Abstract
Background
Several studies investigated the utility of inflammation and nutritional markers in predicting the prognosis in patients with gastric cancer; however, the markers with the best predictive ability remain unclear. This retrospective study aimed to determine inflammation and nutritional markers that predicted prognosis in elderly patients over 75 years of age undergoing curative gastrectomy for gastric cancer.
Methods
Between January 2005 and December 2015, 497 consecutive elderly gastric cancer patients aged over 75 years underwent curative gastrectomy in 12 institutions. The geriatric nutritional risk index (GNRI), prognostic nutritional index, neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and C-reactive protein/albumin ratio were examined as prognostic markers for overall survival (OS) and disease-specific survival (DSS) using area under the curve (AUC) using receiver operating characteristic (ROC) curve analysis.
Results
The GNRI had the highest AUC and predictive value for both OS (0.637, p < 0.001) and DSS (AUC 0.645, p < 0.001). The study cohort was categorized into the high and low GNRI groups based on the optimal GNRI cut-off values for OS (97.0) and DSS (95.8) determined with the ROC analysis. For both OS and DSS, there was a significant correlation between the GNRI and several clinicopathological factors including age, body mass index, albumin, American Society of Anesthesiologists physical status score, depth of tumor invasion, lymph node metastasis, lymphatic invasion, pathological stage, operation duration, bleeding, procedure, approach, death due to primary disease, and death due to other disease. The GNRI remained a crucial independent prognostic factor for both OS (Hazard ratio [HR] = 1.905, p < 0.001) and DSS in multivariate analysis (HR = 1.780, p = 0.043).
Conclusions
Among a panel of inflammation and nutritional markers, the GNRI exhibited the best performance as a prognostic factor after curative gastrectomy in elderly patients with gastric cancer, indicating its utility as a simple and promising index for predicting OS and DSS in these patients.
Similar content being viewed by others
Background
Aging is an inevitable process for all humans, and the global aging population is increasing in parallel to improved quality of life and medical advances [1]. Elderly individuals constitute one of the most vulnerable populations and are at high risk for various nutritional issues and comorbidities including gastric cancer [2,3,4]. Indeed, the rate of elderly patients with gastric cancer is increasing and their high mortality rate is an issue [5, 6]. Therefore, prognostic prediction is important in elderly patients with gastric cancer. Importantly, staging, which is generally used to predict cancer prognosis [7], is not sufficient in elderly patients with gastric cancer [6, 8].
In recent years, various inflammation markers and nutritional indicators, such as the neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR), prognostic nutritional index (PNI), and C-reactive protein (CRP)/albumin ratio (CAR), have been demonstrated to exhibit high utility in predicting surgical complications and prognosis in various cancers [9,10,11]. Although several studies have investigated the utility of these markers in patients with gastric cancer [12,13,14,15,16], the identification of those markers having the best predictive ability in elderly patients with gastric cancer remains unclear. In particular, no studies to date have determined the most useful factors for prognosis in patients over 75 years of age undergoing curative gastrectomy for gastric cancer. The recently developed geriatric nutritional risk index (GNRI) has been shown to exhibit utility as a prognostic factor in various carcinomas [17,18,19]. Importantly, the GNRI can be easily calculated from routine hematological data including serum albumin, height, and weight. These readily available parameters can reflect the survival risk in various cancers and have demonstrated utility in elderly patients [17, 20].
In the present retrospective multicenter study, we aimed to determine inflammation markers and nutritional indicators with the best prognostic utility for outcomes after gastrectomy in elderly patients over 75 years of age with gastric cancer.
Methods
Patients
From January 1, 2005 to December 31, 2015, 864 consecutive patients aged 75 years or older who were diagnosed with gastric cancer underwent gastrectomy in 14 institutions participating in the present study. Among these, 34 patients who underwent procedures other than standard gastrectomy such as local resection, 72 patients who underwent non-curative gastrectomy, and 261 patients with missing data on blood sampling results or surgical factors were excluded (Fig. 1). A total of 497 patients were included in the final analysis. The clinicopathological findings were determined according to the Japanese gastric cancer treatment guidelines [21]. Clinical data, including age, sex, American Society of Anesthesiologists physical status (ASA-PS) score, histology, depth of tumor invasion, lymph node metastasis, pathological stage, operation duration, bleeding, procedure, approach, and adjuvant chemotherapy, were collected from the databases of the participating institutions. Included comorbidities were cardiac and respiratory diseases and diabetes mellitus. In this study, cardiac disease was defined as the diagnosis of New York Heart Association class III or IV cardiac disease or arrhythmia requiring mechanical support, pulmonary disease was defined as % vital capacity of less than 60% or percent predicted forced expiratory volume in 1 s of less than 50%, and diabetes mellitus was defined as the use of oral hypoglycemic drugs or insulin with a higher hemoglobin A1c level above the reference value for each study institution. Patients were periodically checked for cancer recurrence via physical examination and blood tests performed every 3 months following hospital discharge. Computed tomography (CT) was performed at least every 6 months after surgery. Data on recurrence patterns and causes of death were extracted from the clinical records and positron emission tomography/CT results. In patients with difficulty in obtaining follow-up data, direct inquiries were made with their families.
Inflammation and nutritional factors
Peripheral counts of neutrophils, lymphocytes, and platelets and serum levels of albumin, carcinoembryonic antigen, and carbohydrate antigen 19–9 were collected from the medical records. Preoperative blood tests were performed within 7 days before surgery. The GNRI was calculated using the following formula: GNRI = 14.89 × serum albumin level (g/dL) + 41.7 × [current body weight (kg)/ideal body weight (kg)], where ideal weight was defined as 22 × [height (m)]2 [22]. In patients with greater than the ideal body weight, the ratio of the actual body weight to the ideal body weight was set to 1 [22]. The NLR and PLR were calculated by dividing the peripheral neutrophil and platelet counts by the peripheral lymphocyte count, respectively [12, 23]. The PNI was calculated using the following formula: PNI = 10 × serum albumin level + 0.005 × total lymphocyte count [24]. The CRP/albumin ratio was calculated by dividing the serum CRP level by the serum albumin level [12]. The following modified Glasgow prognostic scoring system, which combines CRP and serum albumin levels, was used: score of 0, normal albumin (≥3.5 g/L) and normal CRP (≤10 mg/L); score of 1, elevated CRP (> 10 mg/L) and low albumin (< 3.5 g/L); and score of 2, elevated CRP level (> 10 mg/L) and low albumin (< 3.5 g/L) [25].
Statistical analysis
Continuous variables were expressed as means ± standard deviation. The χ2 or Fisher’s exact test was used to compare categorical variables, and Student’s t-test was used to compare continuous variables. Survival curves were calculated using the Kaplan–Meier method, and differences between survival curves were examined using the log-rank test. The Cox proportional hazards model was used to perform univariate and multivariate analyses of prognostic factors for overall survival (OS) and disease-specific survival (DSS). Receiver operating characteristic (ROC) analysis for OS was performed to determine the cutoff value of age, operation duration, and bleeding amount. A p value of < 0.05 was considered to indicate statistical significance. SPSS for Windows version 24 (IBM, Armonk, NY, USA) was used for all statistical analyses.
Results
The utility of GNRI according to OS
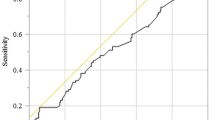
Table 1 shows the area under the curve (AUC) for each potential prognostic factor, which was determined using ROC curve analysis for OS. Among these, the GNRI had the highest AUC and the highest predictive value. Using the optimal GNRI cutoff value of 97.0 for OS determined with the ROC analysis (Fig. 2a), the study patients were divided into the high GNRI (GNRIHigh, n = 269) and low GNRI (GNRILow; n = 228) groups. Table 2 summarizes the relationship between the GNRI and characteristics of the study patients based on OS; GNRI was significantly correlated with age (p < 0.001), sex (p = 0.048), body mass index (BMI) (p < 0.001), albumin (p < 0.001), pulmonary disease (p = 0.026), ASA-PS score (p = 0.002), depth of tumor invasion (p < 0.001), lymph node metastasis (p < 0.001), lymphatic invasion (p < 0.001), and pathological stage (p < 0.001). Table 3 summarizes the relationship between the GNRI and operation-related factors based on OS, the GNRI was significantly correlated with operation duration (p < 0.001), bleeding amount (p = 0.009), type of procedure (p = 0.001), type of approach (p < 0.001), death due to primary disease (p = 0.001), and death due to other disease (p < 0.001) (Table 3).
The OS rates were significantly worse in the GNRILow group than in the GNRIHigh group (p < 0.001) (Fig. 3a). The univariate analysis revealed that the OS was significantly worse in patients aged ≥80 years, and those with undifferentiated adenocarcinoma, positive lymphatic invasion, positive venous invasion, T2 or deeper tumor invasion, positive lymph node metastasis, positive venous invasion, bleeding ≥206 mL, and low GNRI (Table 4). By multivariate analysis, low GNRI, age ≥ 80 years, positive lymph node metastasis, and bleeding ≥206 mL were independent prognostic factors for OS (Table 4).
The utility of GNRI according to DSS
Table 1 shows the AUC for each potential prognostic factor based on the ROC curve analysis for DSS. Among these, the GNRI had the highest AUC and the highest predictive value. Using the optimal GNRI cutoff value of 95.8 determined with the ROC analysis (Fig. 2b), the patients were divided into the high GNRI (GNRIHigh, n = 289) and low GNRI (GNRILow, n = 208) groups. As shown in Table 2, the GNRI was significantly correlated with the following clinicopathological factors: age (p < 0.001), BMI (p < 0.001), albumin (p < 0.001), ASA-PS score (p = 0.002), depth of tumor invasion (p < 0.001), lymph node metastasis (p < 0.001), lymphatic invasion (p = 0.004), and pathological stage (p < 0.001). In addition, the GNRI was significantly correlated with the following operative factors: operation duration (p < 0.001), bleeding amount (p = 0.002), type of procedure (p = 0.002), type of approach (p < 0.001), death due to primary disease (p < 0.001), and death due to other disease (p = 0.011) (Table 3).
The DSS rates were significantly worse in the GNRILow group than in the GNRIHigh group (p < 0.001) (Fig. 3b). The univariate analysis indicated that the DSS was significantly worse in patients with undifferentiated adenocarcinoma, higher tumor size, positive lymphatic invasion, positive venous invasion, T2 or deeper tumor invasion, positive lymph node metastasis, positive venous invasion, bleeding ≥206 mL, and low GNRI (Table 5). The multivariate analysis indicated that low GNRI, undifferentiated adenocarcinoma, positive venous invasion, T2 or deeper tumor invasion, positive lymph node metastasis, and bleeding ≥206 mL were independent prognostic factors for DSS (Table 5).
Discussion
In the present retrospective multicenter study, the GNRI emerged as a prognostic factor for OS and DSS with the best predictive performance among several inflammation and nutritional markers. Although there have been reports to identify the utility of GNRI in elderly gastric cancer patients aged over 65 years, no studies currently address patients over 75 years. To the best of our knowledge, this is the first multicenter study report to clarify the usefulness of GNRI as a prognostic marker in this patient population. Prediction of prognosis in elderly patients is fraught with issues due to the increased frequency of comorbidities and death due to other causes unique to the elderly. Therefore, the GNRI is a clinically useful tool that may be considered for prognostic prediction in conjunction with the TNM classification and other measures of disease progression.
The utility of inflammation and nutritional markers, such as CRP-based CAR and platelet-based PLR related to inflammation, lymphocyte-based NLR related to immunity, and albumin-based PNI related to nutrition, has been extensively investigated in patients with gastric cancer; however, few reports focused on the elderly patients with gastric cancer [14, 23, 24, 26]. In the present multicenter study with a relatively large number of elderly patients with gastric cancer, the AUC values of albumin-based markers related to nutrition, such as the GNRI, PNI, and the modified Glasgow prognostic score, were higher for both OS and DSS, indicating the importance of nutritional status on these outcomes. Malnutrition can be caused by physical, psychological, or physiological changes associated with aging, leading to decreased resistance to infection, immune function, and quality of life [27, 28]. In addition, gastric cancer can easily lead to malnutrition due to impaired food passage. CAR, NLR, and PNI are well-known prognostic factors for gastric cancer; however, the AUC value was higher for the GNRI than CAR, NLR, and PNI in the present study, indicating that CRP, which is related to inflammation, and lymphocyte count, which is related to immunity, might have less utility in predicting the prognosis of elderly patients with gastric cancer compared to the GNRI, which includes strong nutritional components. The GNRI was developed as an objective and simple screening tool to assess nutrition-related risk of morbidity and mortality in elderly hospitalized patients. The GNRI can be easily calculated using albumin, body weight, and height. Albumin, a major protein in human serum, reflects the nutritional status of an individual, and hypoalbuminemia has been demonstrated to be associated with poor prognosis in patients with various cancer [29,30,31]. Body weight has been reported as an indicator of both systemic disease severity and protein and calorie stores; its association with prognosis in patients with cancer has also been reported [32,33,34]. BMI, which takes weight and height into account, is another commonly used parameter to assess the nutritional status of individuals [35, 36]. The GNRI can be easily calculated using albumin and BMI and may be useful as a prognostic indicator for gastric cancer in the elderly.
Our main finding of the GNRI as a prognostic factor in patients with gastric cancer is in agreement with the outcomes of a study by Hirahara et al., who retrospectively examined 297 elderly patients over 65 years of age who underwent curative laparoscopic gastrectomy for gastric cancer. While the age cutoff was 65 years in that study, similar to our findings, the authors reported that the GNRI was significantly associated with OS and cancer-specific survival in elderly patients with gastric cancer and that the GNRI was an independent predictor of OS [37]. Similarly, Sugawara et al. reported that the GNRI was a prognostic factor in gastric cancer based on its significant association with OS and cancer-specific survival in a retrospective analysis of 1166 patients who underwent curative gastrectomy, although the study included patients across all ages and was not restricted to older patients [38]. Nevertheless, our study adds to the accumulating evidence that the GNRI might be considered as a prognostic factor in patients with gastric cancer while confirming its utility in elderly patients.
In this study, the prognosis was worse in the GNRILow group for both OS and DSS; however, the mechanism underlying this outcome is unclear. It is possible that the stage of cancer, one of the clinicopathological factors, might have been more advanced in patients with low GNRI. Previous studies reported the association of low GNRI with advanced stage in various cancers [19, 37, 39,40,41], similar to our findings. Various cytokines are released in advanced cancer [42,43,44], and some studies reported that increased inflammation markers and decreased nutritional marker were associated with increased catabolism, resulting in anorexia and a negative effect on nutritional status [45, 46], which may also account for a low GNRI.
In this study, the low GNRI was significantly correlated with deeper depth of tumor invasion, positive lymph node metastasis, and advanced pathological stage. Generally, advanced cancer tends to be treated with open surgery, which may explain the lower rate of patients undergoing laparoscopic surgery in the GNRILow group than in the GNRIHigh group [47]. Laparoscopic surgery is reported to require long operative time and results in low blood loss [48], which may be the reason for the longer operation duration and lower blood loss in the GNRIHigh group than in the GNRILow group.
This study has several limitations. First, this was a retrospective analysis; however, the study included multiple institutions. Second, the optimal GNRI cutoff value in elderly patient with gastric cancer is unknown. Third, the study did not include data on patients younger than 75 years of age. Fourth, the definition of elderly used in the present study was different from that used in some of the other studies. Currently, the Japanese Geriatrics Society has proposed to redefine the elderly as 75 years of age or older, and thus we adopted a threshold of 75 years [49].
Conclusion
The GNRI exhibited the best prognostic performance among several inflammation and nutritional markers in elderly patients with gastric cancer undergoing curative gastrectomy. As a simple and cost-effective tool, the GNRI is a promising index for predicting OS and DSS in elderly patients with gastric cancer.
Availability of data and materials
The datasets used and analyzed in the present study are not publicly available due to the information that could compromise the privacy of research participants but are available from the corresponding author on reasonable request.
Abbreviations
- ASA-PS:
-
American Society of Anesthesiologists physical status
- BMI:
-
Body mass index
- CAR:
-
C-reactive protein/albumin ratio
- CEA:
-
Carcinoembryonic antigen
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- GNRI:
-
Geriatric nutritional risk index
- NLR:
-
Neutrophil/lymphocyte ratio
- OS:
-
Overall survival
- PLR:
-
Platelet/lymphocyte ratio
- PNI:
-
Prognostic nutritional index
References
Harper S. Economic and social implications of aging societies. Science. 2014;346(6209):587–91.
Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449–60.
Sehl M, Sawhney R, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part II. Cancer J. 2005;11(6):461–73.
Marano L, Carbone L, Poto GE, Gambelli M, Nguefack Noudem LL, Grassi G, et al. Handgrip strength predicts length of hospital stay in an abdominal surgical setting: the role of frailty beyond age. Aging Clin Exp Res. 2022;34:811–7.
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16(1):1–27.
Joharatnam-Hogan N, Shiu KK, Khan K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev. 2020;85:101980.
Wittekind C. 2010 TNM system: on the 7th edition of TNM classification of malignant tumors. Pathologe. 2010;31(5):331–2.
Roberto M, Botticelli A, Strigari L, Ghidini M, Onesti CE, Ratti M, et al. Prognosis of elderly gastric cancer patients after surgery: a nomogram to predict survival. Med Oncol. 2018;35(7):111.
Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18(1):360.
Cho U, Park HS, Im SY, Yoo CY, Jung JH, Suh YJ, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS One. 2018;13(7):e0200936.
Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal Cancer. Ann Surg. 2020;271(4):693–700.
Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, et al. Prognostic significance of the preoperative ratio of C-reactive protein to albumin and neutrophil-lymphocyte ratio in gastric Cancer patients. World J Surg. 2018;42(6):1819–25.
Zhang X, Zhao W, Chen X, Zhao M, Qi X, Li G, et al. Combining the fibrinogen-to-pre-albumin ratio and prognostic nutritional index (FPR-PNI) predicts the survival in elderly gastric Cancer patients after Gastrectomy. Onco Targets Ther. 2020;13:8845–59.
Yue L, Lu Y, Li Y, Wang Y. Prognostic value of C-reactive protein to albumin ratio in gastric Cancer: a meta-analysis. Nutr Cancer. 2021;73(10):1864–71.
Takahashi T, Kaneoka Y, Maeda A, Takayama Y, Fukami Y, Uji M. The preoperative prognostic nutrition index is a prognostic indicator for survival in elderly gastric cancer patients after gastrectomy: a propensity score-matched analysis. Updat Surg. 2020;72(2):483–91.
Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21(2):204–12.
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96.
Lidoriki I, Schizas D, Frountzas M, Machairas N, Prodromidou A, Kapelouzou A, et al. GNRI as a prognostic factor for outcomes in Cancer patients: a systematic review of the literature. Nutr Cancer. 2021;73(3):391–403.
Kubo N, Sakurai K, Tamura T, Toyokawa T, Tanaka H, Muguruma K, et al. The impact of geriatric nutritional risk index on surgical outcomes after esophagectomy in patients with esophageal cancer. Esophagus. 2019;16(2):147–54.
Matsunaga T, Miyata H, Sugimura K, Motoori M, Asukai K, Yanagimoto Y, et al. Prognostic significance of C-reactive protein-to-prealbumin ratio in patients with esophageal Cancer. Yonago Acta Med. 2020;63(1):8–19.
Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):1–21.
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–83.
Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19(1):672.
Gao QL, Shi JG, Huang YD. Prognostic significance of pretreatment prognostic nutritional index (PNI) in patients with nasopharyngeal carcinoma: a Meta-analysis. Nutr Cancer. 2021;73(9):1657–67.
Walsh SM, Casey S, Kennedy R, Ravi N, Reynolds JV. Does the modified Glasgow prognostic score (mGPS) have a prognostic role in esophageal cancer? J Surg Oncol. 2016;113(7):732–7.
Ye Z, Yu P, Cao Y, Chai T, Huang S, Cheng X, et al. Prediction of peritoneal Cancer index and prognosis in peritoneal metastasis of gastric Cancer using NLR-PLR-DDI score: a retrospective study. Cancer Manag Res. 2022;14:177–87.
Lin YM, Wang M, Sun NX, Liu YY, Yin TF, Chen C. Screening and application of nutritional support in elderly hospitalized patients of a tertiary care hospital in China. PLoS One. 2019;14(3):e0213076.
Calderon C, Carmona-Bayonas A, Beato C, Ghanem I, Hernandez R, Majem M, et al. Risk of malnutrition and emotional distress as factors affecting health-related quality of life in patients with resected cancer. Clin Transl Oncol. 2019;21(5):687–91.
Huang R, Greenky M, Kerr GJ, Austin MS, Parvizi J. The effect of malnutrition on patients undergoing elective joint arthroplasty. J Arthroplast. 2013;28(8 Suppl):21–4.
McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–3.
Conrad LB, Awdeh H, Acosta-Torres S, Conrad SA, Bailey AA, Miller DS, et al. Pre-operative core muscle index in combination with hypoalbuminemia is associated with poor prognosis in advanced ovarian cancer. J Surg Oncol. 2018;117(5):1020–8.
Morishita R, Franco Mdo C, Suano-Souza FI, Solé D, Puccini RF, Strufaldi MW. Body mass index, adipokines and insulin resistance in asthmatic children and adolescents. J Asthma. 2016;53(5):478–84.
Heimans L, van den Broek M, le Cessie S, Siegerink B, Riyazi N, Han KH, et al. Association of high body mass index with decreased treatment response to combination therapy in recent-onset rheumatoid arthritis patients. Arthritis Care Res. 2013;65(8):1235–42.
Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Tech Coloproctol. 2016;20(8):517–35.
Naidu AN, Rao NP. Body mass index: a measure of the nutritional status in Indian populations. Eur J Clin Nutr. 1994;48(Suppl 3):S131–40.
Gastelurrutia P, Lupón J, Domingo M, Ribas N, Noguero M, Martinez C, et al. Usefulness of body mass index to characterize nutritional status in patients with heart failure. Am J Cardiol. 2011;108(8):1166–70.
Hirahara N, Matsubara T, Fujii Y, Kaji S, Hyakudomi R, Yamamoto T, et al. Preoperative geriatric nutritional risk index is a useful prognostic indicator in elderly patients with gastric cancer. Oncotarget. 2020;11(24):2345–56.
Sugawara K, Yamashita H, Urabe M, Okumura Y, Yagi K, Aikou S, et al. Geriatric nutrition index influences survival outcomes in gastric carcinoma patients undergoing radical surgery. JPEN J Parenter Enteral Nutr. 2021;45(5):1042–51.
Shu W, Tao W, Chunyan H, Jie F, Yuan L, Yan X, et al. Preoperative nutritional evaluation of prostate cancer patients undergoing laparoscopic radical prostatectomy. PLoS One. 2022;17(2):e0262630.
Liao CK, Chern YJ, Hsu YJ, Lin YC, Yu YL, Chiang JM, et al. The clinical utility of the geriatric nutritional risk index in predicting postoperative complications and long-term survival in elderly patients with colorectal Cancer after curative surgery. Cancers (Basel). 2021;13(22):5852.
Ide S, Okugawa Y, Omura Y, Yamamoto A, Ichikawa T, Kitajima T, et al. Geriatric nutritional risk index predicts cancer prognosis in patients with local advanced rectal cancer undergoing chemoradiotherapy followed by curative surgery. World J Surg Oncol. 2021;19(1):34.
Kurzrock R. Cytokine deregulation in cancer. Biomed Pharmacother. 2001;55(9–10):543–7.
Rutkowski P, Kamińska J, Kowalska M, Ruka W, Steffen J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: correlations with local tumor extent and prognosis. J Surg Oncol. 2003;84(3):151–9.
Kaminska J, Kowalska M, Kotowicz B, Fuksiewicz M, Glogowski M, Wojcik E, et al. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis. M-CSF - an independent prognostic factor. Oncology. 2006;70(2):115–25.
Márton S, Garai J, Molnár V, Juhász V, Bogár L, Köszegi T, et al. Kinetics of inflammatory markers following cancer-related bowel and liver resection. Ups J Med Sci. 2011;116(2):124–8.
Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Kustrzeba-Wojcicka I, et al. Acute-phase response proteins are related to cachexia and accelerated angiogenesis in gastroesophageal cancers. Clin Chem Lab Med. 2008;46(3):359–64.
Antonakis PT, Ashrafian H, Isla AM. Laparoscopic gastric surgery for cancer: where do we stand? World J Gastroenterol. 2014;20(39):14280–91.
Wang JF, Zhang SZ, Zhang NY, Wu ZY, Feng JY, Ying LP, et al. Laparoscopic gastrectomy versus open gastrectomy for elderly patients with gastric cancer: a systematic review and meta-analysis. World J Surg Oncol. 2016;14:90.
Ouchi Y, Rakugi H, Arai H, Akishita M, Ito H, Toba K, et al. Redefining the elderly as aged 75 years and older: proposal from the joint Committee of Japan Gerontological Society and the Japan geriatrics society. Geriatr Gerontol Int. 2017;17(7):1045–7.
Acknowledgments
We thank Enago for editing a draft of this manuscript.
Funding
The authors declare no financial supports.
Author information
Authors and Affiliations
Contributions
TM contributed to the conception and design of the study. TO, ST, TY, AI, KF, KT, HK, TT, KS, KK, and NS contributed to the quality control of data and algorithms. HS and KS contributed to the data analysis and interpretation. TM contributed to the manuscript preparation and editing. YF contributed to the manuscript review and final approval of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The project was performed in accordance with the Declaration of Helsinki, and the study protocol was approved by each institutional review board of all participating hospitals (Supplemental Table 1). In addition, all retrospective data included in this study were anonymous, and the requirement for informed consent was waived by each institutional review board of all participating hospitals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table.
Names of the ethics committees of all participating institutions and the reference numbers of this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Matsunaga, T., Saito, H., Osaki, T. et al. Impact of geriatric nutritional risk index on outcomes after gastrectomy in elderly patients with gastric cancer: a retrospective multicenter study in Japan. BMC Cancer 22, 540 (2022). https://doi.org/10.1186/s12885-022-09638-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09638-6