Abstract
Objective
To analyze associations of suprapatellar fat pad (SPFP) hyperintense signal alterations and mass effect with progression of patellofemoral osteoarthritis (OA) and clinical symptoms over 48 months.
Materials and methods
Subjects from the Osteoarthritis Initiative (n = 426; 51.8 ± 3.8 years; 49.8% women) without radiographic tibiofemoral OA underwent 3T-MRI of their right knees and clinical evaluation using the Knee Injury and Osteoarthritis Outcome Score at baseline and at 48 months. Elevated SPFP signal was assessed on intermediate-weighted, fat-saturated turbo spin-echo (TSE) images. Mass effect was defined as a convex posterior contour. Patellofemoral cartilage, bone marrow lesions (BML), and subchondral cysts were assessed using the Whole-Organ Magnetic Resonance Imaging Score (WORMS). Associations of SPFP imaging findings with MRI and clinical progression were assessed using general linear models and logistic regressions.
Results
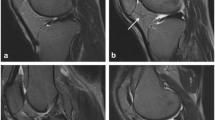
Baseline SPFP signal alterations were found in 51% of the subjects (n = 217), of whom 11% (n = 23) additionally had a mass effect. Progression of cartilage lesions was significantly higher in subjects with signal alteration versus without (adjusted mean increases, 95% CI; patella: 0.29, −0.07 to 0.64 vs −0.04, −0.40 to 0.31; p < 0.001; trochlea: 0.47, 0.16 to 0.77 vs 0.31, 0.01 to 0.61; p = 0.007). BML progression was also more likely in subjects with signal alteration (OR 1.75, 95% CI 1.09 to 2.82; p = 0.021). Mass effect was not associated with joint degeneration and SPFP findings were not associated with clinical worsening (p > 0.18 for all).
Conclusion
Patellofemoral joint degeneration over 48 months was significantly increased in subjects with SPFP signal alteration, suggesting an association between SPFP abnormalities and the progression of patellofemoral OA.




Similar content being viewed by others
References
Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15(6):225.
Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage. 2010;18(7):876–82.
Han W, Aitken D, Zhu Z, Halliday A, Wang X, Antony B, et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis. 2016;75(10):1783–8.
Saddik D, McNally EG, Richardson M. MRI of Hoffa’s fat pad. Skeletal Radiol. 2004;33(8):433–44.
Jacobson JA, Lenchik L, Ruhoy MK, Schweitzer ME, Resnick D. MR imaging of the infrapatellar fat pad of Hoffa. Radiographics. 1997;17(3):675–91.
Gallagher J, Tierney P, Murray P, O’Brien M. The infrapatellar fat pad: anatomy and clinical correlations. Knee Surg Sports Traumatol Arthrosc. 2005;13(4):268–72.
Pan F, Han W, Wang X, Liu Z, Jin X, Antony B, et al. A longitudinal study of the association between infrapatellar fat pad maximal area and changes in knee symptoms and structure in older adults. Ann Rheum Dis. 2015;74(10):1818–24.
Cowan SM, Hart HF, Warden SJ, Crossley KM. Infrapatellar fat pad volume is greater in individuals with patellofemoral joint osteoarthritis and associated with pain. Rheumatol Int. 2015;35(8):1439–42.
Cai J, Xu J, Wang K, Zheng S, He F, Huan S, et al. Association between infrapatellar fat pad volume and knee structural changes in patients with knee osteoarthritis. J Rheumatol. 2015;42(10):1878–84.
Roth C, Jacobson J, Jamadar D, Caoili E, Morag Y, Housner J. Quadriceps fat pad signal intensity and enlargement on MRI: prevalence and associated findings. AJR Am J Roentgenol. 2004;182(6):1383–7.
Shabshin N, Schweitzer ME, Morrison WB. Quadriceps fat pad edema: significance on magnetic resonance images of the knee. Skeletal Radiol. 2006;35(5):269–74.
Staeubli HU, Bollmann C, Kreutz R, Becker W, Rauschning W. Quantification of intact quadriceps tendon, quadriceps tendon insertion, and suprapatellar fat pad: MR arthrography, anatomy, and cryosections in the sagittal plane. AJR Am J Roentgenol. 1999;173(3):691–8.
Schweitzer ME, Falk A, Pathria M, Brahme S, Hodler J, Resnick D. MR imaging of the knee: can changes in the intracapsular fat pads be used as a sign of synovial proliferation in the presence of an effusion? AJR Am J Roentgenol. 1993;160(4):823–6.
Hoffa A. Influence of adipose tissue with regard to the pathology of the knee joint. JAMA. 1904;43:795–6.
Metheny JA, Mayor MB. Hoffa disease: chronic impingement of the infrapatellar fat pad. Am J Knee Surg. 1988;1:134–9.
Kumar D, Alvand A, Beacon JP. Impingement of infrapatellar fat pad (Hoffa’s disease): results of high-portal arthroscopic resection. Arthroscopy. 2007;23(11):1180–6. e1181
Tsavalas N, Karantanas AH. Suprapatellar fat-pad mass effect: MRI findings and correlation with anterior knee pain. AJR Am J Roentgenol. 2013;200(3):W291–6.
Wang J, Han W, Wang X, Pan F, Liu Z, Halliday A, et al. Mass effect and signal intensity alteration in the suprapatellar fat pad: associations with knee symptoms and structure. Osteoarthritis Cartilage. 2014;22(10):1619–26.
Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2010;18(6):776–86.
Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the Osteoarthritis Initiative. Radiology. 2010;254(2):509–20.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
Bucknor MD, Nardo L, Joseph GB, et al. Association of cartilage degeneration with four year weight gain--3T MRI data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2015;23:525–531. https://doi.org/10.1016/j.joca.2014.10.013.
Gersing AS, Solka M, Joseph GB, et al. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2016. https://doi.org/10.1016/j.joca.2016.01.984.
Joseph GB, Hou SW, Nardo L, et al. MRI findings associated with development of incident knee pain over 48 months: data from the osteoarthritis initiative. Skelet Radiol. 2016;45:653–660. https://doi.org/10.1007/s00256-016-2343-5.
Jungmann PM, Nevitt MC, Baum T, et al. Relationship of unilateral total hip arthroplasty (THA) to contralateral and ipsilateral knee joint degeneration - a longitudinal 3T MRI study from the Osteoarthritis Initiative (OAI). Osteoarthr Cartil. 2015; 23:1144–1153. https://doi.org/10.1016/j.joca.2015.03.022.
Kretzschmar M, Heilmeier U, Yu A, et al. Longitudinal analysis of cartilage T2 relaxation times and joint degeneration in African American and Caucasian American women over an observation period of 6 years -data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2016. https://doi.org/10.1016/j.joca.2016.03.002.
Kretzschmar M, Lin W, Nardo L, et al. Association of Physical Activity Measured by Accelerometer, Knee Joint Abnormalities, and Cartilage T2 Measurements Obtained From 3T Magnetic Resonance Imaging: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2015;67:1272–1280. https://doi.org/10.1002/acr.22586.
Lin W, Alizai H, Joseph GB, et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2013;21:1558–1566. https://doi.org/10.1016/j.joca.2013.06.022.
Peterfy CG, Schneider E, Nevitt M. The Osteoarthritis Initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41.
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90.
Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19(8):990–1002.
Hunter DJ, Zhang YQ, Niu JB, Felson DT, Kwoh K, Newman A, et al. Patella malalignment, pain and patellofemoral progression: the health ABC study. Osteoarthritis Cartilage. 2007;15(10):1120–7.
Draper CE, Besier TF, Santos JM, Jennings F, Fredericson M, Gold GE, et al. Using real-time MRI to quantify altered joint kinematics in subjects with patellofemoral pain and to evaluate the effects of a patellar brace or sleeve on joint motion. J Orthop Res. 2009;27(5):571–7.
Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;282:170–6.
Van Huyssteen AL, Hendrix MR, Barnett AJ, Wakeley CJ, Eldridge JD. Cartilage-bone mismatch in the dysplastic trochlea. An MRI study. J Bone Joint Surg. 2006;88(5):688–91.
Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19–26.
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96.
Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res. 2011;63 [Suppl 11]:S208–28.
Schwaiger BJ, Gersing AS, Mbapte Wamba J, Nevitt MC, McCulloch CE, Link TM. Can signal abnormalities detected with MR imaging in knee articular cartilage be used to predict development of morphologic cartilage defects? 48-Month data from the osteoarthritis initiative. Radiology 2016:152308. https://doi.org/10.1148/radiol.2016152308.
Baum T, Stehling C, Joseph GB, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. J Magn Reson Imaging 2012;35:370–378. https://doi.org/10.1002/jmri.22834.
Baum T, Joseph GB, Arulanandan A, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64:248–255. https://doi.org/10.1002/acr.2067.
Stefanik JJ, Gross KD, Guermazi A, Felson DT, Roemer FW, Zhang Y, et al. The relation of MRI-detected structural damage in the medial and lateral patellofemoral joint to knee pain: the multicenter and Framingham osteoarthritis studies. Osteoarthritis Cartilage. 2015;23(4):565–70.
Vahlensieck M, Linneborn G, Schild H, Schmidt HM. Hoffa’s recess: incidence, morphology and differential diagnosis of the globular-shaped cleft in the infrapatellar fat pad of the knee on MRI and cadaver dissections. Eur Radiol. 2002;12(1):90–3.
Duri ZA, Aichroth PM, Dowd G. The fat pad. Clinical observations. Am J Knee Surg. 1996;9(2):55–66.
Morini G, Chiodi E, Centanni F, Gattazzo D. Hoffa’s disease of the adipose pad: magnetic resonance versus surgical findings. Radiol Med. 1998;95(4):278–85.
Chowdhury TT, Salter DM, Bader DL, Lee DA. Signal transduction pathways involving p38 MAPK, JNK, NFkappaB and AP-1 influences the response of chondrocytes cultured in agarose constructs to IL-1beta and dynamic compression. Inflamm Res. 2008;57(7):306–13.
Gosset M, Berenbaum F, Levy A, Pigenet A, Thirion S, Saffar JL, et al. Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene. Arthritis Res Ther. 2006;8(4):R135.
Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–34.
Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;423:17–26.
Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004;(427 Suppl):S37–46.
Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2(6):459–65.
Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535–40.
Roemer FW, Jarraya M, Felson DT, Hayashi D, Crema MD, Loeuille D, et al. Magnetic resonance imaging of Hoffa’s fat pad and relevance for osteoarthritis research: a narrative review. Osteoarthritis Cartilage. 2016;24(3):383–97.
Funding
The OAI is a public–private partnership comprising five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. The analyses in this study were funded through the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants P50-AR060752 and R01-AR064771).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Schwaiger, B.J., Mbapte Wamba, J., Gersing, A.S. et al. Hyperintense signal alteration in the suprapatellar fat pad on MRI is associated with degeneration of the patellofemoral joint over 48 months: data from the Osteoarthritis Initiative. Skeletal Radiol 47, 329–339 (2018). https://doi.org/10.1007/s00256-017-2771-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2771-x