Abstract
Microorganisms and organic compounds (humic and fulvic acid) offer viable alternatives to insecticides and mineral fertilizers. Even though many studies have shown the effects of biofertilizers and organic substances separately, little information is available on plant responses to the combined application of these bio-stimulants, even though these biological inputs have a high potential for simultaneous action. A two-year (2020/21–2021/22) field experiment was conducted to investigate the impact of organic and biofertilizers application on the growth, yield, and biochemical attributes of wheat (cv. Misr-1). Pre-planting, wheat seeds were inoculated with two biofertilizers including Mycorrhizae, and Azotobacter, and their combination (MIX), and control (un-inoculation) were considered the main plot factor. The subplot factor contained the foliar sprays of humic acid, fulvic acid, and control (no spray). The results revealed that the seed inoculation with mycorrhizae and azotobacter in combination with foliar-applied humic acid markedly (p ≤ 0.05) affected the growth, yield, and seed biochemical composition of wheat. Combination of mycorrhiza and azotobacter significantly (p ≤ 0.05) increased) plant height (100 cm), crop growth rate (18.69 g), number of spikelets per spike (22), biological yield (13.4 ton ha-1), grain yield (5.56 ton ha-1), straw yield (8.21 ton ha-1),), nitrogen (2.07%), phosphorous (0.91%), potassium (1.64%), protein content (12.76%), starch (51.81%), and gluten content (30.90%) compared to control. Among organic fertilizers, humic acid caused the maximum increase in plant height (93 cm), crop growth rate ( 15 g day-1 m-2),1000 grain weight (51 g), biological yield ( 11ton ha-1), grain yield (4.5 ton ha-1), protein content (11%), chlorophyll content (46 SPAD), and gluten (29.45%) as compared to all other treatments. The foliar application of humic acid combined with the mycorrhizae or azotobacter seed inoculation was efficient to induce wheat vegetative growth development, as well as yield and its components.
Similar content being viewed by others
Introduction
Wheat is one of the most cultivated crops in the world because a third of the world's population depends on it in their food. It is characterized by the ability to grow under different environmental conditions and variant agricultural systems [1]. The global average of grain yield productivity is currently at 3.3 t ha−1, but this rate will need to nearly double in order to meet rising food demands [2, 3]. Egypt consumes 16 million tons of wheat per year but only produces 9 million tons, so they have set a national target of increasing wheat output to meet domestic demand via the implementation of novel agricultural practices and the use of wheat varieties with the potential to increase grain production (FAO, 2019). Intensive farming practices, which permit high yield and quality, require extensive use of chemical fertilizers, which are costly chemical fertilizers. Moreover, the application of unrenewable substance inputs causes ecological damage, such as adulteration of surface water and soil water and alteration of denitrification processes [4]. In this regard, interest in ecologically friendly, sustainable, and organic agriculture techniques has lately increased [5]. To reduce environmental pollution, it is essential to develop and use sustainable agriculture methods and biofertilization [6]. The usage of bio stimulants for plant development, whose purpose is to enhance physiological processes in plants, boost nutrient acquisition, and raise tolerance against abiotic and biotic challenges, is also required by this agroecological paradigm [7,8,9].
For the development of wheat and other crop species, biofertilizers are being explored as a potential substitute method to get rid of environmental residues of chemical fertilizers. These biofertilizers are primarily based on beneficial microbes added to soil or seed to increase the quantity and biological activity of desirable microorganisms in the rhizosphere, hence improving soil fertility and plant development [10]. Because soil is a complex system that can be influenced by a variety of factors [11, 12], Strengthening the beneficial microbial populations in the soil, particularly in the rhizosphere area, is essential for the circulation of both organic and inorganic nutrients. This can boost nutrient availability to plants while also enhancing soil quality [13]. Bio-fertilizers can enhance plant growth via nitrogen fixation, phytohormone, phosphate, and potassium solubilization [14]. The use of azotobacter chroococcum as biofertilizers has a promising effect on maize growth and yield when compared to non-inoculated plants, according to this study.
Arbuscular mycorrhizal fungi (AMF) are advantageous microorganisms that form a symbiotic relationship with plant roots, enhancing the uptake of essential nutrients such as phosphorus (P), nitrogen (N), sulfur (S), potassium (K), calcium (Ca), iron (Fe), copper (Cu), and zinc (Zn). Additionally, they boost the absorption of nitrogen and phosphorus, increasing crop yield [15,16,17,18]. Arbuscular mycorrhizae can improve the stomatal conductance in shoots and thereby improve the photosynthetic rate in particular under drought conditions [19]. Arbuscular mycorrhiza can increase yield. Recently, in most sustainable food production systems, the application of AMF took more interest [20,21,22], to improve the plant nutrient, and lessen the excessive demand for chemical fertilizers [23]. It was noticed that arbuscular mycorrhizae can improve the soil structure by raising the soil water-holding capacity [24] and supplying the plants with water and nutrients [25]. Underwater scarcity, it was noticed that treating maize with arbuscular mycorrhizae improved the stomatal conductance and the plant biomass [26]. Treating wheat with arbuscular mycorrhizal stimulates changes in the composition of the leaf amino acid, and harvest index [27].
Azotobacter bacteria can use atmospheric nitrogen in the synthesis of their cellular protein and consequently improved the plant crop [28]. Besides, azotobacter could also increase the availability of iron and its absorption [29, 30]. Besides, Azotobacter can fix atmospheric nitrogen and turn it into ammonia, which is easy to be absorbed and utilized by plants [31]. Moreover, Azotobacter could improve plant protection against root pathogens [32, 33], encouraging soil beneficial microorganisms and consequently improving crop productivity [34]. Azotobacter can fix nitrogen, and produce siderophore, polysaccharides, and indole acetic acid ( IAA) that raise plant health [35,36,37]. Inoculation of wheat with azotobacter increased the grain yield compared with untreated plants [38]. It was found by many authors that the usage of azotobacter might encourage o the production of plant growth hormones such as auxins and gibberellins and therefore it could ameliorate the development of the plant roots, and thus could increases nodulation, nitrogen fixation and crop productivity [39,40,41]. Furthermore, azotobacter can improve plant health by boosting the production of Indole-3-Acetic Acid, enhancing resistance to abiotic and biotic stress and pesticides, fixing atmospheric nitrogen, increasing soil fertility, reducing soil clumps, and mitigating soil degradation [42].
Humic and fulvic acids are organic compounds that are generated from the breakdown of organic matter, such as plants and animals [43, 44]. These acids have been found to have a significant impact on soil fertility and plant nutrition, leading to their widespread use as natural soil amendments and plant growth boosters in agriculture [45]. Humic acid can soluble easily in water, and has several potential benefits for soil microbial populations and soil structure such as improving nutrient uptake, and plant growth [46]. In corn, humic acid reduces the negative impacts of water shortage and boosts the survival of droughtstress in corn [47]. Spraying of humic acid can raise the survival of plants to drought stresses [48, 49]. Besides, it was documented by Szczerski et al. [50] and Haider et al. [51] that the application of humic acid can increase the element's absorption and usage and thus the obtained crop yielding. Moreover, the same authors added that spraying of humic materials could also boost the plant height, leaf no., shoot fresh and dry weight, total sugar, carbohydrates, amino acids, minerals, and yield. Moreover, humic acid can improve the root system development, and element absorption [52], and stimulate plant growth and development and thus the obtained yield because its effect is like to the influence of the plant growth hormones; cytokinin and auxin, and gibberellin [53]. Besides, it can also increase the absorption of iron and zinc, which were involved in the synthesis of indole acetic acid [54].
On the other hand, fulvic acid is rich in macro and microelements as well as amino acids and can raise the rate of nutrient absorption from the soil as it works as a carrier to the substances from external parts to the internal parts of the plants. Because of the low molecular weight of fulvic acid, it could pass through the pores of membranes easily [55, 56], and can stay steadily in the soil solution under high salt concentrations as well as in a broad range of pH [57], so it could stimulate the developing of lateral roots and shoots and increase the crop quality attributes [58,59,60,61]. Fulvic acid can arrange plant growth development by ameliorating the photosynthetic rate and reducing transpiration conductance [62, 63]. Moreover, it is also important to boost plant growth by raising the fertilizers utilization efficiency [44], reducing the heavy metal influence [64], and increasing the yield by improving the soil's nutritional status [65].
Considering the importance of biofertilizers and organic fertilizer in the agricultural sector, this research was conducted to investigate the individual and combined application of grains inoculation with biofertilizers; mycorrhizae, and azotobacter and the exogenous application of organic fertilizer; humic acid, and fulvic acid on the performance, productivity, yield components, and quality of wheat.
Materials and methods
Study area
A field experiment was organized at Hosh Isa district, El-Beheira Governorate, Egypt (27°12′16.7"N 31°09′36.9" E). wheat grains (Cv. Misr 1) were sown on the 18th and 20th of November in 2020–2021 and 2021–2022, respectively. Physicochemical analysis of the experimental soil during the study seasons was shown in Table 1. Soil samples were taken from every plot at two using a spiral auger of 2.5 cm diameter. The soil samples were dried at 40 °C and ground to a size of < 2 mm. The organic matter content and soil N content were determined through wet oxidation determination and the Kjeldhal method, respectively [66]. The phosphorus and potassium contents were determined by spectrophotometry and flame photometer, respectively.
Treatments and experimental design layout
In this experiment, four bio-fertilizer treatments including: Control (CK), Azotobacter, Mycorrhizae, and Mycorrhizae + Azotobacter (MIX), were randomly assigned to the main plots, where the organic fertilizer, humic acid (Humic Acid 70% powder, humate (Tianjin) International Limited, Tianjin, China), fulvic acid (Fulvic Acid-100% Water Soluble Fulvic Acid Powder Organic Fertilizer Hebei, China), and control, were allocated to the subplot in a split-plot design with three replicates. The soil was prepared by two orthogonal plowings, followed by leveling the soil and dividing it into the experimental plots (4 × 3 m). Nitrogen fertilizer in the form of urea (46%N) at the rate of 100 kg urea/hectare (50% of the recommended dose) was applied in two equal doses in which the first dose was applied before the first irrigation, whereas the second dose was applied before the second irrigation. During the soil preparation, 200 kg/hectare (or 50% of the advised dose) of calcium superphosphate (15.5 percent P2O5) was applied. Along with the initial dosage of nitrogen fertilizer, 50 kg/hectare (or 50% of the necessary dose) of potassium fertilizer in the form of potassium sulfate (48 percent K2O) was administered. Wheat grains were inoculated before sowing with Azotobacter chroococcum bacteria (Biogen), conc.106 cells/mL. Biogein is produced by the Bio-fertilizers Unit, General Organization of Agriculture Equalization Fund, Agricultural Research Centre, Giza, Egypt. Using a mixture of Glomus mosseae, Glomus fusciulatum, and Glomus clarum, the mycorrhizae, or arbuscular mycorrhizae fungi, were replicated in pot cultures with onion and maize cultivated for four months in a 1:1:1 ratio (v:v:v) Vermiculite is perlite.: peat according to Badr El-Din et al. [67]. The growing medium, spores, hyphae, and roughly cut root pieces made up the mycorrhizae inoculums. Mycorrhizae were acquired from the Plant Pathology Research Institute, Agricultural Research Center, Ministry of Agriculture, and Land Reclamation. Prior to planting, wheat grains were coated with each of the product’s mycorrhizae and Azotobacter using a sticking agent (5 percent Arabic gum). Two regimens of organic fertilizer 4% humic acid and 4% fulvic acid were utilized in this study. Additionally, two applications of foliar fertilizers were made: once at the start of spikes and again 30 days later.
Data recording
Plant samples
The first sampling for estimation of crop growth was made 40 days after sowing (DAS). Subsequently, the sampling was made at 14 days intervals. To record the growth parameters, plants from an area of one square meter were harvested at ground level. The fresh weight of the whole sample was recorded, and plants were divided into their component fractions (leaves, stem, and spikes when they appeared) and weighed in fresh status. A subsample of 10 g from each fraction was taken and dried in the electrical oven maintaining a constant temperature of ± 70 °C to get constant weight. Crop growth rate (CGR) was determined based on seasonal growth data using the formulae suggested by [68] and opted by Rafiq et al. [69], and values are shown in g m−2 d−1:
Wt1 and Wt2 are the total dry weights of samples (g m−2) at the first and second sampling, and T2 and T1 are the duration (days) between the two sampling dates.
Chlorophyll content (SPAD) measurements were taken from the base, the middle, and the tip of the flag leaf on each tiller three times during each growing season [70]. Chlorophyll concentration was calculated by averaging SPAD data (Konica Minolta Optics Inc., Tokyo, Japan). The height (in cm) of 10 plants from the ground up in each plot were measured at harvest. The lengths of ten randomly selected spikes were measured (in centimeters). By counting the amount of fertile and sterile spikelets on 10 randomly selected spikes, the NSS was computed. The term "1000 grain weight" refers to the weight of 1000 grains (TGW, g). After being harvested, each plot's worth of grain was packed, threshed, and measured in tons per hectare (GY). Straw yield (SY) was calculated by weighing the straw that was collected from a given subplot after threshing and then converting that weight to tons per hectare. Before threshing, plants were collected from a designated area within each subplot, and their total weight was recorded as the biological yield (BY), expressed as tons per hectare (ton ha 1). The Harvest index (HI) was calculated according to the following formula
Qualitative analysis
Various quality characteristics were determined by the methods described by the American Association of Cereal Chemists [71] and [72]. Grain samples were taken from each treatment, and N, P, and K percentages were determined, grains were dried then, they were crushed and stored for further analysis. A 0.5 g of the grains powder was wet-digested with an H2SO4–H2O2 mixture. Using Nessler's approach, total nitrogen in digested plant matter was calculated calorimetrically. The measurement was taken at 420 nm, and N was calculated using the following formula:
The protein content in the wheat grains was calculated using the Formula as follows:
Using a JENWAY 6305 UV/Visible Spectrophotometer at 643 nm (OD643) and the colorimeter technique, grain phosphorus was measured (ammonium molybdate). Using a flame photometer (BWB Model BWB-XP, 5 Channel), the potassium content of seeds was assessed by Motsara and Roy [73].
Starch contents were determined by Omeg Analyzer G, where an 18-mm sample spacer was used to fill wheat grains in a machine sample hopper and digital reading of starch content was noted from the screen display, according to the procedure [72]. Gluten values of grains were estimated by glutomatic 2200 apparatus, by using sodium chloride solution [74].
Data analysis
The general linear model (GLM) algorithm of the SAS 9.4 program for Windows was used to perform the analysis of variance (ANOVA) for all analyzed features [75]. The data were examined at a 0.05 level using Fisher's least significant difference (LSD) test. To show the variety of foliar fertilizer and biofertilizer applications, boxplots were established. The ggplot2 package was used to build boxplots in the R project (version 3.4.5). The relationships between characteristics relating to growth, yield, and biochemical content of kernels were explored using Pearson correlation coefficients.
Results
Response of wheat to individual applications of fertilization
Studied traits performance under biofertilizer inoculation
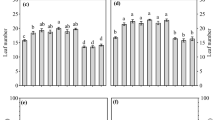
Analysis of variance (ANOVA) for the effect of biofertilizer was shown in Table 2. The results showed that the application of biofertilizer affected highly significantly (p ≤ 0.001) plant height (PH), crop growth rate(GCR), number of spikelets per spike (NSS),1000 grain weight (TGW), biological yield (BY), grain yield (GY), straw yield (SY), harvest index (HI), chlorophyll content (CHL), phosphorous (P), potassium (K), protein content (PC), starch, and gluten content during two experimental seasons. The performance of biofertilizers on yield, growth, and seed biochemical traits is presented in Fig. 1. The application of mycorrhizae combined with azotobacter (mix) recorded the highest value of plant height (100 cm), crop growth rate (18.69 g), number of spikelets per spike (22), biological yield (13.4 ton ha−1), grain yield (5.56 ton ha−1), straw yield (8.21 ton ha−1),), nitrogen (2.07%), phosphorous (0.91%), potassium (1.64%), protein content 12.76%), starch (51.81%), and gluten content (30.90%). The results also showed that the differences between the application of mycorrhizae and azotobacter on chlorophyll content was so slight not enough to be significant, whereas there was no significant difference in grain yield revealed between biofertilizer treatments. Moreover, the obtained results also cleared that the harvest index was greatly increased by the addition of mycorrhizae, whereas the weight of 1000 grain was remarkably enhanced by the application of azotobacter during studying seasons.
Effects of biofertilizer treatments (control, mycorrhizae, azotobacter, and mix) based on information from the seasons of 2020 and 2021 coupled with findings from 15 wheat analyzed qualities identified at field tests. Different lowercase letters on error bars indicate statistically significant differences between treatments (p ≤ 0.05), as performed by the least significant difference (Fisher’s LSD) test. plant height (PH), crop growth rate(CGR), number of spikelets per spike (NSS),1000 grain weight (TGW), biological yield (BY), grain yield (GY), straw yield (SY), harvest index (HI), chlorophyll content (CH), Nitrogen(N), phosphorous (P), potassium (K), protein content (PC), starch, and gluten content
Effect of organic fertilizer on different studied traits
The results in Table 2 indicated that the application of organic fertilizer (humic and fulvic acid) increased significantly (p ≤ 0.001) plant height (PH), crop growth rate(GCR), number of spikelets per spike (NSS),1000 grain weight (TGW), biological yield (BY), grain yield (GY), straw yield (SY), harvest index (HI), chlorophyll content (CHL), phosphorous (P), potassium (K), protein content (PC), starch, and gluten content in the two seasons.
The results exhibited in Fig. 2 showed that the application of humic acid increased markedly PH (93.5 cm), CGR ( 15.19 g day−1 m−2, respectively), TGW (51.12 g,), BY( 11.80 ton ha−1), GY (5.21 ton ha−1), SY (6.53 ton ha−1), HI (46.11%), protein content (11.8%), K (1.56%), P (0.84%), N (1.71%), chlorophyll content (46.48 SPAD), starch (56.72%), and gluten (29.45%) compared to control and the influence of humic was higher than that of fulvic acid.
Effects of organic fertilizer treatments (control, Humic, and fulvic) on 15 studied traits for wheat were determined at field experiments. Combined analysis of 2 successive seasons of 20/21 and 2021/22. Different lowercase letters on error bars indicate statistically significant differences between treatments (p ≤ 0.05), as performed by the least significant difference (Fisher’s LSD) test. plant height (PH), crop growth rate(CGR), number of spikelets per spike (NSS),1000 grain weight (TGW), biological yield (BY), grain yield (GY), straw yield (SY), harvest index (HI), chlorophyll content (CH), Nitrogen(N), phosphorous (P), potassium (K), protein content (PC), starch, and gluten content
Response of wheat to the interaction between biofertilizers and organic fertilizer treatments
Yield and yield component traits
The combined application of bio and organic fertilizers increased greatly the yield and yield components during the two experimental seasons (Table 2). The response of yield and yield components traits to the interaction between biofertilizers and organic fertilizer is shown in Table 3. From the results in Table 3, it could be noticed that the application of humic acid in combination with biofertilizer (Mix) recorded the highest NSS (24.05, and 24.30) in the first and second seasons, respectively over control which exhibited (15.95) in the 2020/21 season, while application of fulvic indicated the lowest NSS (16.10) in the 2021/22 season.
The application of humic acid in combination with biofertilizer (mix) enhanced markedly BY (15.48, and 15.63 ton ha−1), SY (10.61 and 10.73 ton ha−1), and TGW (58.71 and 59.30 g) in the two seasons of study, respectively. In contrast, the lowest values of BY (7.37 and 7.40ton ha−1), SY (3.03, and 3.06 ton ha−1), and TGW (36.96 and 35.65 g) were obtained by control treatment in the two seasons. For GY, the highest value (6.27, and 6.33 ton/ha) was obtained when the azotobacter inoculation along with control in both seasons. The application of fulvic acid combined with mycorrhizae gave the highest value for HI (52.02, and 52.56%) as compared to the application of fulvic acid, which gave the lowest values (26.25 and 26.53%) when it was applied individually in both seasons.
Growth parameters
The combined application of biofertilization and organic fertilizer positively influenced growth parameters (Table 4). The highest value was recorded by the application of humic with a combination of in both seasons. The combined application of humic acid and biofertilizer significantly increased PH (105 and 102 cm) and CGR (24.05, and 24.83), while the lowest plant height(76.19, and 76.22 cm) was observed by control and fulvic acid treatment in both study seasons. Besides, the control treatment gave the lowest values from CGR. Moreover, chlorophyll content was markedly enhanced by the inoculation of wheat seeds by mycorrhizae combined with fulvic acid (50.82 and 51.33) in the two study seasons.
Biochemical composition (NPK, protein, Starch, and Gluten)
The combined application of biofertilization and organic fertilizer had a highly significant effect (p ≤ 0.01) on all seed biochemical traits in both seasons of study (Table 2).
The difference in the performance of wheat grains’ biochemical composition under various regimes of biofertilizers combined with different organic fertilizers is shown in Table 5. The application of biofertilizer and humic acid increased statistically nitrogen content (2.09, and 2.11%), grain starch (60.33 and 60.93), and potassium content (1.73 and 1.76%) in both seasons of the study. Regarding phosphorus content, it was remarkably enhanced by the application of mycorrhiza with humic acid exhibiting the highest level of P (0.98 and 1%) in both seasons, followed by the individual application of mycorrhiza. For gluten, fulvic acid with MIX treatment exhibited the highest values of (31.99, and 32.33) while the lowest values were observed by control treatment in the two seasons. protein content was not influenced significantly by the interaction of bio-organic fertilization in both growing seasons.
Correlation between studied traits
Correlation analysis among all 15 examined attributes showed strong positive correlations (Fig. 3). Amongst the yield trait pairs, the correlation between SY and BY (0.55), TGW and GY(0.55), SY and NSS (0.47), BY and NSS(0.66) were greatest, while the least correlation was observed between BY and NSS(0.39). Also, correlations among the seed biochemical traits were significantly positive, whereas other pairs of traits showed non-significant correlations, including N with each of TGW, GY, chlorophyll, Gluten, and SY (Fig. 3). Among the growth traits, the correlation between PH and CHL (0.58), followed by CGR and PH (0.89), and CHL and CGR (0.61) showed the highest significant positive coefficients (Fig. 3). As regards the correlation among TGW shows a non-significant correlation with other traits except grain yield and biological yield. As regards the correlation among different types of traits, including yield, growth, and seed biochemical parameters, highly significant positive correlations were exhibited between P and NSS (0.79), K and NSS (0.59), N and NSS (0.50), Gluten and Hi (0.87), PC and HI (0.62) (Fig. 3). protein content has a highly significant correlation with SY (0.78), and BY (0.78).
Pearson's correlation coefficients among 15 studied traits under different bio-fertilizers and organic fertilizers(Combined analysis of 2 successive seasons of 20/21 and 2021/22). plant height (PH), crop growth rate(GCR), number of spikelets per spike (NSS),1000 grain weight (TGW), biological yield (BY), grain yield (GY), straw yield (SY), harvest index (HI), chlorophyll content (CHL), nitrogen (N), phosphorous (P), potassium (K), protein content (PC), starch, and gluten
Discussion
From the obtained results, it was clear that the inoculation of wheat plants with mycorrhiza or azotobacter individually or in combination significantly enhanced the total chlorophyll, dry weight, and crop growth rate of wheat plants (Fig. 1). These results are in the same trend as the previous findings of [76,77,78,79], they reported that mycorrhizae could assist in the intake of nutrients and consequently the yield. Moreover, mycorrhizae improved the plant's capacity in absorbing minerals; N, Ca, Mg, Fe, Cu, and Mn under salinity stress [80]. Furthermore, there was a relatively significant increase in the plant height in the tested plants [81], due to the inoculation of mycorrhiza fungi, they demonstrated higher overall morphological characteristics in soybean plants. In addition, the wheat plants significantly outperformed the control plants in terms of the number of spikelets per spike, 100-grain weight, biological yield, and grain yield. In addition, a high wheat yield in plants treated with a mixture of biofertilizers may be caused by a greater moisture content, which helps to boost the nutrient supply to plants and, as a result, raises the total yield [82]. Besides, the inoculation with Arbuscular mycorrhizae helps relieve the undesirable impacts of salinity on wheat, as well as lowers the sodium uptake, whereas it raised the availability of nitrogen, phosphorous, potassium, and magnesium and stimulated the photosynthesis process, chlorophyll, carbohydrates, and protein and thus the productivity [83, 84]. Also, it can help plants to survive drought [85] and can increase stomatal conductance, cellular and plant growth [86], and raise water uptake [87]. The association of AMF with the plant could increase the soil exploration capacity and nutritional status by increasing the absorption of potassium and reducing the Na+/K+ ratio and avoiding damaging the soil biological system [88]. Mycorrhizae raise the plant nutrient intake, and soil fertility relieves the side effect of salinity, minimizes the chemical inputs, and helps the plants to overcome the water shortage, and phytotoxic elements [89].
Azotobacter species are largely related to the composition of numerous hormones such as gibberellin, auxin, and cytokinin [90]. It could raise the wheat germination rate from 20 to 30% and associate with the absorption of nitrogen, phosphorous, iron, and zinc [91]. Azotobacter chroococcum could encourage wheat growth development and its element absorption [92]. Azotobacter spp. could induce growth and crop productivity by activating the synthesis of biological materials, encouraging the rhizosphere microbes, producing phytopathogenic controllers, and increasing the elements absorption and nitrogen fixation [93]. Azotobacter bacteria might fix about 20 kg N a year, so it could assist in ameliorating crop production [94, 95] and can minimize the demand for nitrogen fertilizers up to fifty percent [96]. Inculcation of the root with Azotobacter chroococcum increased the root system and the production of indole acetic acid [97]. Besides, inoculation of strawberries with Azotobacter spp. induced the leaf total chlorophyll content [98], improved plant nutrition, and amelioration soil fertility [99, 100].
According to the obtained results in the current study, it was obvious that humic acid application improved the growth performance, yield, and yield components of wheat. These results were previously confirmed by the findings of Muscolo et al. [90] they reported that humic acid can raise the elements absorption efficacy, and gas exchange rate in the soil, as well as can arrange the rate of stomata conductance and photosynthesis process in the plants. Moreover, humic substances have a positive impact on plant nutrition by improving N, P, mg, and Ca uptake, and thus consequently it increases the yield [101]. Besides, humic acid contains numerous nutrients that assist in improving soil fertility [102,103,104,105]. As humic acid can change positively the soil composition and its physical characteristics, so it can ameliorate plant growth and productivity by raising the chelation and availability of numerous nutrients [106,107,108,109,110]. It was noticed by many authors that humic acid increased beneficial microbes in the soil [111], and improved the efficiency of the used fertilizers and soil airing, so it can help in developing plant growth [112]. Potassium humate can affect positively on developing the growth, productivity, and fruit chemical composition of wheat [113]. Merwad [114] documented that humic acid can increase the absorption of NPK in wheat under salinity stress.
Fulvic acid can attract water and facilitate the mobility of nutrients like Ca, Mg, Fe, Cu, and Zn to the plants roots [45, 55]. As fulvic acid assists the transferring the elements into the plant cell, chlorophyll content, photosynthesis process rate, and minimizing the stomatal conductance and the transpiration conductance, it is considered a plant growth regulator [62, 63], and its effect is like to the influence of cytokinin, auxin and gerbilline [53, 115, 116]. Besides, it also helps in chelating mineral nutrients and increases their absorption and photosynthesis process [117,118,119], increasing antioxidants, gibberellic acid, cytokines and vitamins, therefore it progress the plant growth development [119,120,121]. Priya et al. [122] reported that applying fulvic acid can raise the intake of K and therefore it can improve the starch metabolism.
A considerable increase in plant height, crop growth rate, 100-grain weight, grain NPK content, gluten, starch, and protein were discovered in this study when mycorrhiza and azotobacter were combined with humic acid (Tables 3, 4 and 5). This result might perhaps be explained by the fact that mycorrhiza stimulates plant growth and the absorption of various crucial nutrients, such as nitrogen and phosphorus, under challenging environments. Mycorrhiza's widespread distribution throughout the coating system is responsible for this growth promotion [58]. A prior study found that the greatest harvest index was obtained when organic and biofertilizers were applied together, but there were no statistically significant differences between nitrogen applied as a biofertilizer and nitrogen applied as a chemical fertilizer [123]. When organic and biofertilizers are used in conjunction with fennel, the harvest index of fennel is lowered when compared to the control [124], which is not consistent with the findings of the current research.
Conclusions
The current study showed that bio and organic fertilizers significantly impact the growth, yield, and grain biochemical composition of wheat plants. The best results were seen with a combination of biofertilizers and humic acid, with increased plant height, growth rate, yield, and biochemical composition. This improvement can be attributed to the better plant nutrition and nutrient efficiency provided by organic fertilizers and their synergistic effects with biofertilizers. Using humic acid in conjunction with azotobacter and mycorrhiza is a promising approach for improving wheat yields and quality.
Availability of data and materials
The data can be made available upon reasonable request from the Corresponding author.
References
Marris E. Agronomy: five crop researchers who could change the world. J Nature. 2008;456(7222):563–9.
Dixon J, Braun HJ, Kosina P, Crouch JH, editors. Wheat facts and futures 2009. Cimmyt; 2009.
Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. J Plant soil. 2003;255(2):571–86.
Khan M, et al. Fertilizers and their contaminants in soils, surface and groundwater. Encycl Anthr. 2018;5:225–40.
Esitken A, Ercisli S, Karlidag H, Sahin F. Potential use of plant growth promoting rhizobacteria (PGPR) in organic apricot production. In: Proceedings of the international scientific conference: Environmentally friendly fruit growing, Polli, Estonia, 7-9 September, 2005. Tartu University Press; 2005. p. 90–7.
Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant soil science. 2003;255(2):571–86.
Olivares FL, et al. Plant growth promoting bacteria and humic substances: crop promotion and mechanisms of action. Chem Biol Technol Agric Chem. 2017;4(1):1–13.
Adesemoye AO, Kloepper JW. Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol. 2009;85(1):1–12.
Ekin Z. Integrated use of humic acid and plant growth promoting rhizobacteria to ensure higher potato productivity in sustainable agriculture. Sustainability. 2019;11(12):3417.
Subba Roa NS. Soil microbiology. Oxford: IBH Publ; 1997.
AlhajHamoud Y, et al. Impact of alternative wetting and soil drying and soil clay content on the morphological and physiological traits of rice roots and their relationships to yield and nutrient use-efficiency. J Agric Water Manag. 2019;223(C):1–1.
AlhajHamoud Y, et al. Effect of irrigation regimes and soil texture on the potassium utilization efficiency of rice. J Agronomy. 2019;9(2):100.
Jeffries P, et al. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. J Biol Fertil Soils. 2003;37(1):1–16.
Wu SC, et al. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. J Geoderma. 2005;125(1–2):155–66.
Smit S, Read D. Mycorrhizal Symbiosis. 3rd ed. New York, London, Burlington, San Diego: Elsevier and Academic; 2008.
Smith FA, Smith SEJ. What is the significance of the arbuscular mycorrhizal colonisation of many economically important crop plants? Plant Soil. 2011;348(1):63–79.
Hijri M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza. 2016;26(3):209–14.
Zhang L, Feng G, Declerck S. Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 2018;12(10):2339–51.
Augé RM, Toler HD, Saxton AM. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza. 2015;25(1):13–24.
Rillig MC, et al. Towards an integrated mycorrhizal technology: harnessing mycorrhiza for sustainable intensification in agriculture. Front Plant Sci. 2016;7:1625.
Igiehon NO, Babalola OO. Biofertilizers and sustainable agriculture: exploring arbuscular mycorrhizal fungi. Appl Microbiol Biotechnol. 2017;101(12):4871–81.
Thirkell TJ, et al. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J Ecol. 2017;105(4):921–9.
Püschel D, et al. Arbuscular mycorrhiza stimulates biological nitrogen fixation in two Medicago spp. through improved phosphorus acquisition. Front Plant Sci. 2017;8:390.
Ji L, Tan W, Chen X. Arbuscular mycorrhizal mycelial networks and glomalin-related soil protein increase soil aggregation in Calcaric Regosol under well-watered and drought stress conditions. Soil Tillage Res. 2019;185:1–8.
Wilkinson TD, et al. Aphids can acquire the nitrogen delivered to plants by arbuscular mycorrhizal fungi. Funct Ecol. 2019;33(4):576–86.
Quiroga G, et al. The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant Cell Environ Exp Bot. 2019;42(7):2274–90.
Pons C, et al. Effects of drought and mycorrhiza on wheat and aphid infestation. Ecol Evol. 2020;10(19):10481–91.
Aquilanti L, Favilli F, Clementi F. Comparison of different strategies for isolation and preliminary identification of Azotobacter from soil samples. Soil Biol Biochem. 2004;36(9):1475–83.
Palanché T, et al. Bacterial Iron Transport: Coordination Properties of Azotobactin, the Highly Fluorescent Siderophore of Azotobacter v inelandii. Inorg Chem. 2004;43(3):1137–52.
Youssef MA, et al. Exogenously applied nitrogenous fertilizers and effective microorganisms improve plant growth of stevia (Stevia rebaudiana Bertoni) and soil fertility. J AMB Express. 2021;11(1):1–10.
Prajapati K, Yami K, Singh A. Plant growth promotional effect of Azotobacter chroococcum, Piriformospora indica and vermicompost on rice plant. Nepal J Sci Technol. 2008;9:85–90.
Verma S, Kumar V, Narula N, Merbach W. Studies on in vitro production of antimicrobial substances by Azotobacter chroococcum isolates/mutants/In vitro-Produktion von antimikrobiellen Substanzen durch Azotobacter chroococcum-Isolate/Mutanten. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/J Plant Dis Prot. 2001:152–65.
Sindhu SS, Rakshiya YS, Sahu G. Biological control of soilborne plant pathogens with rhizosphere bacteria. Pest Technol. 2009;3(1):10–21.
Wu CH, et al. Developing microbe–plant interactions for applications in plant-growth promotion and disease control, production of useful compounds, remediation and carbon sequestration. Microb Biotechnol. 2009;2(4):428–40.
Gauri SS, Mandal SM, Pati BR. Impact of Azotobacter exopolysaccharides on sustainable agriculture. Appl Microbiol Biotechnol. 2012;95(2):331–8.
El-Sorady GA, et al. Response of Bread Wheat Cultivars Inoculated with Azotobacter Species under Different Nitrogen Application Rates. J Sustainability. 2022;14(14):8394.
Elmardy NA, et al. Photosynthetic performance of rocket (Eruca sativa. Mill.) grown under different regimes of light intensity, quality, and photoperiod. J PLos One. 2021;16(9):e0257745.
Mahato S, Kafle A. Comparative study of Azotobacter with or without other fertilizers on growth and yield of wheat in Western hills of Nepal. Ann Agrar Sci. 2018;16(3):250–6.
Verma JP, et al. Evaluation of plant growth promoting activities of microbial strains and their effect on growth and yield of chickpea (Cicer arietinum L.) in India. Soil Biol Biochem. 2014;70:33–7.
Kyaw EP, et al. Study on plant growth promoting activities of Azotobacter isolates for sustainable agriculture in Myanmar. Biotech Biores. 2019;1(5):1–6.
Wani SA, et al. Azotobacter chroococcum–a potential biofertilizer in agriculture: an overview. Soil science: agricultural environmental prospectives. 2016. p. 333–48.
Sumbul A, et al. Azotobacter: a potential bio-fertilizer for soil and plant health management. Saudi J Biol Sci. 2020;27(12):3634–40.
Atia MA, Shaban KA, Sallam AM. Role of Humic, Ascorbic Acids with or without Compost to Improve Nutrients Content, Yield Components, and Seed Quality of Sesame. J Soil Sci Agric Eng. 2014;5(7):1049–66.
Ahmad T, Khan R, Nawaz Khattak T. Effect of humic acid and fulvic acid based liquid and foliar fertilizers on the yield of wheat crop. J Plant Nutr. 2018;41(19):2438–45.
Canellas LP, et al. Humic and fulvic acids as biostimulants in horticulture. Sci Hortic. 2015;196:15–27.
Gomaa M, et al. Impact of humic acid application on productivity of some maize hybrids under water stress conditions. J Middle East J Appl Sci. 2014;4(3):668–73.
Moghadam HRT, Khamene MK, Zahedi H. Effect of humic acid foliar application on growth and quantity of corn in irrigation withholding at different growth stages. J Maydica. 2014;59(2):124–8.
Cordeiro FC, et al. Humic acid effect on catalase activity and the generation of reactive oxygen species in corn (Zea mays). Biosci Biotechnol Biochem. 2011;75(1):70–4.
Ali S, et al. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Pollut Res. 2015;22(14):10601–9.
Szczerski C, et al. Short-and long-term effects of modified humic substances on soil evolution and plant growth in gold mine tailings. Wat, Air, Soil Poll. 2013;224(3):1–14.
Haider G, et al. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil Biol Biochem. 2015;395(1):141–57.
Dinçsoy M, Sönmez F. The effect of potassium and humic acid applications on yield and nutrient contents of wheat (Triticum aestivum L. var. Delfii) with same soil properties. J Plant Nutr. 2019;42(20):2757–72.
Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12(7):908–31.
Manaf A, et al. Interactive effect of zinc fertilization and cultivar on yield and nutritional attributes of canola (Brassica napus L). J Soil Sci Plant Nutr. 2019;19(3):671–7.
Bocanegra M, Lobartini JC, Orioli GA. Plant uptake of iron chelated by humic acids of different molecular weights. Commun Soil Sci Plant Anal. 2006;37(1–2):239–48.
Chen Y, Nobili MD, Aviad T. Stimulatory effects of humic substances on plant growth. Soil organic matter in sustainable agriculture. 2004. p. 103–29.
Zimmerli L, et al. The xenobiotic β-aminobutyric acid enhances Arabidopsis thermotolerance. Plant J. 2008;53(1):144–56.
Aminifard M, et al. Fulvic acid affects pepper antioxidant activity and fruit quality. Afr J Biotech. 2012;11(68):13179–85.
Dobbss L, et al. Changes in root development of Arabidopsis promoted by organic matter from oxisols. Ann Appl Biol. 2007;151(2):199–211.
El-Helaly M. Effect of foliar application of humic and fulvic acids on yield and its components of some carrot (Daucus carota l.) cultivars. J Hortic Sci Ornam Plants. 2018;10(3):159–66.
Eyheraguibel B, Silvestre J, Morard P. Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Biores Technol. 2008;99(10):4206–12.
Anjum S, et al. Fulvic acid application improves the maize performance under well-watered and drought conditions. J Agron Crop Sci. 2011;197(6):409–17.
Huang S, Xiong B, Sun G, He S, Liao L, Wang J, et al. Effects of fulvic acid on photosynthetic characteristics of citrus seedlings under drought stress. InIOP Conf Ser Earth Environ Sci. 2020;474(3):032007. IOP Publishing.
Yildirim E, et al. Humic+ Fulvic acid mitigated Cd adverse effects on plant growth, physiology and biochemical properties of garden cress. J Sci Rep. 2021;11(1):1–8.
Ali EF, et al. Ginger extract and fulvic acid foliar applications as novel practical approaches to improve the growth and productivity of Damask Rose. J Plants. 2022;11(3):412.
Nelson DA, Sommers LE. Total carbon, organic carbon, and organic matter. J Methods Soil Anal: Part 2 Chem Microbiol Properties. 1983;9:539–79.
Badr El-Din SM, Attia M, Abo-Sedera S. Field assessment of composts produced by highly effective cellulolytic microorganisms. J Biol Fertil Soils. 2000;32(1):35–40.
Hunt R. Plant growth analysis: the rationale behind the use of the fitted mathematical function. J Ann Bot. 1979;43(2):245–9.
Spiertz J, De Vos N. Agronomical and physiological aspects of the role of nitrogen in yield formation of cereals. J Plant soil. 1983;75(3):379–91.
Liu XJ, et al. Physiological response of flag leaf and yield formation of winter wheat under different spring restrictive irrigation regimes in the Haihe Plain, China. J Integr Agric. 2021;20(9):2343–59.
Borrás L, Slafer GA, Otegui ME. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: a quantitative reappraisal. J Field Crops Res. 2004;86(2–3):131–46.
Taylor S, Payton M, Raun W. Relationship between mean yield, coefficient of variation, mean square error, and plot size in wheat field experiments. J Commun Soil Sci Plant Anal. 1999;30(9–10):1439–47.
Motsara MR. Guide to laboratory establishment for plant nutrient analysis. Scientific Publishers; 2015.
Zörb C, et al. Quantitative proteome analysis of wheat gluten as influenced by N and S nutrition. J Plant Soil. 2010;327(1):225–34.
SAS, SAS Institute. The SAS system for Windows. Release 9.4. 2013.
Smith SE, Smith AF. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol. 2016;62:227–50.
Bucher M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2007;173(1):11–26.
Pellegrino E, et al. Responses of wheat to arbuscular mycorrhizal fungi: a meta-analysis of field studies from 1975 to 2013. Soil Biol Biochem. 2015;84:210–7.
Porcel R, Aroca R, Ruiz-Lozano JM. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustain Dev. 2012;32(1):181–200.
Mardukhi B, et al. Arbuscular mycorrhizas enhance nutrient uptake in different wheat genotypes at high salinity levels under field and greenhouse conditions. CR Biol. 2011;334(7):564–71.
Copetta A, Lingua G, Berta G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. J Mycorrhiza. 2006;16(7):485–94.
Singh A. Mineral nutrition of groundnut. J Adv Plant Physiol. 1999;2:161–200.
Talaat NB, Shawky BT. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot. 2014;98:20–31.
Abdel-Fattah GM, Asrar A-WA. Arbuscular mycorrhizal fungal application to improve growth and tolerance of wheat (Triticum aestivum L) plants grown in saline soil. Acta Physiol Plant. 2012;34(1):267–77.
Ruiz-Lozano JM, et al. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016;39(2):441–52.
Dar ZM, et al. Review on arbuscular mycorrhizal fungi: an approach to overcome drought adversities in plants. Int J Curr Microbiol Appl Sci. 2018;7:1040–9.
Symanczik S, et al. Effects of two contrasted arbuscular mycorrhizal fungal isolates on nutrient uptake by Sorghum bicolor under drought. Mycorrhiza. 2018;28(8):779–85.
Evelin H, et al. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Front Plant Sci. 2019;10:470.
Ganugi P, et al. A review of studies from the last twenty years on plant–arbuscular mycorrhizal fungi associations and their uses for wheat crops. Agronomy. 2019;9(12):840.
Kukreja K, et al. Phytohormone production by Azotobacter-a review. Agric Rev-Agric Res Commun Centre India. 2004;25(1):70–5.
Chen JH. The combined use of chemical and organic fertilizers and/or biofertilizer for crop growth and soil fertility. In: International workshop on sustained management of the soil-rhizosphere system for efficient crop production and fertilizer use, vol. 16, no. 20. Bangkok: Land Development Department; 2006. p. 1–11.
Khan M, Zaidi A. Synergistic effects of the inoculation with plant growth-promoting rhizobacteria and an arbuscular mycorrhizal fungus on the performance of wheat. Turk J Agric For. 2007;31(6):355–62.
Lenart A. Occurrence, characteristics, and genetic diversity of Azotobacter chroococcum in various soils of Southern Poland. Pol J Environ Stud. 2012;21(2).
Kizilkaya R. Nitrogen fixation capacity of Azotobacter spp. strains isolated from soils in different ecosystems and relationship between them and the microbiological properties of soils. Environ Biol. 2009;30(1):73–82.
Esmailpour A, Hassanzadehdelouei M, Madani A. Impact of livestock manure, nitrogen and biofertilizer (Azotobacter) on yield and yield components wheat (Triticum Aestivum L.). Cercet Agron Mold. 2013;46(2):5–15.
Romero-Perdomo F, et al. Azotobacter chroococcum as a potentially useful bacterial biofertilizer for cotton (Gossypium hirsutum): Effect in reducing N fertilization. Rev Argent Microbiol. 2017;49(4):377–83.
Duca D, et al. Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek. 2014;106(1):85–125.
Rueda D, et al. Effect of Azospirillum spp. and Azotobacter spp. on the growth and yield of strawberry (Fragaria vesca) in hydroponic system under different nitrogen levels. J Appl Pharm Sci. 2016;6(1):048–54.
Kurrey DK, et al. Effect of Azotobacter on physio-chemical characteristics of soil in onion field. Pharma Inn J. 2018;7(2):108–13.
Gothandapani S, Sekar S, Padaria JC. Azotobacter chroococcum: utilization and potential use for agricultural crop production: an overview. Int J Adv Res Biol Sci. 2017;4(3):35–42.
Shaaban S, Manal F, Afifi M. Humic acid foliar application to minimize soil applied fertilization of surface-irrigated wheat. World J Agric Sci. 2009;5(2):207–10.
Yildirim E. Foliar and soil fertilization of humic acid affect productivity and quality of tomato. Acta Agric Scand Section B-Soil Plant Sci. 2007;57(2):182–6.
Fahramand M, Moradi H, Noori M, Sobhkhizi A, Adibian M, Abdollahi S, et al. Influence of humic acid on increase yield of plants and soil properties. Int J Farming Allied Sci. 2014;3(3):339–41.
Canellas LP, Olivares FL. Physiological responses to humic substances as plant growth promoter. Chem Biol Technol Agric. 2014;1(1):1–11.
Khaled H, Fawy HA. Effect of different levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 2011;6(1):21–9.
Szczepanek M, Wilczewski E. Effect of humic substances on germination of wheat and barley under laboratory conditions. Acta Sci Polonorum Agric. 2011;10(1).
Khattab MM, et al. Effect of humic acid and amino acids on pomegranate trees under deficit irrigation. I: Growth, flowering and fruiting. J Hortic Sci Ornam Plants. 2012;4(3):253–9.
Cavalcante I, et al. Foliar spray of humic substances on seedling production of yellow passion fruit. J Food Agric Environ Exp Bot. 2013;11(2):301–4.
Mosa W, El-Megeed N, Paszt LS. The effect of the foliar application of potassium, calcium, boron and humic acid on vegetative growth, fruit set, leaf mineral, yield and fruit quality of’Anna’apple trees. Am J Exp Agric. 2015;8(4):224–34.
Shiva KN, Srinivasan V, Zachariah TJ, Leela NK. Integrated nutrient management on growth, yield and quality of paprika alike chillies (Capsicum annuum L.). J Spices Aromatic Crops. 2015;24(2).
Puglisi E, et al. Rhizosphere microbial diversity as influenced by humic substance amendments and chemical composition of rhizodeposits. J Geochem Explor. 2013;129:82–94.
Canellas LP, Olivares FL, Aguiar NO, Jones DL, Nebbioso A, Mazzei P, et al. Humic and fulvic acids as biostimulants in horticulture. Sci Hortic. 2015;30(196):15–27.
Kandil A, et al. Role of humic acid and amino acids in limiting loss of nitrogen fertilizer and increasing productivity of some wheat cultivars grown under newly reclaimed sandy soil. Int J Adv Res Biol Sci. 2016;3(4):123–36.
Merwad AR. Using humic substances and foliar spray with moringa leaf extract to alleviate salinity stress on wheat. Sustainability of Agricultural Environment in Egypt: Part II, 2018: p. 265-286.
El Hamied SAA. Improving growth and productivity of “Sukkary” mango trees grown in North Sinai using extracts of some brown marine algae, yeasts and effective microorganisms 2-Productivity and fruit quality. Middle East J Agric Res. 2014;3(2):318–29.
Samavat S, Samavat S. The effects of fulvic acid and sugar cane molasses on yield and qualities of tomato. Int Res J Appl Basic Sci. 2014;8(3):266–8.
Yang S, et al. Effect of fulvic acid on the phosphorus availability in acid soil. J Soil Sci Plant Nutr. 2013;13(3):526–33.
Lotfi R, et al. Physiological responses of Brassica napus to fulvic acid under water stress: Chlorophyll a fluorescence and antioxidant enzyme activity. Crop J. 2015;3(5):434–9.
Wang Y, et al. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol Environ Saf. 2019;167:10–9.
Abd El-Hameed M, et al. Reducing mineral N fertilizer partially in Thompson seedless vineyards by using fulvic acid and effective microorganisms. World Rural Obser. 2014;6(4):36–42.
Justi, M., E.G. Morais, and C.A. Silva, Fulvic acid in foliar spray is more effective than humic acid via soil in improving coffee seedlings growth. Archives of Agronomy Soil science: agricultural environmental prospectives, 2019.
Priya B, et al. Fulvic acid (FA) for enhanced nutrient uptake and growth: insights from biochemical and genomic studies. J Crop Improv. 2014;28(6):740–57.
Naik PS, Chanemougasoundharam A, Khurana SP, Kalloo G. Genetic manipulation of carotenoid pathway in higher plants. Curr Sci. 2003:1423–30.
Kapoor R, Giri B, Mukerji KG. Improved growth and essential oil yield and quality in Foeniculum vulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. J Bioresour Technol. 2004;93(3):307–11.
Acknowledgements
Authors would like to acknowledge their universities for supporting the research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, S.F.L, A.I, and W.F.A.M.; methodology, S.F.L.; software, S.F.L.; validation, W.F.A.M.; formal analysis W.F.A.M, AND A.I; investigation, S.F.L.; resources, S.F.L.; data curation W.F.A.M; writing—original draft preparation, S.F.L., and W.F.A.M.; writing—review and editing, S.F.L., A.I, and, W.F.A.M; visualization, W.F.A.M.; supervision, W.F.A.M.; project administration, S.F.L; funding acquisition, S.F.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human or animal subjects. The current experimental research and field study including plant material, is comply with relevant institutional, national, and international guidelines and legislation and used for research and development.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lamlom, S.F., Irshad, A. & Mosa, W.F.A. The biological and biochemical composition of wheat (Triticum aestivum) as affected by the bio and organic fertilizers. BMC Plant Biol 23, 111 (2023). https://doi.org/10.1186/s12870-023-04120-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04120-2