Abstract
Background
Mutations in driver genes such as IDH and BRAF have been identified in gliomas. Meanwhile, dysregulations in the p53, RB1, and MAPK and/or PI3K pathways are involved in the molecular pathogenesis of glioblastoma. RAS family genes activate MAPK through activation of RAF and PI3K to promote cell proliferation. RAS mutations are a well-known driver of mutation in many types of cancers, but knowledge of their significance for glioma is insufficient. The purpose of this study was to reveal the frequency and the clinical phenotype of RAS mutant in gliomas.
Methods
This study analysed RAS mutations and their clinical significance in 242 gliomas that were stored as unfixed or cryopreserved specimens removed at Kyoto University and Osaka National Hospital between May 2006 and October 2017. The hot spots mutation of IDH1/2, H3F3A, HIST1H3B, and TERT promoter and exon 2 and exon 3 of KRAS, HRAS, and NRAS were analysed with Sanger sequencing method, and 1p/19q codeletion was analysed with multiplex ligation-dependent probe amplification. DNA methylation array was performed in some RAS mutant tumours to improve accuracy of diagnosis.
Results
RAS mutations were identified in four gliomas with three KRAS mutations and one NRAS mutation in one anaplastic oligodendroglioma, two anaplastic astrocytomas (IDH wild-type in each), and one ganglioglioma. RAS-mutant gliomas were identified with various types of glioma histology.
Conclusion
RAS mutation appears infrequent, and it is not associated with any specific histological phenotype of glioma.
Similar content being viewed by others
Background
Glioma is a common tumour originating in brain [1]. Glioblastoma is the most aggressive subtype and the most common in adult glioma [1]. Other than glioblastoma, diffuse gliomas include astrocytomas and oligodendrogliomas. and anaplastic astrocytomas and anaplastic oligodendrogliomas show poor prognosis compared in each subtype [1]. These subtypes had been classified mainly by histological diagnosis [2]. Recent intensive genomic and molecular biological analyses of gliomas have identified several significant driver gene mutations in IDH, BRAF, or H3F3 [3, 4]. Dysregulations in the p53, RB1, and MAPK / PI3K pathways have also been suggested to be involved in the molecular pathogenesis of glioblastoma [5, 6]. The importance of the molecular information to an understand the biological properties and pathogenesis of glioma is well recognized. The new 2016 World Health Organization (WHO) classification for central nervous system tumours has introduced the concept of multi-layered integrated diagnosis using a combination of traditional histopathological classification and information obtained from modern molecular analytical methods; therefore, the necessity for molecular information will increase in the neuro-oncological field [7].
RAS genes including KRAS, HRAS, and NRAS are well-known oncogenic genes, and are involved in the ERK pathway, a subgroup of the MAPK pathway. Ligand-mediated activation of receptor tyrosine kinases, such as epidermal growth factor receptor (EGFR), activate RAS proteins and initiate the cascade of the ERK signalling pathway. Activated RAS proteins activate the RAF, which can activate MEK just upstream of ERK [8, 9]. In addition, RAS genes also activate PI3K [10]. Through these several pathways, RAS genes promote cell proliferation, survival, and growth.
Mutations in RAS genes have been found in various cancer cells and lead to dysregulation of cell proliferation to promote oncogenesis [11, 12]. RAS proteins are bound to GDP in a stable state, and switch to an activated state when bound to GTP [12, 13]. GTPase switches GTP-bound RAS back to GDP-bound RAS [13]. RAS mutations have an impaired intrinsic GTPase and are insensitive to GTPase-activating proteins; therefore, inhibiting the conversion of GTP to GDP resulting in dysregulated cell proliferation and oncogenesis [11,12,13]. RAS mutations are mainly observed in codons 12, 13 and 61, and often in pancreatic, colorectal, lung and thyroid cancers [14, 15]. KRAS-activating mutations are widely effective as predictors of resistance to anti-EGFR monoclonal antibodies in colorectal and lung cancer patients [15,16,17,18]. Anti-KRAS drugs have been under development [19, 20], and some clinical trials are ongoing [21]. RAS mutation is now an important biomarker and therapeutic target in these solid cancers.
In terms of central nervous system diseases, a recent study showed an important relationship between RAS mutations and cerebral arterio-venous malformations as a non-neoplastic pathology [22]. Although several reports have found a small number of cases bearing RAS mutations in various gliomas, the clinicopathological properties of these mutations have not been fully addressed [23,24,25,26]. This study analysed RAS mutations and their clinical significance in gliomas.
Methods
Patients and samples
Inclusion criteria for the present study were the local initial diagnosis of gliomas according to the 2007 WHO classification of central nervous system tumours, and frozen or fresh tumour tissues available for genetic analysis. The exclusion criteria were insufficient quality of results of genetic analysis, or clinical data, but no case was excluded. A total of 242 cases were enrolled, including 167 tumours operated on from July 2008 to October 2017 in Kyoto University Hospital, and 75 tumours operated on from May 2006 to March 2017 in Osaka National Hospital. Clinical data collected from each institution included age, sex, tumour location, extent of resection, clinical course including treatment protocol and dates of surgery, recurrence or progression, and death. Ki-67 index were analysed in 167 tumours which was operated in Kyoto University Hospital.
Sanger sequencing
Tumour DNA was extracted from tumour specimens with NucleoSpin® Tissue (MACHEREY-NAGEL, Düren, Germany). Regions of interest for driver genes [23, 27,28,29,30] were amplified by PCR with gene-specific primers (Supplementary Table 1) and TaKaRa Ex Taq® (TAKARA BIO, Shiga, Japan) (IDH1/2, H3F3A, and HIST1H3B) or AmpliTaq Gold 360 (Thermo Fisher Scientific, Waltham, MA) (TERTp, KRAS, HRAS, and NRAS) using Applied Biosystems GeneAmp PCR System 9700 (Thermo Fisher Scientific). PCR products were purified by ExoSAP-IT (Affymetrix, Santa Clara, CA), then sequenced with sequencing primer (IDH1) or PCR forward primer as a sequencing primer (IDH2, H3F3A, HIST1H3B, TERTp, and exons 2 and 3 of KRAS, HRAS, and NRAS) and BigDye® Terminator V1.1 Cycle Sequencing Kit (Thermo Fisher Scientific) using the ABI 3130xL Genetic Analyzer (Thermo Fisher Scientific).
MGMT promoter methylation analysis
O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation was assessed by quantitative methylation-specific PCR (qMSP), in accordance with previous reports [31, 32]. Genomic DNA samples were processed using the EZ DNA Methylation Gold Kit (Zymo Research Corporation, Irvine, CA). The methylation status of samples was analysed by qMSP using the QuantStudio 12 K Flex Real-Time PCR System (Thermo Fisher Scientific) with POWER SYBR® Green PCR Master Mix (Thermo Fisher Scientific) and specific primers (Supplementary Table 1) [33] by the standard curve method. The cut-off for determining a hypermethylated state was set as > 1% [32].
1p/19q co-deletion
1p/19q copy number analyses were performed with multiplex ligation-dependent probe amplification (MLPA) according to the instructions from the manufacturer (SALSA MLPA KIT probemix P088; MRC-Holland, Amsterdam, the Netherlands [32, 34]. Raw data were analysed by Coffalyser.NET software (MLC-Holland).
Integrated diagnosis
Using all molecular pathological information, all cases received integrated diagnoses according to the 2016 WHO classification for central nervous system tumours.
DNA methylation array
DNA methylation profiles were examined by Filgen, Inc. (Aichi, Japan) using the Infinium® MethylationEPIC BeadChip system (illumina, San Diego, CA). Raw methylation data (idat files) were uploaded onto the MolecularNeuropathology.org website and compared to a reference cohort to then be classified into subgroups of the highest calibrated score for each sample [35].
Statistical analysis
All statistical analyses were performed using JMP version 15 software (SAS institute INC). The continuous variates were analyses by Student’s t-test. For survival analysis, overall survival (OS) was defined as the interval between the initial operative day and the date of death or last follow-up date on which the patient was known to be alive. Survival data were analysed using the log-rank test and Cox regression analyses. Differences were considered significant for values of p < 0.05.
Results
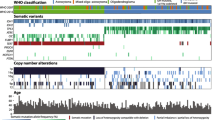
All 242 cases were classified by the 2016 WHO classification, and mutation status is shown in Table 1. The average age of all patients was 51.3 years (range, 4–85 years; standard deviation, 19.2 years), with 153 men and 89 women. RAS mutations were detected in four gliomas (1.65% of total cases).
Mutations in KRAS were revealed in three tumours: an anaplastic astrocytoma, IDH-wildtype; an anaplastic oligodendroglioma, IDH-mutant with a 1p/19q codeletion; and a ganglioglioma. Another anaplastic astrocytoma, IDH-wildtype, showed NRAS mutation. No HRAS mutations were found in the present study. The clinical courses of four cases with RAS mutation are presented below, and summarized in Table 2. All four gliomas occurred in patients under 55 years old (average age, 41.5 years; range, 31–54 years) and were in the supratentorial area. RAS-mutant gliomas accounted for 6.25% of cases of anaplastic astrocytoma, IDH-wildtype (2 of 32), 6.67% of anaplastic oligodendroglioma, IDH-mutant with 1p/19q codeletion (1 of 15), and all gangliogliomas (1 of 1). Ki-67 labelling index of 3 WHO grade III tumours with RAS mutation was higher than that of other grade III tumours (average 29.2% (12.5–40%) vs 15.7% (3.6–50%), p = 0.04), and that of 2 anaplastic astrocytomas was higher than that of other anaplastic astrocytomas (average 37.5% (35–40%) vs 13.9% (3.6–30%), p = 0.0003). Ki-67 labelling index (5%) of the one ganglioglioma with RAS mutation was similar to that (average 3.38% (0.4–10%)) of other WHO grade I tumours.
The clinical courses for each case were not uncommon. But the meaning of RAS mutations in glioma for survival were difficult to be discussed in the present study due to the small number of patients, and the Kaplan-Meyer curve showed no difference in overall survival between anaplastic astrocytoma, IDH-wild type, with and without RAS mutation (Supplementary Fig. 1).
Case presentations
Case 1
A 26-year-old woman presented with a chief complaint of dizziness, and MRI showed left frontal lobe tumour with hyperintensity on T2-weighted imaging without gadolinium enhancement. She elected to follow a “wait and scan” approach (Fig. 1a, b). Five years later, the slowly growing tumour was removed under awake craniotomy. Post-operative MRI showed total resection of the T2-hyperintense lesion. Histopathological examinations detected atypical glia-like cells proliferating densely, cells with round nuclei and clear cytoplasm resembling fried eggs, as well as astrocytic cells, in a substantial area of the tumour. No necrosis or microvascular proliferation was identified (Fig. 1c). FISH detected 1p/19q codeletion, and Ki-67 labelling index of the tumour was 12.5%. The pathological diagnosis was anaplastic oligoastrocytoma, and the patient was followed without post-surgical chemotherapy or radiotherapy. At 45 months after the first surgery, the tumour recurred, and a second surgery was performed to achieve total resection. No re-recurrence was seen until this presentation, 69 months after the first surgery. No anti-tumour treatment had been performed after the second surgery. Genetic analysis of primary tumour showed IDH1 R132H, TERT C250T, and KRAS G12A (Supplementary Fig. 2), and no mutations in IDH2, H3F3A, or HIST1H3B. MGMT promoter was hypomethylated. MLPA analysis showed 1p/19q codeletion and no CDKN2A/B deletion (Fig. 1d). The integrated diagnosis from Sanger sequencing, MLPA, and pathological findings was anaplastic oligodendroglioma, IDH-mutant and 1p/19q codeleted. Interestingly, genetic analysis of recurrent tumour showed the same result about IDH1/2, TERTp, H3F3A and HIST1H3B, but KRAS mutation was not detected.
Neuroimaging, histopathological and molecular properties in Case 1. a, b Presurgical MRI for Case 1. A lesion is apparent in the left frontal lobe, with the appearance of widespread hyperintensity on fluid-attenuated inversion recovery (FLAIR) image (a) without any enhanced lesions on gadolinium-enhanced images (b). c Histopathological examination using hematoxylin and eosin staining. Findings typical of oligodendroglioma are observed. Some mitoses are evident. Scale bar, 100 μm. d MLPA analysis shows 1p/19q codeletion and no deletion of CDKN2A/B
Case 2
A 54-year-old woman presented with a 3-month history of increasing headache and dizziness. MRI showed a gadolinium-enhanced lesion in the genu of the corpus callosum and a T2 hyperintensity lesion spreading to bilateral frontal lobes (Fig. 2a, b). Emergent endoscopic surgery was performed because of progressing hydrocephalus and achieved partial removal of the tumour. Histopathological examinations showed increased atypical glial cells and numerous mitoses, but no microvascular proliferation or palisading necrosis in the specimen (Fig. 2c). Ki-67 labelling index was 40%. The pathological diagnosis was high-grade glioma, and post-operative treatment was radiotherapy concomitant with temozolomide [36]. After discharge, she received maintenance therapy with temozolomide and bevacizumab. However, she showed progressive disease 29 months after the first surgery and received bevacizumab in combination with ifosfamide, carboplatin, and etoposide (ICE) [37]. The tumour kept growing slowly, and she died 49 months after the first surgery. Genetic analysis revealed no mutations in IDH1/2, H3F3A, HIST1H3B or TERT promoter, and MGMT promoter was hypermethylated. In addition, KRAS E76D was detected (Supplementary Fig. 2). A DNA methylation array showed MGMT promoter hypermethylation, matching the qMSP result, but did not identify any matching methylation classes with high calibrated scores. The copy number profile showed no special characteristics (Fig. 2d). The final diagnosis was anaplastic astrocytoma, IDH-wildtype. To support this diagnosis, additional Sanger sequencing was performed and TP53 P72R was revealed.
Neuroimaging, histopathological and molecular properties in Case 2. a, b Presurgical MRI for Case 2. Widespread hyperintensity is seen in bilateral frontal lobes and the corpus callosum to the occipital lobe on FLAIR image (a) with enhanced lesions in the genu of the corpus callosum and bilateral frontal lobes (b). c Histopathological examination using hematoxylin and eosin staining. Scale bar, 100 μm. d Copy number profile analysed by Illumina Methylation Epic Beadchip
Case 3
A 45-year-old man presented with simple partial seizures involving the right side of the face. MRI showed a T2-hyperintense lesion without gadolinium enhancement in the left frontoparietal lobe. Histopathological examinations of stereotactic biopsy revealed tumour cells with semiround or round nuclei (Fig. 3a) of various sizes, and areas of mitoses, with a Ki-67 labelling index of 35%. No necrosis or vascular proliferation was seen, and FISH revealed no 1p/19q codeletion. The diagnosis was anaplastic glioma. He received chemoradiotherapy comprising 60 Gy with temozolomide, but MRI showed tumour progression 3 months later (Fig. 3b, c). He was treated with additional radiotherapy and bevacizumab with ICE but died 24 months after the first surgery. Genetic analysis revealed NRAS Q61R (Supplementary Fig. 2), but no mutations in IDH1/2, H3F3A, HIST1H3B or TERT promoter, and MGMT promoter was not hypermethylated. Methylation-based profiling by the DNA methylation array classified this tumour as “methylation class family Glioblastoma, IDH wildtype” with a calibrated score of 0.55. This low score could be a result of low tumour content or low DNA quality in the analysed material, but the classification matched well with the clinical course and pathological findings. The copy number profile showed amplification of PDGFRA and loss of CDKN2A/B and TP53, gain of chromosomes 7, 9q, and 12, and loss of chromosomes 11 and 13 (Fig. 3d). Because there was no evidence of grade 4 histology, the integrated diagnosis was determined as anaplastic astrocytoma, IDH-wildtype.
Neuroimaging, histopathological and molecular properties in Case 3. a Histopathological examination using haematoxylin and eosin staining. Scale bar, 100 μm. b, c MRI for Case 3 at the time of recurrence. Widespread hyperintensity is seen in the left frontal and parietal lobes on FLAIR imaging (b) with an enhanced region in the centre of the lesion (c). d Copy number profile as analysed by Illumina Methylation Epic Beadchip
Case 4
A 36-year-old man was referred after a brain tumour was coincidentally identified on screening CT after a traffic accident. MRI revealed a left medial occipitotemporal tumour with gadolinium enhancement (Fig. 4a, b). Histopathological examination of stereotactic biopsy (Fig. 4c) revealed a dense, invasive proliferation of various-sized glial cells with some mitoses, and a Ki-67 labelling index of 5%. No necrosis or microvascular proliferation was identified. Immunohistochemistry showed positive results for olig2, GFAP, and p53, while FISH showed no 1p/19q codeletion. Based on these findings, the first diagnosis was anaplastic astrocytoma. The patient received chemoradiation and maintenance chemotherapy with temozolomide. As tumour progression was detected 18 months after biopsy, he underwent gross total resection of the tumour. No tumour recurrence was identified after the second surgery, and no additional treatment was performed for 24 months. Genetic analysis of primary tumour revealed KRAS Q61K (Supplementary Fig. 2), wild-type IDH1/2, H3F3A, HIST1H3B and TERT promoter, and no MGMT promoter hypermethylation. The DNA methylation array classified “methylation class family pilocytic astrocytoma” as the methylation class and “methylation class of low-grade glioma, subclass hemispheric pilocytic astrocytoma and ganglioglioma” as the methylation class family member, with calibrated scores of 0.97 and 0.96, respectively. The copy number profile showed gain of chromosomes 7, 9, 11 and 12 (Fig. 4d). Histopathological re-examination revealed many large ganglion cells with anisonucleosis and some double nuclei (Fig. 4e), Nissl bodies and eosinophilic granular bodies (Fig. 4f) in specimens from the second surgery. Given these genetic results and histopathological findings, the final diagnosis was ganglioglioma. Like as case 1, KRAS mutation was not detected in the recurrent tumour.
Neuroimaging, histopathology and molecular properties of Case 4. a, b Presurgical MRI for Case 4. FLAIR hyperintensity and gadolinium-enhanced lesions are seen in the left medial temporal to the occipital lobe. c Histopathological examination using haematoxylin and eosin staining. Scale bar, 100 μm. d Copy number profile as analysed by Illumina Methylation Epic Beadchip. e, f Histopathological examination using haematoxylin and eosin staining at the time of review. In this area, many large, ganglion-like cells are observed (e). Some cells show double nuclei (f) and some eosinophilic bodies are present. Scale bars, 100 μm (e), 20 μm (f)
Review of the previous reported cases
Previous 17 studies presented 44 gliomas with RAS mutations (Table 3). They were 17 glioblastomas (2 were glioblastomas with oligodendroglial component), 1 astrocytoma, 4 oligodendrogliomas, 3 anaplastic oligodendrogliomas, 1 oligoastrocytoma, 9 pilocytic astrocytomas, 2 anaplastic pilocytic astrocytomas, 2 fibrillary astrocytomas, 2 gangliogliomas, 2 pleomorphic xanthoastrocytomas, and 1 gliosarcoma. And they included 14 men and 13 women, and ages at diagnosis were described in 28 patients and they were 1–64 years (average, 33.3 years; standard deviation, 17.1 years). The co-existing mutations were various and IDH1 R132H was the major mutation which detected in 11 cases.
Discussion
Various reports have described RAS mutations in glioma. Chi et al. analysed 214 gliomas, and they found 3 KRAS mutation cases among 164 glioblastomas [24]. Wakimoto et al. found 4 KRAS-mutant IDH-mutant gliomas, comprising 2 oligodendrogliomas, a grade 2 astrocytoma, and a glioblastoma with an oligodendroglial component. These 4 tumours all showed 1p/19q codeletions, and were thus considered to represent grade 2 or 3 oligodendrogliomas based on the 2016 WHO brain tumour classifications [26]. Ballester et al. showed the results of next-generation sequencing of 381 diffuse gliomas [47]. They found a NRAS mutation in 11 oligodendrogliomas, 2 KRAS mutations in 16 anaplastic oligodendrogliomas, and 2 KRAS mutations and 2 NRAS mutations in 226 glioblastomas. Pekmezci et al. detected KRAS mutation in 2 of 40 gangliogliomas [25]. Literature review of RAS-mutant gliomas showed that RAS-mutant gliomas have various histologies and that RAS mutation co-existed with other genetic alterations. They were often reported in young cases. The larger database made by the Cancer Genome Atlas (TCGA) Research Network showed 2 KRAS mutation and 2 NRAS mutation in 590 glioblastomas, and 1 KRAS mutation and 1 NRAS mutation in low grade gliomas with IDH-mutant and 1p/19q codeletion, and 1 KRAS mutation and 2 NRAS mutation in those with IDH-mutant and no 1p/19q codeletion [52]. Summarizing by age group, RAS mutations were found in 1 out of all 93 gliomas under 30 years old, 6 out of 631 cases from 30 to 60 years old, and 1 out of 363 cases in over 60 years old, and there was no significant difference in frequency of RAS mutations [52]. Similar to these studies, we report RAS mutation as a rare occurrence with no association to a particular histological phenotype of glioma. Additionally, copy number analysis in the present study revealed no chromosomal gain or loss.
In this study, RAS-mutant gliomas showed various histology, but all cases were in relatively young adults. RAS mutation was found in an anaplastic oligodendroglioma, two IDH-wildtype anaplastic astrocytomas, and a ganglioglioma. Among the 20- to 60-year-old patients of our present cohort, 14 tumours were anaplastic oligodendrogliomas, 23 were anaplastic astrocytomas (14 were IDH-wildtype), and one was ganglioglioma. Excluding the single ganglioglioma case present in our cohort, IDH-wildtype anaplastic astrocytomas in patients under 60 years old showed RAS mutation the most frequently (14.3%). Genetically, no other major driver mutations were identified in the anaplastic astrocytomas or the ganglioglioma, which had RAS mutations. The case of RAS-mutant anaplastic oligodendroglioma showed IDH1 and TERT promoter mutations, which are known to be detected in almost all oligodendrogliomas [27]. Because of the small number of RAS mutant tumours, clarifying the genetic properties of RAS mutant tumours and discussing associations between RAS mutations and other driver genes is difficult, however, some studies reported the co-existing other genetic alterations in RAS-mutant gliomas. Clinically, the two cases of anaplastic astrocytoma with RAS mutation showed aggressive infiltration during the clinical course with high Ki-67 labelling index, but clinical outcomes did not differ from those of other IDH-wildtype anaplastic astrocytomas (Supplementary Fig. 1). The other two cases of anaplastic oligodendroglioma and ganglioglioma showed benign clinical courses. Some studies have reported RAS mutation as a prognostic factor in some non-neuroepithelial solid cancers [53, 54]. However, we could not explain the clinical significance of RAS mutation occurring in gliomas. The limitation of the present study was the rarity of RAS mutant gliomas due to the infrequency of RAS mutation in glioma. This was why the survival analysis was difficult in our cases, but it was also the same in another previous cohort. These issues should be addressed using larger cohorts in the future.
KRAS mutation has been reported to increase vascular endothelial growth factor (VEGF) expression and to promote the construction of a tumour vascular network [55]. However, the present study found no evidence of an aggressive vascular network such as widespread gadolinium enhancement or intra-tumoral arteriovenous shunt. KRAS G12D is reportedly associated with gliosis [56]. Another report suggested that KRAS signalling is essential for the maintenance of glioblastoma in mice, and inhibition of KRAS expression result in tumour apoptosis [57]. These facts proposed that RAS mutation has some effect on glioma maintenance and proliferation, and MAPK / PI3K pathways, which are activated by RAS mutation, have been suggested to be involved in the molecular pathogenesis of glioblastoma [5, 6]. Although the higher Ki-67 labelling index in the RAS-mutant gliomas had not been discussed previously, this may reflect the tumour proliferation activities. Some anti-RAS drugs are currently under development [19, 20], and these drugs are expected to make contributions to improving the prognosis of RAS-mutant glioma in the near future.
In the presented case series, recurrent tumours of case 1 (AO) and case 4 (ganglioglioma) showed no RAS mutations which were shown in their primary tumour. This fact may imply that tumour with RAS mutation was disappeared by treatment. Through direct comparison of the genomic landscape of gliomas at initial diagnosis and recurrence, a previous study showed that full set of mutations found in the initial tumour do not maintain in the recurrences and suggested that recurrent tumours are originate from cells derived at a very early stage of the evolution of tumours [58]. While IDH1 and TERTp mutations, and 1p/19q codeletion assigned as the truncal events during tumour evolution [3], RAS mutations in glioma may be an additional alterations to development. About the primary tumours, Sanger sequencing revealed TP53 mutation in one of these AAs, and methylation assay showed amplification of PDGFRA and loss of CDKN2A/B and TP53 in the other. This fact proposed that RAS mutation have a potential to be a driver gene of glioma development, but its effect may be supportive compared with major truncal driver mutations like as IDH mutation, TERTp mutation and 1p/19q codeletion. Because RAS mutation could switch at glioma recurrence, the molecular analysis is thought to be essential for recurrent as well as primary tumours when anti-RAS treatment are conducted.
KRAS G12A and KRAS Q61K are present in 0.76 and 0.07% of cases in the Project Genomics Evidence Neoplasia Information Exchange (AACR GENIE) launched by the American Association for Cancer Research [59]. KRAS G12A has been identified in lung, colon, colorectal and rectal adenocarcinoma, and uterine endometrioid carcinoma, while KRAS Q61K has been found in colon, colorectal and pancreatic adenocarcinoma. KRAS G12A and KRAS Q61K are predictive biomarkers for the use of erlotinib, gefitinib, cetuximab, and panitumumab in patients [16,17,18, 60, 61]. Non-small cell lung carcinoma and colorectal carcinoma have the greatest number of therapies targeting KRAS G12A and KRAS Q61K or related pathways. KRAS E76D has not been reported in other types of cancer, and further study was needed whether if it has a role of an activating mutation. NRAS Q61R is present in 0.73% of AACR GENIE cases [59], and has been identified in cutaneous melanoma, melanoma, papillary thyroid cancer, poorly differentiated thyroid gland cancer, and colon adenocarcinoma [59]. NRAS Q61R is a predictive biomarker for the uses of cetuximab and panitumumab in patients [60, 61]. Further, for NRAS-mutant melanoma, binimetinib reportedly improves progression-free survival compared with dacarbazine [62].
Lower grade astrocytomas in our cohort contained a large number of IDH-wild type tumours. This fact partially results from high frequency of TERTp mutation. In our IDH-wild type tumours, 8 out of 18 DAs and 15 out of 32 AAs showed TERTp mutation. Nowadays, IDH-wild type astrocytomas with TERTp mutations are known as a group of astrocytomas with poor prognosis, and these tumours are supposed to be a different group from the group of common lower grade astrocytomas [63]. The diagnosis of lower grade astrocytoma without IDH mutation needs further discussion.
Conclusions
We found 4 RAS mutations in various types of 242 gliomas. All cases involved younger adults. No clear association was identified between RAS mutations and clinical or genetic characteristics of tumours. Clarification of the effectiveness of anti-RAS treatments for gliomas requires further investigations in larger cohorts.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AACR:
-
American Association for Cancer Research
- EGFR:
-
Epidermal growth factor receptor
- ERK:
-
Extracellular signal-regulated kinase
- FISH:
-
Fluorescence in situ hybridization
- GDP:
-
Guanosine diphosphate
- GTP:
-
Guanosine triphosphate
- ICE:
-
Ifosfamide, carboplatin, and etoposide
- MAPK:
-
Mitogen-activated protein kinase
- MEK:
-
MAPK kinase
- MGMT:
-
O6-methylguanine-DNA methyltransferase
- MLPA:
-
Multiplex ligation-dependent probe amplification
- MRI:
-
Magnetic resonance imaging
- PCR:
-
Polymerase chain reaction
- PI3K:
-
Phosphoinositide 3-kinase
- qMSP:
-
Quantitative methylation-specific PCR
- RAF:
-
Rapidly accelerated fibrosarcoma
- RAS:
-
Rat sarcoma
- RB1:
-
Retinoblastoma
- TCGA:
-
The Cancer Genome Atlas
- VEGF:
-
Vascular endothelial growth factor
- WHO:
-
World Health Organization
References
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology. 2014;16(suppl 4):iv1–iv63.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of Tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. https://doi.org/10.1007/s00401-007-0243-4.
Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–68. https://doi.org/10.1038/ng.3273.
Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–12. https://doi.org/10.1038/ng.2611.
Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. https://doi.org/10.1038/nature07385.
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–12. https://doi.org/10.1126/science.1164382.
Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20. https://doi.org/10.1007/s00401-016-1545-1.
Prior IA, Hancock JF. Ras trafficking, localization and compartmentalized signalling. Semin Cell Dev Biol. 2012;23(2):145–53. https://doi.org/10.1016/j.semcdb.2011.09.002.
Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274(5295):2086–9. https://doi.org/10.1126/science.274.5295.2086.
Castellano E, Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2(3):261–74. https://doi.org/10.1177/1947601911408079.
Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348(6297):125–32. https://doi.org/10.1038/348125a0.
Lowy DR, Willumsen BM. Function and regulation of ras. Annu Rev Biochem. 1993;62(1):851–91. https://doi.org/10.1146/annurev.bi.62.070193.004223.
Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, et al. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277(5324):333–8. https://doi.org/10.1126/science.277.5324.333.
De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–62. https://doi.org/10.1016/S1470-2045(10)70130-3.
Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–65. https://doi.org/10.1056/NEJMoa0804385.
Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23(25):5900–9. https://doi.org/10.1200/JCO.2005.02.857.
Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–5. https://doi.org/10.1158/0008-5472.CAN-06-0191.
Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2(1):e17. https://doi.org/10.1371/journal.pmed.0020017.
Ross SJ, Revenko AS, Hanson LL, Ellston R, Staniszewska A, Whalley N, et al. Targeting KRAS-dependent tumors with AZD4785, a high-affinity therapeutic antisense oligonucleotide inhibitor of KRAS. Sci Transl Med. 2017;9(394):eaal5253.
Shin SM, Choi DK, Jung K, Bae J, Kim JS, Park SW, et al. Antibody targeting intracellular oncogenic Ras mutants exerts anti-tumour effects after systemic administration. Nat Commun. 2017;8(1):15090. https://doi.org/10.1038/ncomms15090.
Furuse J, Kurata T, Okano N, Fujisaka Y, Naruge D, Shimizu T, et al. An early clinical trial of Salirasib, an oral RAS inhibitor, in Japanese patients with relapsed/refractory solid tumors. Cancer Chemother Pharmacol. 2018;82(3):511–9. https://doi.org/10.1007/s00280-018-3618-4.
Nikolaev SI, Vetiska S, Bonilla X, Boudreau E, Jauhiainen S, Rezai Jahromi B, et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N Engl J Med. 2018;378(3):250–61. https://doi.org/10.1056/NEJMoa1709449.
Bleeker FE, Lamba S, Leenstra S, Troost D, Hulsebos T, Vandertop WP, et al. IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat. 2009;30(1):7–11. https://doi.org/10.1002/humu.20937.
Chi AS, Batchelor TT, Dias-Santagata D, Borger D, Stiles CD, Wang DL, et al. Prospective, high-throughput molecular profiling of human gliomas. J Neuro-Oncol. 2012;110(1):89–98. https://doi.org/10.1007/s11060-012-0938-9.
Pekmezci M, Villanueva-Meyer JE, Goode B, Van Ziffle J, Onodera C, Grenert JP, et al. The genetic landscape of ganglioglioma. Acta Neuropathol Commun. 2018;6(1):47. https://doi.org/10.1186/s40478-018-0551-z.
Wakimoto H, Tanaka S, Curry WT, Loebel F, Zhao D, Tateishi K, et al. Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin Cancer Res. 2014;20(11):2898–909. https://doi.org/10.1158/1078-0432.CCR-13-3052.
Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126(2):267–76. https://doi.org/10.1007/s00401-013-1141-6.
Arita H, Yamasaki K, Matsushita Y, Nakamura T, Shimokawa A, Takami H, et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun. 2016;4(1):79. https://doi.org/10.1186/s40478-016-0351-2.
Chang YS, Yeh KT, Hsu NC, Lin SH, Chang TJ, Chang JG. Detection of N-, H-, and KRAS codons 12, 13, and 61 mutations with universal RAS primer multiplex PCR and N-, H-, and KRAS-specific primer extension. Clin Biochem. 2010;43(3):296–301. https://doi.org/10.1016/j.clinbiochem.2009.10.007.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73. https://doi.org/10.1056/NEJMoa0808710.
Okita Y, Nonaka M, Shofuda T, Kanematsu D, Yoshioka E, Kodama Y, et al. (11)C-methinine uptake correlates with MGMT promoter methylation in nonenhancing gliomas. Clin Neurol Neurosurg. 2014;125:212–6. https://doi.org/10.1016/j.clineuro.2014.08.004.
Umehara T, Arita H, Yoshioka E, Shofuda T, Kanematsu D, Kinoshita M, et al. Distribution differences in prognostic copy number alteration profiles in IDH-wild-type glioblastoma cause survival discrepancies across cohorts. Acta Neuropathol Commun. 2019;7(1):99. https://doi.org/10.1186/s40478-019-0749-8.
Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793–7.
Jeuken J, Cornelissen S, Boots-Sprenger S, Gijsen S, Wesseling P. Multiplex ligation-dependent probe amplification. J Mol Diagnostics. 2006;8(4):433–43. https://doi.org/10.2353/jmoldx.2006.060012.
Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–74. https://doi.org/10.1038/nature26000.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. https://doi.org/10.1056/NEJMoa043330.
Arakawa Y, Mizowaki T, Murata D, Fujimoto K, Kikuchi T, Kunieda T, et al. Retrospective analysis of bevacizumab in combination with ifosfamide, carboplatin, and etoposide in patients with second recurrence of glioblastoma. Neurol Med Chir (Tokyo). 2013;53(11):779–85. https://doi.org/10.2176/nmc.oa2013-0211.
Maltzman TH, Mueller BA, Schroeder J, Rutledge JC, Patterson K, Preston-Martin S, et al. Ras oncogene mutations in childhood brain tumors. Cancer Epidemiol Biomark Prev. 1997;6(4):239–43.
Knobbe CB, Reifenberger J, Reifenberger G. Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and BRAF in glioblastomas. Acta Neuropathol. 2004;108(6):467–70. https://doi.org/10.1007/s00401-004-0929-9.
Janzarik WG, Kratz CP, Loges NT, Olbrich H, Klein C, Schäfer T, et al. Further evidence for a somatic KRAS mutation in a pilocytic astrocytoma. Neuropediatrics. 2007;38(2):61–3. https://doi.org/10.1055/s-2007-984451.
Jeuken J, van den Broecke C, Gijsen S, Boots-Sprenger S, Wesseling P. RAS/RAF pathway activation in gliomas: the result of copy number gains rather than activating mutations. Acta Neuropathol. 2007;114(2):121–33. https://doi.org/10.1007/s00401-007-0239-0.
Schiffman JD, Hodgson JG, Vandenberg SR, Flaherty P, Polley M-YC YM, et al. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant Astrocytomas. Cancer Res. 2010;70(2):512–9. https://doi.org/10.1158/0008-5472.CAN-09-1851.
Cin H, Meyer C, Herr R, Janzarik WG, Lambert S, Jones DTW, et al. Oncogenic FAM131B–BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 2011;121(6):763–74. https://doi.org/10.1007/s00401-011-0817-z.
Theeler BJ, Ellezam B, Sadighi ZS, Mehta V, Tran MD, Adesina AM, et al. Adult pilocytic astrocytomas: clinical features and molecular analysis. Neuro-Oncology. 2014;16(6):841–7. https://doi.org/10.1093/neuonc/not246.
Milinkovic VP, Skender Gazibara MK, Manojlovic Gacic EM, Gazibara TM, Tanic NT. The impact of TP53 and RAS mutations on cerebellar glioblastomas. Exp Mol Pathol. 2014;97(2):202–7. https://doi.org/10.1016/j.yexmp.2014.07.009.
Samal F, Stanek L, Filip M, Haninec P, Vicha A, Musil Z, et al. Complete diagnostics and clinical approach for a female patient with unusual glioblastoma: a case study. Mol Clin Oncol. 2016;5(1):161–4. https://doi.org/10.3892/mco.2016.891.
Ballester LY, Fuller GN, Powell SZ, Sulman EP, Patel KP, Luthra R, et al. Retrospective analysis of molecular and Immunohistochemical characterization of 381 primary brain tumors. J Neuropathol Exp Neurol. 2017;76(3):179–88. https://doi.org/10.1093/jnen/nlw119.
Reinhardt A, Stichel D, Schrimpf D, Sahm F, Korshunov A, Reuss DE, et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018;136(2):273–91. https://doi.org/10.1007/s00401-018-1837-8.
Chau LQ, Levy ML, Crawford JR. Unusual KRAS missense mutation (p.E63K) in patient with juvenile pilocytic astrocytoma of the tectum. BMJ Case Rep. 2019;12(2):e228128.
Schittenhelm J, Krischker N, Gepfner-Tuma I, Behling F, Noell S, Eckert F, et al. Oncogenic KRAS hotspot mutations are rare in IDH-mutant gliomas. Brain Pathol. 2019;29(3):321–4. https://doi.org/10.1111/bpa.12709.
Zou H, Duan Y, Wei D, Zhang Y, Dai J, Li J, et al. Molecular features of pleomorphic xanthoastrocytoma. Hum Pathol. 2019;86:38–48. https://doi.org/10.1016/j.humpath.2018.08.038.
Ceccarelli M, Floris, Tathiane, Thais, Sofie, Bradley, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–63. https://doi.org/10.1016/j.cell.2015.12.028.
Tao LY, Zhang LF, Xiu DR, Yuan CH, Ma ZL, Jiang B. Prognostic significance of K-ras mutations in pancreatic cancer: a meta-analysis. World J Surg Oncol. 2016;14(1):146. https://doi.org/10.1186/s12957-016-0888-3.
Xie MZ, Li JL, Cai ZM, Li KZ, Hu BL. Impact of primary colorectal Cancer location on the KRAS status and its prognostic value. BMC Gastroenterol. 2019;19(1):46. https://doi.org/10.1186/s12876-019-0965-5.
Figueras A, Arbos MA, Quiles MT, Vinals F, Germa JR, Capella G. The impact of KRAS mutations on VEGF-A production and tumour vascular network. BMC Cancer. 2013;13(1):125. https://doi.org/10.1186/1471-2407-13-125.
Ryu MJ, Liu Y, Zhong X, Du J, Peterson N, Kong G, et al. Oncogenic Kras expression in postmitotic neurons leads to S100A8-S100A9 protein overexpression and gliosis. J Biol Chem. 2012;287(27):22948–58. https://doi.org/10.1074/jbc.M112.357772.
Holmen SL, Williams BO. Essential role for Ras signaling in glioblastoma maintenance. Cancer Res. 2005;65(18):8250–5. https://doi.org/10.1158/0008-5472.CAN-05-1173.
Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–93. https://doi.org/10.1126/science.1239947.
Consortium APG. AACR Project GENIE: Powering precision medicine through an international consortium. Cancer Discov. 2017;7(8):818–31.
Hsu HC, Thiam TK, Lu YJ, Yeh CY, Tsai WS, You JF, et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget. 2016;7(16):22257–70. https://doi.org/10.18632/oncotarget.8076.
Peeters M, Oliner KS, Price TJ, Cervantes A, Sobrero AF, Ducreux M, et al. Analysis of KRAS/NRAS mutations in a phase III study of Panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal Cancer. Clin Cancer Res. 2015;21(24):5469–79. https://doi.org/10.1158/1078-0432.CCR-15-0526.
Dummer R, Schadendorf D, Ascierto PA, Arance A, Dutriaux C, Di Giacomo AM, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(4):435–45. https://doi.org/10.1016/S1470-2045(17)30180-8.
Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–10. https://doi.org/10.1007/s00401-018-1913-0.
Acknowledgements
This study included the result based upon data generated by The Cancer Genome Atlas (TCGA) Research Network and American Association for Cancer Research (AACR). We would like to acknowledge TCGA and AACR and their financial and material support in the development of the TCGA and AACR Project GENIE registry, as well as members of the consortium for their commitment to data sharing. Interpretations are the responsibility of the study authors.
Funding
This work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Project No. 16 K10754, 17 K19724, 19 K09505, and 19 K22685).
Author information
Authors and Affiliations
Contributions
Y.A. and Y.Ka. designed the study. Y.M., E.Y., T.S., T. K, and D.K. performed genetical analysis and data analysis. M.T., M.N., Y.O., Y.M., and S.Miy. collected samples. S.Min., Y.Ko., M.M. and T. H performed histological analyses and diagnosis. Y.M., Y.A. and Y.Ka. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was carried out in accordance with the principles of the Declaration of Helsinki, and approval was obtained from the institutional review boards at Kyoto University Hospital (approval number: G1124) and Osaka National Hospital (approval number: 0713). Informed consent was obtained from all patients.
Competing interests
The authors declare that they have no competing interests in relation to this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. 1.
Kaplan-Meier curve for anaplastic astrocytomas without IDH mutation in the present study. The black line shows wild-type RAS and the red line shows mutant-type RAS. The p value is calculated as 0.98 by log-rank test.
Additional file 2: Supplementary Fig. 2.
Chromatograms made by Sanger sequencing showing RAS mutations in the four tumours. Case 1) KRAS c.35 G > C, p.G12A (red arrow) in exon 2 of KRAS. Case 2) KRAS c.228 G > C, p.E76D (red arrow) in exon 3 of KRAS. Case 3) NRAS c.182 A > G, p.Q61R (red arrow) in exon 3 of NRAS. Case 4) KRAS c.180–181 TC > AA, p.Q61K (red arrows) in exon 3 of KRAS.
Additional file 3: Supplementary Table 1.
Gene-specific primers used for PCR amplifications of the regions of interest in driver genes and quantitative methylation-specific PCR (qMSP) of MGMT promoter.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Makino, Y., Arakawa, Y., Yoshioka, E. et al. Infrequent RAS mutation is not associated with specific histological phenotype in gliomas. BMC Cancer 21, 1025 (2021). https://doi.org/10.1186/s12885-021-08733-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08733-4