Abstract
Background
Meniscal morphological changes are associated with knee OA. However, the correlation of meniscal height and OA-related knee structural abnormalities is still not well understood. The purpose of present study is to investigate whether and how meniscal anterior and posterior horn heights are associated with structural abnormalities in knees with symptomatic OA.
Methods
Our sample consisted of 106 patients (61 female, aged 40–73 years) with symptomatic knee OA. Kellgren-Lawrence system was used for radiographic evaluation. On sagittal sequence, medial meniscal posterior horn height (MPH), lateral meniscal anterior horn height (LAH) and lateral meniscal posterior horn height (LPH) were measured on the middle slice through the medial/lateral compartment. Knee structural abnormalities were assessed using the modified whole-organ magnetic resonance imaging score (WORMS). Associations between meniscal anterior and posterior horn heights and knee structural abnormalities were assessed using linear regression analysis.
Results
Higher MPH was significantly associated with higher WORMS score for medial meniscal anterior horn lesion (P = 0.016) but did not have a statistical association with other WORMS parameters. Increased LAH was statistically correlated with decreased WORMS scores for lateral compartmental cartilage lesions (P = 0.001–0.004) and lateral compartmental bone marrow edema patterns (BMEPs) (P = 0.021–0.027). Moreover, LPH was negatively associated with WORMS scores for lateral compartmental cartilage lesions (P = 0.007–0.041) and lateral compartmental BMEPs (P = 0.022–0.044). Additionally, higher MPH was statistically associated with lower trochlea cartilage WORMS score and higher LAH was significantly correlated with higher WORMS score for trochlea subarticular cysts.
Conclusions
Changes of LAH and LPH were inversely associated with the severity of lateral compartmental cartilage lesions and BMEPs, while higher MPH was only significantly correlated with more severe medial meniscal anterior horn lesions. Besides, MPH and LAH were also significantly associated with patellofemoral structural abnormalities. The present study provided novel information for understanding the role of meniscal morphological changes in knee OA, which would be helpful in identifying and evaluating knees with or at risks for OA.
Similar content being viewed by others
Background
The medial and lateral menisci are asymmetric, wedge-shaped fibrocartilaginous discs, playing an essential role in absorbing, transmitting, and distributing mechanical stress [1]. Damage to a meniscus may compromise its functions and may then increase the risks for knee osteoarthritis (OA). A large number of studies have reported that meniscal damage and malposition are associated with OA-related knee structural abnormalities such as cartilage loss, bone marrow edema patterns (BMEPs) and subchondral cysts [2,3,4,5,6,7]. Meanwhile, investigators were also becoming more concerned about meniscus geometry of OA knees. For example, a study by Jung et al. suggested that the meniscus may be hypertrophied in OA knees [8]. Moreover, using three-dimensional quantitative methods, meniscal shape and position were found to be different between individuals with and without knee OA [9,10,11]. However, the association of meniscal shape, in particular meniscal height, with OA-related knee structural abnormalities is still not well understood. In a prior study, we found that both medial and lateral meniscal body heights were correlated with structural abnormalities in symptomatic knee OA [12]. Given that both medial meniscal body height and posterior horn height were previously revealed to be different between knees with and without OA, whether medial meniscal posterior horn height is also associated with OA-related knee structural abnormalities has become a question to be further explored [11]. Although it is currently unknown whether there is a difference in lateral meniscal heights between knees with and without OA, however, considering the potent role of changed meniscal shape on knee OA incidence and development, further investigation into the association of medial/lateral meniscal anterior/posterior horn heights and OA-related knee structural abnormalities is important because this may help us more efficiently identify individuals with or at risks for knee OA.
Thus, the purpose of this study was to investigate whether and how meniscal anterior and posterior horn heights are associated with OA-related knee structural abnormalities in middle-aged and elderly patients with symptomatic knee OA.
Materials and methods
Subjects
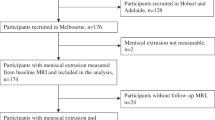
Our institutional review board approved this retrospective study. Informed consent was waived because the present study involved no potential risk to patients. One hundred and thirty-five consecutive patients who underwent knee X-ray and MRI examinations at the second Xiangya hospital between September 2020 and October 2020 and met the American College of Rheumatology (ACR) clinical criteria for knee OA were included [13]. In brief, patients who had knee pain and at least three of the following items were diagnosed to have symptomatic knee OA: age > 50 years; stiffness < 30 min; crepitus; bony tenderness; bony enlargement; no palpable warmth. For patients who underwent bilateral knee scans, we only included the knee presenting with more severe symptoms. The following exclusion criteria were applied: (1) history of rheumatoid arthritis or other inflammatory arthropathy; (2) history of knee surgery or trauma of the index knee. The patient selection process is described in Fig. 1.
X-ray and MRI examinations
Weight-bearing PA using the protocol of Buckland-Wright, weight-bearing skyline and weight-bearing lateral radiographs were obtained for all kness [14, 15]. MRI scans were performed using a 3-T MRI scanner (uMR 790, Shanghai United Imaging Healthcare, Shanghai, China) with quadrature transmit-receive coils. The following sequences were used in the present study: (a) a sagittal intermediate-weighted (IW) fat-saturated (FS) 2D fast spin-echo (FSE) sequence (repetition time [TR] / echo time [TE], 2682/39 msec; in plane spatial resolution, 0.69 × 0.63 mm2; section thickness, 3.5 mm); (b) a sagittal T2-weighted FS 2D FSE sequence (TR/TE, 3800/68 msec; in plane spatial resolution, 0.74 × 0.63 mm2; section thickness, 3.5 mm); (c) a coronal IW FS 2D FSE sequence (TR/TE, 2482/38 msec; in plane spatial resolution, 0.69 × 0.56 mm2; section thickness, 3.5 mm); (d) a coronal T1-weighted 2D FSE sequence (TR/TE, 518/10 msec; in plane spatial resolution, 0.59 × 0.50 mm2; section thickness, 3.5 mm) and (e) an axial IW FS 2D FSE sequence (TR/TE, 2722/38 msec; in plane spatial resolution, 0.65 × 0.59 mm2; section thickness, 3.5 mm).
Image analysis
A radiologist (Y.L. with 7 years of experience) who was blinded to subject characteristics assessed and measured all X-ray and MRI images.
Radiographic evaluation
Knee OA was assessed using the Kellgren-Lawrence (K&L) grades [16]. Based on this grading scheme, knee OA was divide&d into 5 grades: 0 = definite absence of X-ray changes; 1 = doubtful narrowing of joint space and possible osteophytic lipping; 2 = definite osteophytes and possible narrowing of joint space; 3 = moderate multiple osteophytes, definite narrowing of joint space, and some sclerosis and possible deformity of bone ends; and 4 = large osteophytes, marked narrowing of joint space, severe sclerosis, and definite deformity of bone ends.
Measurements of meniscal anterior and posterior horn heights
Medial meniscal posterior horn height (MPH), lateral meniscal anterior horn height (LAH) and lateral meniscal posterior horn height (LPH) were measured on the sagittal 2D IW FS FSE sequence. By counting the total number of slices through the medial/lateral compartment, the middle slice was selected for the measurements. Heights of the anterior and posterior horns were defined as the largest dimensions in the longitudinal axis of the anterior and posterior horns. Due to the variability in insertion patterns of the anterior horn of the medial meniscus, we were unable to find a reproducible way to measure the height of medial meniscal anterior horn [17, 18]. Hence, for the lateral meniscus we measured the heights of anterior and posterior horns, and for the medial meniscus we only measured its posterior horn (Figs. 2 and 3).
Heights of lateral meniscal anterior and posterior horn were measured on the sagittal 2D IW FS FSE sequence and were defined as the largest dimension in the longitudinal axis of the anterior and posterior horn of the lateral meniscus obtained in a middle slice through the lateral compartment. (IW, intermediate-weighted; FS, fat-saturated; FSE, fast spin-echo; LAH, lateral meniscal anterior horn height; LPH, lateral meniscal posterior horn height)
Height of medial meniscal posterior horn was measured on the sagittal 2D IW FS FSE sequence and was defined as the largest dimension in the longitudinal axis of the posterior horn of the medial meniscus obtained in a middle slice through the medial compartment. (IW, intermediate-weighted; FS, fat-saturated; FSE, fast spin-echo; MPH, Medial meniscal posterior horn height)
WORMS scoring
The University of California San Francisco (UCSF)-modified whole-organ MRI score (WORMS) system was used to semi-quantitatively assess OA-related knee morphological abnormalities [19,20,21]. Meniscal lesions were graded from 0 to 4 in each of 6 regions (anterior/body/posterior regions of the medial and lateral menisci) using the following 4-point scale: 0 = normal, 1 = intrasubstance signal, 2 = nondisplaced tear, 3 = displaced or complex tear, and 4 = complete destruction/maceration. Knee cartilage lesions were evaluated in medial compartment (medial femur and medial tibia), lateral compartment (lateral femur and lateral tibia), and patellofemoral compartment (patella and trochlea) with an 8-point scale: 0 = normal cartilage, 1 = normal thickness but increased or otherwise abnormal signal on fluid sensitive sequences, 2 = partial thickness focal defect < 1 cm in greatest width, 2.5 = full thickness focal defect < 1 cm in greatest width, 3 = multiple areas of grade 2 lesions intermixed with areas of normal thickness, or grade 2 lesion wider than 1 cm but < 75% of the entire region, 4 = diffuse (≥ 75% of the region) partial thickness lesion, 5 = multiple areas of grade 2.5 lesions or a full thickness lesion wider than 1 cm but < 75% of the region and 6 = diffuse (≥75% of the region) full thickness loss. BMEPs and subarticular cysts were scored from 0 to 3 in each of 6 regions (patella, trochlea, medial/ lateral femur, and medial/lateral tibia). Ligamentous/tendinous abnormalities of the anterior cruciate ligament, posterior cruciate ligament, medial collateral ligament, lateral collateral ligament, patellar tendon, and popliteal tendon as well as other findings (subchondral cysts, effusion, loose bodies, and popliteal cysts) were graded according to UCSF-modified WORMS as previously described [12, 21]. In addition, we calculated sum and maximum scores for medial and lateral meniscus individually. We also calculated sum and maximum scores for cartilage lesions, BMEPs and subarticular cysts individually for medial compartment, lateral compartment and patellofemoral compartment, respectively. The WORMS maximum score was based on the most severe lesion within all regions analyzed in the compartment.
Reproducibility
Reproducibility results for radiographic assessment and WORMS gradings have been previously described by our group [12]. The intraclass correlation coefficients (ICCs) for intrareader agreement in K&L grades were 0.88 and 0.91, and 0.90 for interreader agreement. For WORMS gradings, ICCs range between 0.86 and 0.96 for intrareader agreement and 0.86 and 0.96 for interreader agreement.
We used ICCs to evaluate intra- and inter-reader reproducibility for meniscal anterior and posterior horn heights measurements. Ten subjects were randomly selected and two radiologists (Y.L. and G.D. with 7 and 9 years of experience, respectively) performed meniscal anterior and posterior horn heights measurements twice independently. The two readers blinded to each other’s and their own previous measurements and the two readings of each reader were at least 14 days apart. For MPH measurement, the ICCs for intrareader agreement were 0.98 and 0.96, and ICCs for interreader agreement was 0.98. The ICCs for intrareader agreement in LAH measurement were 0.95 and 0.91, and 0.96 and 0.90 for LPH measurement. The ICCs for inter-reader agreement in LAH and LPH measurements were 0.97 and 0.99, respectively.
Statistical analysis
Statistical analyses were performed with SPSS v. 24 software (Chicago, IL), using a two-sided, 0.05 level of significance. For the variables in the characteristics of the study sample, summary statistics were constructed employing frequencies and proportions for categorical data and means and standard deviations (SDs) for continuous variables. The association between meniscal anterior/posterior horn heights and OA-related knee structural abnormalities were assessed using linear regression analysis. The analyses were adjusted for age, sex, BMI, K&L grades and were then further adjusted for meniscal anterior/posterior WORMS scores.
Results
Our sample consisted of 106 patients (57.5% women, mean age 54.4 ± 8.8 years [range 40–73 years], mean body mass index [BMI] 24.3 ± 3.4 kg/m2) (Table 1). In our sample, 26 knees had K&L 0 (24.5%), while 38 knees had K&L 1 (35.8%), 33 knees had K&L 2 (31.1%), and 9 knees had K&L 3 (8.6%). During the process of radiographic evaluation, we did not meet a knee with K&L 4 grade. In addition, no knees among 106 knees were found to have lateral tibiofemoral joint space narrowing. Hence all knees with a K/L score of 3 are those with medial compartmental OA.
Table 2 showed that higher MPH was significantly associated with higher meniscus WORMS score for MMA before and after further adjustment (P = 0.015 and 0.016, respectively). However, MPH was not significantly correlated with the rest medial meniscus WORMS parameters. Moreover, no statistically significant association was found of MPH with WORMS scores for medial compartmental cartilage lesions, medial compartmental BMEPs, medial compartmental subarticular cysts, ligamentous/tendinous abnormalities, effusion, loose bodies and popliteal cysts (P ≥ 0.060).
As shown in Table 3, higher LAH was significantly correlated with lower WORMS scores for LMA, LMB, LMP, LM sum and LM maximum (P = 0.014–0.021), while the associations were no longer significant after further adjustment (P ≥ 0.136). In addition, increased LAH was significantly associated with decreased lateral compartmental cartilage WORMS scores (P ≤ 0.031), however, after further adjustment increased LAH was only significantly associated with decreased cartilage WORMS scores for LF (P = 0.001) and LC sum (P = 0.004). Higher LAH was statistically correlated with lower BMEPs WORMS scores for LF, LT and LC sum (P = 0.002–0.040) and was still statistically correlated with lower BMEPs WORMS scores for LF (P = 0.027) and LC sum (P = 0.021) when fully adjusted. The correlations of LAH with WORMS scores for lateral compartmental subarticular cysts, ligamentous/tendinous abnormalities, effusion, loose bodies and popliteal cysts did not reach the statistical significance (P ≥ 0.119).
Before adjusting for lateral meniscal posterior horn WORMS scores, increased LPH was statistically associated with decreased meniscus WORMS scores for LMB, LMP, and LM sum (P = 0.024–0.037), however, after further adjustment the statistical significances disappeared (P ≥ 0.289) (Table 4). Additionally, higher LPH was significantly correlated with lower scores for all cartilage WORMS parameters (P ≤ 0.009) and was still statistically correlated with cartilage WORMS scores for LF, LT and LC sum after further adjustment (P = 0.007–0.041). Similarly, increased LPH was statistically associated with decreased WORMS outcome parameters for lateral compartmental BMEPs (P = 0.002–0.032) before full adjustment and was still significantly associated with decreased BMEPs WORMS scores for LF (P = 0.044) and LC sum (P = 0.022) when fully adjusted. No statistically significant correlation was found between LPH and WORMS scores for lateral compartmental subarticular cysts, ligamentous/tendinous abnormalities, effusion, loose bodies and popliteal cysts (P ≥ 0.266).
As shown in Supplementary Table 1, higher MPH was significantly associated with lower trochlea WORMS cartilage score (P = 0.048). No significant association was found between MPH and other WORMS parameters for patellofemoral joint (P ≥ 0.209). Supplementary Table 2 showed that higher LAH was significantly correlated with higher WORMS score for trochlea subarticular cysts before and after further adjustment (P = 0.019 and 0.006). LAH did not have a statistical association with other WORMS parameters for patellofemoral joint (P ≥ 0.068). Supplementary Table 3 demonstrated that the associations between LPH and patellofemoral structural abnormalities were not statistically significant (P ≥ 0.058).
Discussion
In the current study, we investigated the association of meniscal anterior and posterior horn heights with OA-related knee structural change. This study demonstrated that higher LAH and LPH were significantly associated with less severe lateral compartmental cartilage lesions and BMEPs, while increased MPH was significantly associated with more severe medial meniscal anterior horn lesions.
Morphological changes of meniscus were suggested to be one of the features of knee OA [9]. Previous studies revealed that meniscal volume, width, and height were different between patients with and without knee OA [9, 11]. A longitudinal study by Katja et al. reported that meniscal volume and width were reduced during a 2-year period in patients with medial joint space narrowing at baseline [22]. Recently, morphological changes of the meniscus were found to be related to subsequent knee replacement in knees with rapidly progressing OA [23]. The above findings indicated the important role of meniscal morphological changes in the development of knee OA. To date, the association of meniscal morphological changes and knee structural abnormalities is still not fully understood. In our previous work, we found that medial and lateral meniscal body heights were associated with multiple OA-related structural abnormalities [12]. Based on that, the present study extended the scope of the investigation to MPH, LAH and LPH, which would be a beneficial supplement in understanding the association of meniscal morphological changes and knee OA.
By comparing knees with and without radiographic OA, Wolfgang et al. found that both medial meniscal body height and posterior horn height were both higher in knees with radiographic OA [11]. Higher medial meniscal body height has already been reported to had association with meniscal lesions, cartilage lesions, BMEPs, ligamentous abnormalities and loose bodies. However, in the present study, MPH only showed a statistical correlation with medial meniscal anterior horn lesions [12]. This fact suggested that the change of MPH was less closely associated with OA-related knee structural abnormalities when compared with medial meniscal body height. A recent study by Kawahara et al. reported a statistically significant association between MPH and cartilage lesions, which conflicted with our findings [24]. A possible reason was that their results did not adjust for the common confounders such as meniscal lesions. In addition, as highly flexed knee may develop more compression on meniscus, the special lifestyle of Japanese (for example, often sit down on knees), as a special confounder, may also be a possible reason for the inconsistency of the results from two studies [25, 26].
Laterally, the changes of LAH and LPH were inversely correlated with the severity of OA-related knee structural abnormalities. A hypothetical theory raised by Wenger et al. may be helpful for explaining this finding [9]. As medial compartment of knee usually carries more loading than lateral compartment in load transmission, medial meniscus, with time, may partly or totally extrude beyond the margin of medial tibia plateau. The extruded part of medial meniscus may potentially swell as it becomes unloaded, which may cause an increase in medial meniscal height. Meanwhile, due to the narrowing of medial joint space, the relative unloaded lateral compartment is likely to develop a more open-angled shape. Lateral meniscus may therefore slightly hypertrophied and bulged, resulting in higher LAH and LPH. This hypothesis provided a possible explanation to the inverse correlation between LAH/LPH and the severity of knee structural abnormalities because higher LAH and LPH may partly resulted from the relatively decreased loading.
Interestingly, higher MPH was significantly associated with less severe trochlea cartilage lesions and higher LAH was significantly correlated with more severe trochlea subarticular cysts. Previous studies have suggested that the development of radiographic knee OA in symptomatic adults beginning in the patellofemoral compartment [27, 28]. A study by Hart et al. showed that meniscus pathology was associated with an elevated prevalence of patellofemoral cartilage lesions and was associated with worsening of patellofemoral OA-related abnormalities 2 years later [29]. However, it is not well known how meniscal height is correlated with patellofemoral OA. As mechanics plays an important role in patellofemoral pathology, further investigation on mechanical stress alteration of OA knees may be needed in exploring the association between meniscal height and patellofemoral structural abnormalities [30].
We acknowledge that this study has some limitations. First, we assessed the OA-related knee structural abnormalities using the UCSF-modified WORMS scoring system which was helpful to a detailed assessment of the knee structures [19,20,21]. However, as a semi-quantitative method, this scoring system may not be sensitive enough to monitor subtle changes such as cartilage compositional degeneration [21]. Hence, although in the present study meniscal anterior and posterior horn heights did not show a statistical association with some knee structural changes, quantitative study is required to further explore whether there is a potential correlation between them. Second, limited by the cross-sectional nature, this study was not able to clarify whether the changes of MPH, LAH and LPH are the causes or consequences of OA related knee structural abnormalities. A longitudinal study may contribute to further reveal the association between meniscal height and the incidence and development of knee OA and will be our future research direction. Third, our results did not adjust for meniscal anterior/posterior extrusion because no widely used measurement method and threshold has been proposed so far. Forth, we did not meet K&L 4 grade knees during the process of data collection, so our sample did not contain K&L 4 grade knees. However, since K&L 4 knees were often macerated or destroyed, a partial or total loss of meniscal substance is not uncommon in those knees. Therefore, in K&L 4 knees, the morphological changes of the meniscus may be greatly affected by the loss of meniscal substance. Regrettably, the extent of the loss of meniscal substance and how it affects the morphological changes of the meniscus were complex questions and beyond the scope of this study. Despite the above limitations, we believe this study is valuable and provides some novel information for understanding the role of meniscal morphological changes in knee OA.
Conclusion
This study explored the association of meniscal anterior and posterior horn heights with OA-related knee structural abnormalities. We found that increased LAH and LPH were significantly associated with less severe lateral compartmental cartilage lesions and BMEPs, while MPH only had a statistical correlation with medial meniscal anterior horn lesions. Moreover, increased MPH was statistically associated with less severe trochlea cartilage score and increased LAH was significantly correlated with more severe trochlea subarticular cysts.
Availability of data and materials
Datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- ACR:
-
The American College of Rheumatology
- BMEPs:
-
Bone marrow edema patterns
- CI:
-
Confidence interval
- FS:
-
Fat-saturated
- FSE:
-
Fast spin-echo
- ICCs:
-
Intraclass correlation coefficients
- IW:
-
Intermediate-weighted
- K&L:
-
Kellgren-Lawrence
- LAH:
-
Lateral meniscal anterior horn height
- LC:
-
Lateral compartment
- LCL:
-
Lateral collateral ligament
- LF:
-
Lateral femur
- LM:
-
Lateral meniscus
- LMA:
-
Lateral meniscal anterior horn
- LMB:
-
Lateral meniscal body
- LMP:
-
Lateral meniscal posterior horn
- LPH:
-
Lateral meniscal posterior horn height
- LT:
-
Lateral tibia
- MC:
-
Medial compartment
- MCL:
-
Medial collateral ligament
- MF:
-
Medial femur
- MM:
-
Medial meniscus
- MT:
-
Medial tibia
- MMA:
-
Medial meniscal anterior horn
- MMB:
-
Medial meniscal body
- MPH:
-
Medial meniscal posterior horn height
- MMP:
-
Medial meniscal posterior horn
- OA:
-
Osteoarthritis
- PCL:
-
Posterior cruciate ligament
- PL:
-
Patellar ligament
- PT:
-
Popliteal tendon
- SD:
-
Standard deviation
- TR:
-
Repetition time
- TE:
-
Echo time
- WORMS:
-
Whole-organ MRI score
References
Swamy N, Wadhwa V, Bajaj G, Chhabra A, Pandey T. Medial meniscal extrusion: detection, evaluation and clinical implications. Eur J Radiol. 2018;102:115–24.
Crema MD, Guermazi A, Li L, Nogueira-Barbosa MH, Marra MD, Roemer FW, et al. The association of prevalent medial meniscal pathology with cartilage loss in the medial tibiofemoral compartment over a 2-year period. Osteoarthr Cartil. 2010;18(3):336–43.
Englund M, Guermazi A, Roemer FW, Yang M, Zhang Y, Nevitt MC, et al. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: the MOST study. Ann Rheum Dis. 2010;69(10):1796–802.
Bloecker K, Wirth W, Guermazi A, Hunter DJ, Resch H, Hochreiter J, et al. Medial meniscal extrusion relates to cartilage loss in specific Femorotibial subregions- data from the osteoarthritis initiative. Arthritis Care Res. 2015;67(11):1545–52.
Liu Y, Joseph GB, Foreman SC, Li X, Lane NE, Nevitt MC, et al. Determining a threshold of medial meniscal extrusion for prediction of knee pain and cartilage damage progression over 4 years: data from the osteoarthritis initiative. AJR Am J Roentgenol. 2021;216(5):1318–28.
Liu Y, Du G, Li X. Threshold for lateral meniscal body extrusion on MRI in middle-aged and elderly patients with symptomatic knee osteoarthritis. Diagn Interv Imaging. 2020;101(10):677–83.
Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54(3):795–801.
Jung KA, Lee SC, Hwang SH, Yang KH, Kim DH, Sohn JH, et al. High frequency of meniscal hypertrophy in persons with advanced varus knee osteoarthritis. Rheumatol Int. 2010;30(10):1325–33.
Wenger A, Wirth W, Hudelmaier M, Noebauer-Huhmann I, Trattnig S, Bloecker K, et al. Meniscus body position, size, and shape in persons with and persons without radiographic knee osteoarthritis: quantitative analyses of knee magnetic resonance images from the osteoarthritis initiative. Arthritis Rheum. 2013;65(7):1804–11.
Bloecker K, Guermazi A, Wirth W, Benichou O, Kwoh CK, Hunter DJ, et al. Tibial coverage, meniscus position, size and damage in knees discordant for joint space narrowing - data from the osteoarthritis initiative. Osteoarthr Cartil. 2013;21(3):419–27.
Wirth W, Frobell RB, Souza RB, Li X, Wyman BT, Le Graverand MP, et al. A three-dimensional quantitative method to measure meniscus shape, position, and signal intensity using MR images: a pilot study and preliminary results in knee osteoarthritis. Magn Reson Med. 2010;63(5):1162–71.
Liu Y, Du G. The association of meniscal body height with knee structural changes in middle-aged and elderly patients with symptomatic knee osteoarthritis. Br J Radiol. 2021;0(0):20210152.
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039–49.
Buckland-Wright C. Protocols for precise radio-anatomical positioning of the tibiofemoral and patellofemoral compartments of the knee. Osteoarthr Cartil. 1995;3 Suppl A:71–80.
McAlindon T, Zhang Y, Hannan M, Naimark A, Weissman B, Castelli W, et al. Are risk factors for patellofemoral and tibiofemoral knee osteoarthritis different? J Rheumatol. 1996;23(2):332–7.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
Berlet GC, Fowler PJ. The anterior horn of the medial Meniscus. Clin J Sport Med. 1998;7(3):540–3.
Puig L, Monllau JC, Corrales M, Pelfort X, Melendo E, Caceres E. Factors affecting meniscal extrusion: correlation with MRI, clinical, and arthroscopic findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):394–8.
Schwaiger BJ, Mbapte Wamba J, Gersing AS, Nevitt MC, Facchetti L, McCulloch CE, et al. Hyperintense signal alteration in the suprapatellar fat pad on MRI is associated with degeneration of the patellofemoral joint over 48 months: data from the osteoarthritis initiative. Skelet Radiol. 2018;47(3):329–39.
Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the osteoarthritis initiative. Arthritis Care Res. 2012;64(2):248–55.
Liu Y, Foreman SC, Joseph GB, Neumann J, Tien PC, Li X, et al. Is treated HIV infection associated with knee cartilage degeneration and structural changes? A longitudinal study using data from the osteoarthritis initiative. BMC Musculoskelet Disord. 2019;20(1):190.
Bloecker K, Wirth W, Guermazi A, Hitzl W, Hunter DJ, Eckstein F. Longitudinal change in quantitative meniscus measurements in knee osteoarthritis--data from the Osteoarthritis Initiative. Eur Radiol. 2015;25(10):2960–8.
Roth M, Emmanuel K, Wirth W, Kwoh CK, Hunter DJ, Hannon MJ, et al. Changes in medial meniscal three-dimensional position and morphology as predictors of knee replacement in rapidly progressing knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res. 2021;73(7):1031–7.
Kawahara T, Sasho T, Katsuragi J, Ohnishi T, Haneishi H. Relationship between knee osteoarthritis and meniscal shape in observation of Japanese patients by using magnetic resonance imaging. J Orthop Surg Res. 2017;12(1):97.
Li G, Zayontz S, DeFrate LE, Most E, Suggs JF, Rubash HE. Kinematics of the knee at high flexion angles: an in vitro investigation. J Orthop Res. 2004;22(1):90–5.
Kim E, Kim YJ, Cha JG, Kim MY, Lee DH, Cho SG, et al. Kinematic change of the meniscus and the tibiofemoral joint space in asymptomatic volunteers using a wide bore 3T closed MRI system. Skelet Radiol. 2015;44(10):1441–51.
Duncan R, Peat G, Thomas E, Hay EM, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011;70(11):1944–8.
Stefanik JJ, Guermazi A, Roemer FW, Peat G, Niu J, Segal NA, et al. Changes in patellofemoral and tibiofemoral joint cartilage damage and bone marrow lesions over 7 years: the multicenter osteoarthritis study. Osteoarthr Cartil. 2016;24(7):1160–6.
Hart HF, Crossley KM, Felson D, Jarraya M, Guermazi A, Roemer F, et al. Relation of meniscus pathology to prevalence and worsening of patellofemoral joint osteoarthritis: the multicenter osteoarthritis study. Osteoarthr Cartil. 2018;26(7):912–9.
Lee TQ, Morris G, Csintalan RP. The influence of tibial and femoral rotation on patellofemoral contact area and pressure. J Orthop Sports Phys Ther. 2003;33(11):686–93.
Acknowledgements
We would like to extend our sincerest thanks to the entire Department of Radiology (XX. Hospital, XX. University) for their assistance.
Funding
This work has been supported by: National Natural Science Foundation of China (Grant/Award Number: 82102030, 61971451); Clinical Research Center for Medical Imaging in Hunan Province (Grant/Award Number: 2020SK4001); Innovative Province special construction foundation of Hunan Province (Grant/Award Number: 2019SK2131); Leading talents of scientific and technological innovation in Hunan Province in 2021 (Grant/Award Number: 2021RC4016). These funding bodies did not have a role in the design of the study, collection, analysis, and interpretation of data; and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. YL. and JL. take responsibility for the integrity of the work as a whole, from inception to finished article. Conception and design of the study: YL. and JL. Acquisition of data: YL. Analysis and interpretation of data: YL., JL. and GD.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complied with the Declaration of Helsinki. The institutional review board (IRB) of our hospital (The Second Xiangya Hospital, Central South University, Changsha, Hunan, China) approved this retrospective study. Informed consent was waived by the IRB of the Second Xiangya Hospital because the present study involved no potential risk to patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Du, G. & Liu, J. Meniscal anterior and posterior horn heights are associated with MRI-defined knee structural abnormalities in middle-aged and elderly patients with symptomatic knee osteoarthritis. BMC Musculoskelet Disord 23, 218 (2022). https://doi.org/10.1186/s12891-022-05143-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-022-05143-w