
Vancomycin-resistant Enterococcus
- VSE:
-
Vancomycin-susceptible Enterococcus
- SSTIs:
-
Skin and soft-tissue infections
References
Rajput A, Poudel S, Tsunemoto H, Meehan M, Szubin R, Olson CA, Seif Y, Lamsa A, Dillon N, Vrbanac A. Identifying the effect of vancomycin on health care–associated methicillin-resistant Staphylococcus aureus strains using bacteriological and physiological media. GigaScience. 2021;10(1):giaa156.
Panwala T, Gandhi P, Jethwa D: Inducible Clindamycin resistance and MRSA amongst Staphylococcus aureus isolates: A phenotypic detection.
Thabit AK, Nicolau DP, Kuti JL. In vitro pharmacodynamics of human simulated exposures of telavancin against methicillin-susceptible and-resistant Staphylococcus aureus with and without prior vancomycin exposure. Antimicrob Agents Chemother. 2016;60(1):222–8.
Cascioferro S, Carbone D, Parrino B, Pecoraro C, Giovannetti E, Cirrincione G, Diana P. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. ChemMedChem. 2021;16(1):65–80.
Appelbaum PJCM. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2006;12:16–23.
Selvabai, AP, Sattar, SBA, Jayaraman, P, SHANMUGAM PJJoC, Research D. Detection and Characterisation of Heteroresistant Vancomycin Intermediate Staphylococcus aureus (hVISA) using Phenotypic and Genotypic Methods. J Clin Diagn Res. 2019. https://doi.org/10.7860/JCDR/2019/41127.12868.
Wu Q, Sabokroo N, Wang Y, Hashemian M, Karamollahi S, Kouhsari EJAR, Control I. Systematic review and meta-analysis of the epidemiology of vancomycin-resistance Staphylococcus aureus. Isolates Antimicrob Resist Infect Control. 2021;10(1):1–13.
Frank AL, Marcinak JF, Mangat PD, Tjhio JT, Kelkar S, Schreckenberger Quinn PC, JPJTPidj,. Clindamycin treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatric Infect Dis J. 2002;21(6):530–4.
Blair J, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJJNrm. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51.
Chambers HJPm. Methicillin-resistant Staphylococcus aureus. Mechanisms of resistance and implications for treatment. 2001;109(2 Suppl):43–50.
GM Eliopoulos VG Meka 2004 Gold HSJCID: Antimicrobial resistance to linezolid 39 7 1010 1015
Lewis JS, Jorgensen JHJCID. Inducible clindamycin resistance in staphylococci: should clinicians and microbiologists be concerned? Clin Infect Dis. 2005;40(2):280–5.
Al Jalali V, Zeitlinger M. Clinical pharmacokinetics and pharmacodynamics of telavancin compared with the other glycopeptides. Clin Pharmacokinet. 2018;57(7):797–816.
Saravolatz LD, Stein GE. Oritavancin: a long-half-life lipoglycopeptide. Clin Infect Dis. 2015;61(4):627–32.
Dash RP, Babu RJ, Srinivas NR. Review of the pharmacokinetics of dalbavancin, a recently approved lipoglycopeptide antibiotic. Infect Dis. 2017;49(7):483–92.
Zhanel GG, Calic D, Schweizer F, Zelenitsky S, Adam H, Lagacé-Wiens PR, Rubinstein E, Gin AS, Hoban DJ, Karlowsky JA. New lipoglycopeptides. Drugs. 2010;70(7):859–86.
Zhanel GG, Schweizer F, Karlowsky JA. Oritavancin: mechanism of action. Clin Infect Dis. 2012;54(3):S214–9.
Song Y, Lunde CS, Benton BM, Wilkinson BJ. Further insights into the mode of action of the lipoglycopeptide telavancin through global gene expression studies. Antimicrob Agents Chemother. 2012;56(6):3157–64.
Belley A, McKay GA, Arhin FF, Sarmiento I, Beaulieu S, Fadhil I, Parr TR, Moeck G. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant enterococci to effect rapid bacterial killing. Antimicrob Agents Chemother. 2010;54(12):5369–71.
Burdette SD, Trotman R. Tedizolid: the first once-daily oxazolidinone class antibiotic. Clin Infect Dis. 2015;61(8):1315–21.
Chen K-H, Huang Y-T, Liao C-H, Sheng W-H, Hsueh P-R. In vitro activities of tedizolid and linezolid against Gram-positive cocci associated with acute bacterial skin and skin structure infections and pneumonia. Antimicrob Agents Chemother. 2015;59(10):6262–5.
Mikamo H, Takesue Y, Iwamoto Y, Tanigawa T, Kato M, Tanimura Y, Kohno S. Efficacy, safety and pharmacokinetics of tedizolid versus linezolid in patients with skin and soft tissue infections in Japan–results of a randomised, multicentre phase 3 study. J Infect Chemother. 2018;24(6):434–42.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, Atkins D, Barbour V, Barrowman N, Berlin JA, Clark J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (Chinese edition). Journal of Chinese Integrative Medicine. 2009 Sep;7(9):889–96.
Newcastle O: Newcastle-Ottawa: Scale customized for cross-sectional studies In. In.; 2018.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Abdel-Wahab FB, El Menofy N, El-Batal A, Mosalam F, Abdulall AK. Enhanced Antimicrobial Activity of the combination of Silver Nanoparticles and Different β Lactam antibiotics against Methicillin Resistant Staphylococcus aureus isolates. Azhar Int J Phar Med Sci. 2021;1(1):24–33.
Bradley JS. Which antibiotic for resistant gram-positives, and why? J Infect. 2014;68:S63–75.
Hadadi M, Heidari H, Ebrahim-Saraie HS, Motamedifar M. Molecular characterization of vancomycin, mupirocin and antiseptic resistant Staphylococcus aureus strains. Mediter J Hematol Infect Dis. 2018;10(1):e2018053.
Lee C-R, Cho IH, Jeong BC, Lee SH. Strategies to minimize antibiotic resistance. Int J Environ Res Public Health. 2013;10(9):4274–305.
Wu Q, Sabokroo N, Wang Y, Hashemian M, Karamollahi S, Kouhsari E. Systematic review and meta-analysis of the epidemiology of vancomycin-resistance Staphylococcus aureus isolates. Antimicrob Resist Infect Control. 2021;10(1):1–13.
!!! INVALID CITATION !!! (9, 24–26).
Sader HS, Mendes RE, Duncan LR, Pfaller MA, Flamm RK. Antimicrobial activity of dalbavancin against Staphylococcus aureus with decreased susceptibility to glycopeptides, daptomycin, and/or linezolid from US medical centers. Antimicrob Agents Chemother. 2018;62(3):e20397.
Esposito S, Purrello Sm, Bonnet E, Novelli A, Tripodi F, Pascale R, Unal S, Milkovich G. Central venous catheter-related biofilm infections: An up-to-date focus on meticillin-resistant Staphylococcus aureus. J Glob Antimicrob Resist. 2013;1(2):71–8.
Di Pilato V, Ceccherini F, Sennati S, D’Agostino F, Arena F, D’Atanasio N, Di Giorgio FP, Tongiani S, Pallecchi L, Rossolini GM. In vitro time-kill kinetics of dalbavancin against Staphylococcus spp biofilms over prolonged exposure times. Diagn Microbiol Infect Dis. 2020;96(2):114901.
Guest JF, Esteban J, Manganelli AG, Novelli A, Rizzardini G, Serra M. Comparative efficacy and safety of antibiotics used to treat acute bacterial skin and skin structure infections: results of a network meta-analysis. PLoS ONE. 2017;12(11):e0187792.
Mendes RE, Farrell DJ, Sader HS, Flamm RK, Jones RN. Activity of oritavancin against gram-positive clinical isolates responsible for documented skin and soft-tissue infections in European and US hospitals (2010–13). J Antimicrob Chemother. 2015;70(2):498–504.
Lodise TP, Redell M, Armstrong SO, Sulham KA, Corey GR. Efficacy and safety of oritavancin relative to vancomycin for patients with acute bacterial skin and skin structure infections (ABSSSI) in the outpatient setting: results from the SOLO clinical trials. InOpen forum infectious diseases. 2017 Jan 1;4;1. Oxford University Press.
Hasannejad-Bibalan M, Mojtahedi A, Biglari H, Halaji M, Sedigh Ebrahim-Saraie H. Antibacterial activity of tedizolid, a novel oxazolidinone against methicillin-resistant Staphylococcus aureus: a systematic review and meta-analysis. Microb Drug Resist. 2019;25(9):1330–7.
Biedenbach D, Bouchillon S, Johnson B, Alder J, Sahm D. In vitro activity of tedizolid against Staphylococcus aureus and Streptococcus pneumoniae collected in 2013 and 2014 from sites in Latin American countries, Australia, New Zealand, and China. Eur J Clin Microbiol Infect Dis. 2016;35(12):1933–9.
Betriu C, Morales G, Rodríguez-Avial I, Culebras E, Gómez M, López-Fabal F, Picazo JJ. Comparative activities of TR-700 (torezolid) against staphylococcal blood isolates collected in Spain. Antimicrob Agents Chemother. 2010;54(5):2212–5.
Barber KE, Smith JR, Raut A, Rybak MJ. Evaluation of tedizolid against Staphylococcus aureus and enterococci with reduced susceptibility to vancomycin, daptomycin or linezolid. J Antimicrob Chemother. 2016;71(1):152–5.
Moenster RP, Linneman TW, Call WB, Kay CL, McEvoy TA, Sanders JL. The potential role of newer gram-positive antibiotics in the setting of osteomyelitis of adults. J Clin Pharm Ther. 2013;38(2):89–96.
Lee EY, Caffrey AR. Thrombocytopenia with tedizolid and linezolid. Antimicrob Agents Chemother. 2018. https://doi.org/10.1128/AAC.01453-17.
Aktas G, Derbentli S. In vitro activity of dalbavancin against staphylococci isolated in Istanbul Turkey. Chemotherapy. 2010;56(6):444–7.
Aktas G, Derbentli S. In vitro activity of daptomycin combined with dalbavancin and linezolid, and dalbavancin with linezolid against MRSA strains. J Antimicrob Chemother. 2017;72(2):441–3.
Azrad M, Baum M, Rokney A, Levi Y, Peretz A. In vitro activity of tedizolid and dalbavancin against MRSA strains is dependent on infection source. Int J Infect Dis. 2019;78:107–12.
Citron DM, Tyrrell KL, Goldstein EJ. Comparative in vitro activities of dalbavancin and seven comparator agents against 41 Staphylococcus sp. cultured from osteomyelitis infections and 18 VISA and hVISA strains. Diagn Microbiol Infect Dis. 2014;79(4):438–40.
Corey GR, Arhin FF, Wikler MA, Sahm DF, Kreiswirth BN, Mediavilla JR, Good S, Fiset C, Jiang H, Moeck G. Pooled analysis of single-dose oritavancin in the treatment of acute bacterial skin and skin-structure infections caused by gram-positive pathogens, including a large patient subset with methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2016;48(5):528–34.
Chong YP, Park S-J, Kim HS, Kim ES, Kim M-N, Kim S-H, Lee S-O, Choi S-H, Jeong J-Y, Woo JH. In vitro activities of ceftobiprole, dalbavancin, daptomycin, linezolid, and tigecycline against methicillin-resistant Staphylococcus aureus blood isolates: stratified analysis by vancomycin MIC. Diagn Microbiol Infect Dis. 2012;73(3):264–6.
Guzek A, Rybicki Z, Tomaszewski D. In vitro analysis of the minimal inhibitory concentration values of different generations of anti-methicillin-resistant Staphylococcus aureus antibiotics. Indian J Med Microbiol. 2018;36(1):119–20.
Huang V, Cheung CM, Kaatz GW, Rybak MJ. Evaluation of dalbavancin, tigecycline, minocycline, tetracycline, teicoplanin and vancomycin against community-associated and multidrug-resistant hospital-associated meticillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2010;35(1):25–9.
Jones RN, Flamm RK, Castanheira M, Sader HS, Smart JI, Mendes RE. Activity of telavancin against gram-positive pathogens isolated from bone and joint infections in North American, Latin American, European and Asia-Pacific nations. Diagn Microbiol Infect Dis. 2017;88(2):184–7.
Karlowsky JA, Hackel MA, Bouchillon SK, Alder J, Sahm DF. In vitro activities of tedizolid and comparator antimicrobial agents against clinical isolates of Staphylococcus aureus collected in 12 countries from 2014 to 2016. Diagn Microbiol Infect Dis. 2017;89(2):151–7.
Lee Y, Hong SK, Choi S, Im W, Yong D, Lee K. In vitro activity of tedizolid against gram-positive bacteria in patients with skin and skin structure infections and hospital-acquired pneumonia: a Korean multicenter study. Ann Lab Med. 2015;35(5):523.
Díaz MCL, Ríos E, Rodríguez-Avial I, Simaluiza RJ, Picazo JJ, Culebras E. In-vitro activity of several antimicrobial agents against methicillin-resistant Staphylococcus aureus (MRSA) isolates expressing aminoglycoside-modifying enzymes: potency of plazomicin alone and in combination with other agents. Int J Antimicrob Agents. 2017;50(2):191–6.
Múnera JMV, Ríos AMO, Urrego DM, Jiménez Quiceno JN. In vitro susceptibility of methicillin-resistant Staphylococcus aureus isolates from skin and soft tissue infections to vancomycin, daptomycin, linezolid and tedizolid. Brazilian J Infect Dis. 2017;21(5):493–9.
Mendes RE, Sader HS, Flamm RK, Farrell DJ, Jones RN. Telavancin in vitro activity against a collection of methicillin-resistant Staphylococcus aureus isolates, including resistant subsets, from the United States. Antimicrob Agents Chemother. 2015;59(3):1811–4.
McCurdy SP, Jones RN, Mendes RE, Puttagunta S, Dunne MW. In vitro activity of dalbavancin against drug-resistant Staphylococcus aureus isolates from a global surveillance program. Antimicrob Agents Chemother. 2015;59(8):5007–9.
Mendes R, Sader H, Smart J, Castanheira M, Flamm R. Update of the activity of telavancin against a global collection of Staphylococcus aureus causing bacteremia, including endocarditis (2011–2014). Eur J Clin Microbiol Infect Dis. 2017;36(6):1013–7.
Okado JB, Avaca-Crusca JS, Oliveira AL, Dabul ANG, da Cunha Camargo ILB. Daptomycin and vancomycin heteroresistance revealed among CC5-SCCmecII MRSA clone and in vitro evaluation of treatment alternatives. J Glob Antimicrob Resist. 2018;14:209–16.
Pfaller MA, Sader HS, Rhomberg PR, Flamm RK, Mendes RE. In vitro activity of tedizolid in comparison with other oral and intravenous agents against a collection of community-acquired methicillin-resistant Staphylococcus aureus (2014–2015) in the United States. Microb Drug Resist. 2019;25(6):938–43.
Prokocimer P, Bien P, DeAnda C, Pillar CM, Bartizal K. In vitro activity and microbiological efficacy of tedizolid (TR-700) against Gram-positive clinical isolates from a phase 2 study of oral tedizolid phosphate (TR-701) in patients with complicated skin and skin structure infections. Antimicrob Agents Chemother. 2012;56(9):4608–13.
Rolston KV, Wang W, Nesher L, Coyle E, Shelburne S, Prince RA. In vitro activity of telavancin compared with vancomycin and linezolid against gram-positive organisms isolated from cancer patients. J Antibiot. 2014;67(7):505–9.
Şanal L, Yılmaz N, Uludag H, Öztürk R, Sen S, Cesur S. Detection of synergistic antimicrobial activities of ceftaroline, telavancin, daptomycin, and vancomycin against methicillin-resistant staphylococcus aureus strains in intensive care units. Jundishapur J Microbiol. 2018. https://doi.org/10.5812/jjm.66445.
Schmidt-Malan SM, Quaintance KEG, Karau MJ, Patel R. In vitro activity of tedizolid against staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis. 2016;85(1):77–9.
Shams W, Walker ES, Levy F, Reynolds SA, Peterson SM, Sarubbi FA. Comparative activity of telavancin and other antimicrobial agents against methicillin-resistant Staphylococcus aureus isolates collected from 1991 to 2006. Chemotherapy. 2010;56(5):411–6.
Sweeney D, Shinabarger DL, Arhin FF, Belley A, Moeck G, Pillar CM. Comparative in vitro activity of oritavancin and other agents against methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2017;87(2):121–8.
Smart JI, Corey GR, Stryjewski ME, Wang W, Barriere SL. Assessment of telavancin minimal inhibitory concentrations by revised broth microdilution method in phase 3 complicated skin and skin-structure infection clinical trial isolates. Diagn Microbiol Infect Dis. 2017;87(3):268–71.
Thomson KS, Goering RV. Activity of tedizolid (TR-700) against well-characterized methicillin-resistant Staphylococcus aureus strains of diverse epidemiological origins. Antimicrob Agents Chemother. 2013;57(6):2892–5.
Campanile F, Borbone S, Perez M, Bongiorno D, Cafiso V, Bertuccio T, Purrello S, Nicolosi D, Scuderi C, Stefani S. Heteroresistance to glycopeptides in Italian meticillin-resistant Staphylococcus aureus (MRSA) isolates. Int J Antimicrob Agents. 2010;36(5):415–9.
Bensaci M, Sahm D. Surveillance of tedizolid activity and resistance: in vitro susceptibility of gram-positive pathogens collected over 5 years from the United States and Europe. Diagn Microbiol Infect Dis. 2017;87(2):133–8.
Chen H, Yang Q, Zhang R, He W, Ma X, Zhang J, Xia F, Zhao F, Cao J, Liu Y. In vitro antimicrobial activity of the novel oxazolidinone tedizolid and comparator agents against Staphylococcus aureus and linezolid-resistant Gram-positive pathogens: a multicentre study in China. Int J Antimicrob Agents. 2014;44(3):276–7.
Brown SD, Traczewski MM. Comparative in vitro antimicrobial activities of torezolid (TR-700), the active moiety of a new oxazolidinone, torezolid phosphate (TR-701), determination of tentative disk diffusion interpretive criteria, and quality control ranges. Antimicrob Agents Chemother. 2010;54(5):2063–9.
Yum JH, Choi SH, Yong D, Chong Y, Im WB, Rhee D-K, Lee K. Comparative in vitro activities of torezolid (DA-7157) against clinical isolates of aerobic and anaerobic bacteria in South Korea. Antimicrob Agents Chemother. 2010;54(12):5381–6.
Sahm DF, Deane J, Bien PA, Locke JB, Zuill DE, Shaw KJ, Bartizal KF. Results of the surveillance of tedizolid activity and resistance program: in vitro susceptibility of gram-positive pathogens collected in 2011 and 2012 from the United States and Europe. Diagn Microbiol Infect Dis. 2015;81(2):112–8.
Li S, Guo Y, Zhao C, Chen H, Hu B, Chu Y, Zhang Z, Hu Y, Liu Z, Du Y. In vitro activities of tedizolid compared with other antibiotics against gram-positive pathogens associated with hospital-acquired pneumonia, skin and soft tissue infection and bloodstream infection collected from 26 hospitals in China. J Med Microbiol. 2016;65(10):1215–24.
Viñuela-Prieto JM, de Mendoza DL. Activity of linezolid and tedizolid against clinical isolates of methicillin-resistant and methicillin and linezolid resistant Staphylococcus aureus: an in vitrocomparison. Rev Esp Quimioter. 2016;29(5):255–8.
Pfaller MA, Flamm RK, Jones RN, Farrell DJ, Mendes RE. Activities of tedizolid and linezolid determined by the reference broth microdilution method against 3,032 gram-positive bacterial isolates collected in Asia-Pacific, Eastern Europe, and Latin American countries in 2014. Antimicrob Agents Chemother. 2016;60(9):5393–9.
Acknowledgements
We thank the Research Fund of Changzhi Medical College (BS202023) for your kind support.
Funding
None.
Author information
Authors and Affiliations
Contributions
FL, EK, GHA, SKh, and SR, NO contributed to the work's conception, design, drafting, and extraction of data. Ch. Sh, Kh. A contributed to revising and final approval of the version to be published. All authors agreed and confirmed the manuscript for publication. All authors read and approved the final manuscript.
Author's information
Khalil Azizian; Ph. D of Medical Bacteriology in Kurdistan University of Medical Sciences. His research interests are working on nosocomial infections. He has experience in diagnostics infections and antimicrobial resistance testing.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
The study protocol was approved by the Health Research Ethics Committee of the Golestan University of Medical Sciences (reference no. IR.GOUMS.REC.1401.139).
Consent for publications
Not applicable in this section.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
The quality assessment of included studies in this meta-analysis.
Additional file 2:
Antibacterial activity of dalbavancin telavancin, tedizolid, and dalbavancin against MRSA isolates based on year groups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, F., Rajabi, S., Shi, C. et al. Antibacterial activity of recently approved antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) strains: A systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 21, 37 (2022). https://doi.org/10.1186/s12941-022-00529-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-022-00529-z