Abstract
Background
Zinc is one of the essential trace elements in plants. There are few studies on the phytohormone to rescue the toxicity of excessive zinc to plants. The aim of this research was to evaluate the alleviating effects of brassinosteroids (BR) and gibberellic acid (GA) on the toxicity of Medicago sativa L. (M. sativa) induced by excessive zinc.
Results
After zinc, BR and GA were applied to M. sativa seedlings for 7 weeks, their physiological and biochemical properties and gene expression patterns were evaluated. BR and GA significantly weakened the inhibition effect of zinc stress on growth and biomass of M. sativa. Under zinc stress, the zinc accumulation in M. sativa roots was over 5 times that in shoots. Application of BR and GA reduced zinc accumulation in roots. The content of lipid peroxides in M. sativa decreased and the activity of antioxidant enzymes increased under BR and GA treatments. In addition, BR and GA treatment down-regulated the transcription level of MsZIP1/3/5, the transporters of zinc uptake in root cells. And BR and GA up-regulated the expressions of zinc efflux, chelation, vacuolar storage and long-distance transport related genes: MsZIP7, MsHMA1, MsZIF1, MsMTP1, MsYSL1 and MsNAS1.
Conclusions
Our findings further showed that BR and GA application to M. sativa under zinc stress can reduce zinc accumulation, promote the response of the antioxidant defense system, and actively regulate the mechanism of heavy metal detoxification. Notably, 100 nM BR performed slightly better than 100 nM GA in all aspects of the detoxification of M. sativa by excessive zinc.
Similar content being viewed by others
Introduction
Zinc (Zn) is an essential trace element in plants and a typically transition metal for the synthesis and action of various enzymes, auxin, and chlorophyll [1, 2]. However, excessive Zn can poison plants by inhibiting seed germination and root elongation, reducing photosynthesis and respiration, and damaging plant cell membranes, proteins and genetic material [1, 3,4,5,6]. And plant-enriched Zn can eventually be absorbed through the food chain and harm human health [7]. The continuous development of industrialization and agriculture of human society is accompanied by the emergence of soil heavy metal pollution. Activities such as industrial waste discharge, mining, agricultural use of sludge, and application of Zn-containing fertilizers lead to excessive accumulation of Zn in soil [8, 9]. The alarms of plant Zn toxicity and soil Zn contamination have drawn the attention of the scientific community to measures to mitigate excess Zn-stressed plants. Implementing of artificial auxiliary measures, such as plant hormone application and microbial symbiotic culture, can effectively improve plant tolerance without directly damaging the soil environment.
Medicago sativa L. (M. sativa) is a perennial leguminous with large biomass, high protein and fiber content, and is a high-quality forage grass [10]. Its well-developed roots and ability to fix nitrogen are very conducive to improving soil fertility [10]. Moreover, it is resistant to drought, cold, salt, especially heavy metals, and has strong adaptability. M. sativa is a pioneer plant to repair contaminated soil in mining area [11, 12]. M. sativa can grow in acidic copper mine tailings in arid lands and enrich cuprum (Cu), lead (Pb), cadmium (Cd), Zn, and other heavy metals [13]. M. sativa can also regulate osmotic pressure and redox reaction to activate heavy metal detoxification mechanism to cope with Pb poisoning [14, 15]. The application of sodium bicarbonate and citric acid can significantly improve the hyperaccumulation and purification efficiency of Cd, Cu, mercury (Hg), Pb, and Zn in M. sativa [16]. M. sativa has good tolerance and enrichment ability to heavy metals.
Phytohormone application is considered to be one of the methods to improve plant tolerance to heavy metals [17]. Through the crosstalk of phytohormone, osmotic pressure regulation, oxidative stress, heavy metal transport and other signal transduction pathways, the application of phytohormones can mediate the mechanism of heavy metal detoxification in plants [18]. Gibberellic acid (GA) plays an important role in plant growth and development, including regulating cell elongation and division, breaking dormancy and reproductive development [19]. And now the mechanism of GA alleviating abiotic stress in plants has been gradually revealed. GA could be counteracted the toxicity of Cd and Pb to Vicia faba L. plants [20]. GA can reduce the harmful effect of low concentrations of Pb and Cd to Chlorella vulgaris [21]. The inhibited levels of chromium (VI) on Pisum sativum (L.) seedlings growth, nitrogen assimilation and antioxidant system could be alleviated by GA [22]. Cd stress resulted in in lipid peroxidation and nitric oxide accumulation in Arabidopsis thaliana roots, and GA could effectively reduce them [23].
Brassinosteroids (BR) regulates various physiological functions such as embryogenesis and seed germination, cell division and elongation, differentiation and growth of pollen tubes, and leaf senescence and death [24]. Notably, BR also regulates plant resistance to heavy metal toxicity. BR pre-soaking treatment alleviated the peroxidative caused by Cd and chromium (Cr) in radish (Raphanus sativus L. var. Pusa Chetki) [25]. Exogenous application of BR promoted the development of tomato (Lycopersicon esculentum) seedlings under Cd stress by improving the activities of photosynthetic system and antioxidant system [26]. BR pre-germination treatment improved plant biomass production by blocking Cu uptake and accumulation [27]. At present, GA and BR have mature and common synthetic pathways. Therefore, it is possible to use GA and BR to assist plant remediation of Zn-contaminated soil easily and efficiently.
The effects and coping measures of Zn deficiency on plants have been extensively reported. However, there are few studies on BR and GA on Zn uptake and transport in plants under excessive Zn. With M. sativa as research material, we designed pot experiments, treated seedlings with different Zn concentrations, and applied exogenous GA and BR treatments of the same concentration to observe and measure the physiological and biochemical performance and genes expression patterns, to explore effects of BR and GA on Zn toxicity and transport in plants.
Materials and Methods
Plant materials, Zn treatment and phytohormone application
The M. sativa seeds, cultivar Giulia, were surface-disinfected with 3% sodium hypochlorite for 20 min, and then rinsed with sterile water. The seeds were spread on the sterilized sand, poured into sterile water to cover the surface of the sand, covered with plastic wrap, and placed in a 25°C incubator for dark cultivation. After 3 days, the seeds were germinated, and then transferred to the growth chamber. Growth chamber conditions were 16 h of light (7:00 – 23:00, 25°C, Relative Humidity 60%) and 8 h of darkness (23:00 – 07:00, Relative Humidity 60%, 22°C). When the seedlings had 4 cotyledons, they were transplanted into square pots (7 cm × 7 cm × 7 cm) filled with sterilized sand and began to be irrigated with the completed Hoagland nutrient solution [28]. The control group was treated with nutrient solution with no modification. Zn treatment was changed from 0.8 mM ZnSO47H2O in Hoagland's nutrient solution to 1 mM and 2 mM. The 24-Epibrassinolide (24-epiBL) and GA powder was dissolved in a small amount of anhydrous ethanol, then diluted to 20 mM in sterile water and stored at -20℃. 20 mM 24-epiBL and GA were diluted with nutrient solution to 100 nM respectively to treatment. Zn stress (1 mM or 2 mM) × Phytohormone (BR or GA) two-factor randomized block design was used, with 9 plant biological replicates per treatment.
After 45 days of treatment, M. sativa plants were harvested. And their aboveground and underground biomass were weighed, and then the samples were frozen in liquid nitrogen and transferred to a—80℃ refrigerator for storage.
Biochemical index determination
The samples were dried in an oven at 80°C to constant weight, then weighed (0.1 g or less) and ground to fine powders. Then the samples were completely digested with 5 mL ternary mixtures of HNO3: H2SO4: HClO4 (10:1:4 (V/V/V)). Appropriate amounts of Zn element standard stock solution were diluted step by step to draw a standard curve. The absorption peaks were determined using the inductively coupled plasma atomic emission spectrometer (ICP, 710-ES, Varian, USA). Finally, the Zn concentration of the samples solution was calculated according to the standard curve, and the Zn mass fraction of the samples was calculated based on the mass.
We determined the content of catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), superoxide anion (OFR) and malondialdehyde (MDA) in samples by performing with CAT Kit, POD Kit, SOD Kit, OFR Kit and MDA Kit (Solarbio, Beijing, China) according to the manufacturer's protocol.
Genes expression
Total RNA was isolated by the Plant RNA Kit (Omega Bio-Tek, Georgia, America). cDNA was synthesized from 1 µg of RNA with ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China), and Real-time PCRs were performed using HiScript III RT SuperMix for qPCR (+ gDNA wiper) (Vazyme, Nanjing, China) according to the manufacturer's instructions. The primers design refers to the results of Cardini et al. 's study in 2021 [29], and the detailed primers sequences were presented in table S1.
Statistical analysis
All data were analyzed using SPSS 16 software (SPSS, Chicago, USA) for two-factor analysis of variance (ANOVA) (including Brown-Forsythe test and Bartlett test) and Tukey’s multiple comparisons test. The data were presented as t mean ± standard deviation (SD) of different replicates. And different letters indicate a significant difference at p < 0.05.
Results
BR and GA application improved M. sativa growth under Zn stress
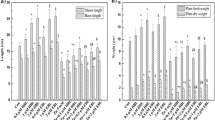
The effect of Zn, BR and GA treatments on M. sativa growth was assessed (Fig. 1). The results showed that Zn treatment (1 mM and 2 mM) had significantly adverse effects on the biomass and phenotype of M. sativa compared to the control group. The biomass under Zn stress, both in roots and shoots, was significantly lower than in untreated plants (Fig. 1A). Moreover, under Zn stress, M. sativa plants type was weaker and smaller, with the old leaves yellowing and wilting, fewer branches, and shorter roots (Fig. 1B).
M. sativa seedlings were treated with Zn, BR and GA for 7weeks. (A) the phenotype of seedlings under different treatments before sampling. Scale bars, 5 cm. (B) The root and shoots biomass of M. sativa upon different treatments as described above. The fresh weight of roots or shoots is expressed as mg/plant. Analysis of variance (ANOVA) (including Brown-Forsythe test and Bartlett test) and Tukey’s multiple comparisons test were performed on the data of 6 biological replicates (n= 6). Vertical bars represent mean ± standard deviation (SD). Averages with the different letter are significantly different at p < 0.05.
Under the treatment of 100 nM BR or GA, the roots and shoots biomass of M. sativa were higher than those of plants under the same Zn stress. In particular, under the same concentration of Zn stress, the shoots biomass treated with 100 nM BR was significantly higher than that treated with 100 nM GA. Consistently, BR and GA showed the same trend for the improvement of M. sativa roots biomass, even though the difference between BR and GA application did not reach the level of significance (Fig. 1B). The application of BR and GA made M. sativa leaves return to green to some extent, and the plant type also partially recovered (Fig. 1A). These results indicated that the application of BR or GA could improve the growth of M. sativa under Zn stress. And under the same Zn stress conditions, the same concentration of BR may have better mitigation effect on plants than GA.
BR and GA application affected Zn uptake and transport in M. sativa shoots and roots
Zn content was measured in the M. sativa roots and shoots after Zn, BR and GA treatments (Fig. 2). In control plants, Zn levels between roots and shoots were similar. The Zn content in M. sativa roots under 2 mM Zn treatment was about 5 times more than that in shoots. And under other treatments, it has reached over 7 times in shoots (Fig. 2). Compared with control plants, Zn content in M. sativa was significantly increased under 2 and 3 mM Zn stress. And the roots Zn content under 3 mM Zn was significantly higher than that under 2 mM Zn. The same trends were also shown in the shoots, but the differences were not significant (Fig. 2). These results suggested that M. sativa mainly enriched Zn in roots to alleviate Zn toxicity in the aboveground tissues to tolerate stress and maintain normal growth.
Effects of Zn, BR and GA application on Zn enrichment and transport in M. sativa. ANOVA (including Brown-Forsythe test and Bartlett test) and Tukey’s multiple comparisons test were performed on the data of 3 biological replicates (n = 3). Vertical bars represent mean ± SD. Averages with the different letter are significantly different at p < 0.05
BR and GA application significantly reduced Zn content in roots under all the Zn treatments. The application of BR and GA also reduced the Zn content in shoots under all the Zn stress, though the difference was not significant under 2 mM Zn stress. Under the same Zn concentration treatment, Zn content in roots and shoots of M. sativa treated with BR was lower than with GA (Fig. 2). These indicated that the application of BR and GA significantly alleviated Zn stress on M. sativa, possibly by reducing roots enrichment. In addition, with the same concentration of BR and GA, the effect of BR on inhibiting excessive Zn accumulation in roots was stronger.
BR and GA application attenuated oxidative stress caused by excess Zn in M. sativa
Lipid peroxides and antioxidant enzymes were regulated by Zn, BR, and GA treatments. Compared with control M. sativa plants, MDA content in roots and shoots increased significantly under 2 mM and 3 mM Zn stress. BR and GA reduced the MDA level of plants under Zn stress, and BR-treated plants had lower MDA content than GA-treated plants, even though these differences were not significant. Moreover, MDA content in roots was higher than shoots under all treatments (Fig. 3A).
Effects of Zn, BR and GA application on peroxide levels in M. sativa. Analysis of MDA content (A), OFR content (B) and its production rate (C) activity in M. sativa roots and shoots after 7 weeks of treatment. ANOVA (including Brown-Forsythe test and Bartlett test) and Tukey’s multiple comparisons test were performed on the data of 3 biological replicates (n = 3). Vertical bars represent mean ± SD. Averages with the different letter are significantly different at p < 0.05
The OFR activity and production rate of M. sativa plants under Zn stress were higher than those of control group. And the OFR activity and production rate of roots treated with 3 mM Zn were significantly different from that of control plants. BR and GA reduced the OFR activity and production rate of Zn-stressed plants to levels that were not significantly different from the control group. A similar pattern of OFR and its production rate was observed in shoots. In particular, the OFR activity difference of the shoots between 2 mM Zn stress and the control group was also significant. In addition, the ORF activity and production rate of plants treated with BR were lower than those treated with GA (Fig. 3 BC).
The effects of Zn stress, BR and GA on M. sativa roots or shoots were similar. 2 mM and 3 mM Zn increased SOD activity of M. sativa, but the difference was not significant compared with the control group. BR and GA increased SOD activity of M. sativa plants under Zn stress. And the SOD activity of M. sativa plants under 3 mM Zn + BR was higher than that of 3 mM Zn + GA (Fig. 4A). The effects of different treatments on POD activity were like that of SOD activity. In particular, under the same Zn concentration, no matter roots or shoots, BR-treated plants showed higher POD activity than GA (Fig. 4B).
Effects of Zn stress, BR and GA application on antioxidant enzyme activity of M. sativa. Analysis of SOD (A), POD (B), and CAT (C) activities in M. sativa roots and shoots after 7 weeks of treatment. ANOVA (including Brown-Forsythe test and Bartlett test) and Tukey’s multiple comparisons test were performed on the data of 3 biological replicates (n = 3). Vertical bars represent mean ± SD. Averages with the different letter are significantly different at p < 0.05
There was no significant difference in CAT activity of M. sativa shoots under different treatments. But, Zn-treated plants shoots CAT activity was higher compared to control. Contrary to the performance of shoot CAT activity, the CAT activity of roots under different treatments showed obvious differences. Under the same Zn concentration, the application of BR and GA increased the CAT activity of roots. It is worth mentioning that the CAT activity of roots was much higher than that of shoots (Fig. 4C).
BR and GA regulated the transcription level of Zn transporters
Zn, BR and GA mediated the expression of M. sativa ZRT-IRT-like protein (MsZIP) related to Zn in and out of cells.
Zn treatments down-regulated MsZIP1, MsZIP3, and MsZIP5 expressions. And this downregulation was reinforced by BR and GA applications. In the control group, the expressions of MsZIP1 were similar in roots and shoots. However, Zn stress significantly down-regulated the expressions of MsZIP1 in roots. Under the same Zn concentration, BR significantly reduced the expressions of MsZIP1 in roots, while GA insignificantly down-regulated its expressions. There was no significant difference in expressions of MsZIP1 in M. sativa shoots under different treatments. And BR and GA down-regulated the expression of MsZIP1 in shoots under 2 mM Zn (Fig. 5A). Different from MsZIP1, the expressions of MsZIP3 were higher in shoots than in roots (Fig. 5B). And, the expressions of MsZIP3 were higher in roots than that in shoots. Compared with the control group, Zn stress, BR and GA did not significantly down-regulate the expressions of MsZIP5 in roots and shoots (Fig. 5C). In contrast, Zn stress upregulated the expressions of MsZIP7, though insignificantly. While this upregulation was reinforced by BR and GA applications. In addition, MsZIP7 expressions in shoots were higher than that in roots (Fig. 5D).
Total RNA was isolated from M. sativa roots and shoots. The MsEF1α from M. sativa was used as the housekeeping gene for normalization. After 7 weeks of treatment, the expressions of MsZIP1/3/5/7 (A-D), that involved in Zn flow in and out of cells, was determined in the roots and shoots of M. sativa. ANOVA (including Brown-Forsythe test and Bartlett test) and Tukey’s multiple comparisons test were performed on the data of 3 biological replicates (n = 3). Vertical bars represent mean ± SD. Averages with the different letter are significantly different at p < 0.05.
Zn, BR and GA mediated the expression of M. sativa metal tolerance protein 1 (MsMTP1) and zinc-induced facilitators 1 (MsZIF1) associated with Zn accumulation in vacuoles.
MsMTP1 was more strongly expressed in shoots than in roots, but there was no significant difference between different treatments in shoots. Zn stress up-regulated the expressions of MsMTP1 on shoots and roots. Further, BR and GA promote this up-regulation. The expressions of MsMTP1 in M. sativa shoots under 3 mM Zn and GA application were significantly higher than that in the control plants. And the expressions of MsMTP1 in roots under 3 mM Zn and its application with BR or GA were significantly higher than that in the control plants (Fig. 6A).
Effects of Zn stress, BR and GA on the expressions of genes related to Zn-efflux. Total RNA was isolated from M. sativa roots and shoots. The MsEF1α gene from M. sativa was used as the housekeeping gene for normalization. The expressions of MsMTP1 (A) and MsZIF1 (B) genes involved in Zn efflux and vacuolar storage were determined in roots and shoots of M. sativa after 7 weeks of treatment. ANOVA (including Brown-Forsythe test and Bartlett test) and Tukey’s multiple comparisons test were performed on the data of 3 biological replicates (n = 3). Vertical bars represent mean ± SD. Averages with the different letter are significantly different at p < 0.05
Unlike MsMTP1, the expressions of MsZIF1 were similar in roots and shoots. Zn stress, BR and GA also up-regulated the expressions of MsZIF1 in shoots, but there was no significant difference among different treatments. The expressions of MsZIF1 in roots under 3 mM Zn and BR or GA was significantly higher than that under other treatments (Fig. 6B).
Zn, BR and GA mediated the expression of M. sativa heavy metal transporters 4, yellow stripe-like protein 1, and nicotianamine synthase 1 (MsHMA4, MsYSL1 and MsNAS1) related to Zn chelation and long-distance transport.
MsHMA4 was strongly induced to express in both roots and shoots. Zn stress, BR and GA up-regulated the expressions of MsHMA4. BR and GA up-regulated MsHMA4 expressions in shoots under the same Zn concentration. And under 3 mM Zn + GA, MsHMA4 expressions were significantly higher than that under control. In particular, the expressions of MsHMA4 in roots showed consistent patterns (Fig.7A).
Effects of Zn, BR and GA on the expressions of Zn chelation and transport genes. Total RNA was isolated from M. sativa roots and shoots. The MsEF1α gene from M. sativa was used as the housekeeping gene for normalization. The expressions of MsHMA4 (A), MsYSL1 (B) and MsNAS1 (C) genes involved in Zn chelation and long-distance transport were determined in the roots and shoots of M. sativa after 7 weeks of treatment. ANOVA (including Brown-Forsythe test and Bartlett test) and Tukey’s multiple comparisons test were performed on the data of 3 biological replicates (n = 3). Vertical bars represent mean ± SD. Averages with the different letter are significantly different at p < 0.05
On the whole, the expressions of MsYSL1 in shoots were slightly higher than that in roots. Zn stress, BR and GA up-regulated the expressions of MsYSL1. The expression level of MsYSL1 in roots under 3 mM Zn and BR or GA treatment was significantly higher than that in the control. Besides this, the differences among other treatments were not significant (Fig.7B).
MsNAS1 was strongly expressed in both roots and shoots. And the expressions of MsNAS1 in root was higher than that in shoots in control group. Zn stress, BR and GA up-regulated the expressions of MsNAS1, significantly. The expressions of MsNAS1 in roots under 2 mM Zn was significantly upregulated by BA and GA. The expressions of MsNAS1 in shoots was up-regulated under Zn stress (Fig. 7C).
Discussion
BR and GA alleviated oxidative stress and growth inhibition of M. sativa under Zn stress
Zn accumulates in the soil and is absorbed and used by plants through their roots [30]. After Zn ions are absorbed by roots, some of them are chelated and stored in vacuoles, some are transported or diffused between cells, and some are transported to xylem step by step [31, 32]. And then, Zn ions are chelated in the xylem by nicotinamide, transported over long distances as free cations, and finally unloaded in mesophyll cells [33, 34]. Then, some of them are chelated and stored in vacuoles or transported and diffused between cells, and some of them are transported to the reproductive organs through the loading and unloading of phloem [35]. With excess Zn, the above series of plant Zn uptake and transport networks are responded to and regulated.
We found that excess Zn significantly inhibited the accumulation of M. sativa biomass. And Zn stress significantly increased MDA and OFR content and production rate. Meanwhile, the activities of CAT, SOD and POD were increased under Zn stress. These also confirmed that oxidative stress caused by excessive heavy metals can significantly inhibit plant growth and biomass accumulation [36, 37]. The results of correlation analysis also showed that Zn accumulation in plants had a significant negative correlation with biomass, and a significant positive correlation with MDA, OFR, CAT, SOD, and POD activities (Table S2-4). Besides, these indicators are also correlated with each other (Table S2-4). We speculate that this is a chain reaction of growth inhibition and oxidative stress triggered by increased Zn accumulation in plants. It is worth mentioning that BR and GA significantly eased this inhibition. Under the same Zn stress, BR and GA increased M. sativa biomass. Moreover, the application of BR and GA further decreased the content of lipid oxides (MDA and OFR), and further increased the activities of antioxidant enzymes (CAT, SOD, POD).
BR significantly increased plant heights, chlorophyll content and enzyme activity of Chlorella vulgaris under Cd stress [38]. Foliar spraying of BR significantly enhanced the biomass, antioxidant enzyme activities and proline content of mung bean (Vigna radiata L. Wilczek) under aluminium (Al) stress [39]. Exogenous BR application up-regulated the transcription levels of antioxidant enzyme-related genes in rice in rice (Oryza sativa L.), Maize (Zea mays L.), and Pepper (Capsicum annuum) under heavy metal stress [40,41,42]. GA treatment up-regulated the activity of various oxidoreductases to alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) [22]. And GA treatment at an appropriate concentration increased proline and antioxidant enzyme levels of spinach (Spinacia oleracea L.) to alleviate Cu stress [43]. These evidences indicated that BR and GA play an important role in alleviating heavy metal toxicity and reducing oxidative stress response to promote plant growth. It is worth mentioning that our study showed that BR application at the same concentration was better than GA in this regard under the same Zn concentration stress.
BR and GA reduced the excessive accumulation of Zn in M. sativa
The results showed that over five times as much Zn content was accumulated in the roots as in the shoots. The roots biomass was reduced more than the shoots because of the more severe stress caused by direct exposure to excessive Zn [36, 44]. Similarly, Namdjoyan et al. found that the roots of safflower (Carthamus tinctorius L.) seedlings accumulated more Zn than those in shoots [37]. These support the view that plants accumulate much more heavy metals in the roots to reduce the toxicity to the aboveground and thus enhance their tolerance.
It has been reported that BR and GA reduce the accumulation of other heavy metals (such as Pb, Cu, Cr, Cd, and Zn) in plants [18, 45]. GA can improve plant tolerance to heavy metal toxicity via reducing accumulation or accelerating excretion [46, 47]. BR and GA application significantly reduced excessive Zn accumulation in M. sativa roots and shoots. And the application of BR and GA decreased the lipid peroxides of M. sativa roots to a greater extent than shoots, and improved the activity of antioxidant enzymes more strongly in roots than in shoots. Particularly, the same concentration BR inhibited the excessive accumulation of Zn more strongly than GA.
BR and GA regulated heavy metal transporters expression patterns in M. sativa with excess Zn
The Neighbor-joining (NJ) phylogenetic trees of ZIP, MTP, ZIF, HMA, YSL and NAS gene sequences of M. sativa and other plant species have been inferred, including Medicago truncatula, Arabidopsis thaliana (L.) Heynh., Glycine max (L.) Merr., Hordeum vulgare L., Oryza sativa L., Triticum aestivum L., and Zea mays L. [29]. First, MsZIP1-7 gene sequences and MtZIP1-7 existed as lineal homologues. In addition, the sequences of MsZIP1/3/5 were closely related to those of AtZIP3/4/7, OsZIP4/5/8, ZmZIP4/5/7, and HvZIP3/5/7. Particularly, the sequence of MsZIP1, OsZIP1/2, ZmZIP2 and HvZIP1/2 were similar to each other. As regards MTP, the sequence of MsMTP1 was also related to those of GmMTP1, AtMTP1/2/3, ZmMTP1, HvMTP1 and OsMTP1/2/3. As regards ZIF, the sequence of MsZIF1 clustered with the sequences of AtZIF1/2, ZmZIF1 and GmZIF1. As regards HMA, the sequence of MsHMA1 was also related to AtHMA1/2/3. Similarly, the sequence of MsYSL1 was most similar to those of AtYSL1/2/3, ZmYSL1 and OsYSL1 and the sequence of MsNAS1 was most similar to those of AtNAS1/2/3, ZmNAS1 and OsNAS1 [29].
ZIP family genes located on the plasma membrane are involved in the flow of Zn in and out of cells [48]. And they can transport a variety of metal ions (Fe, Zn, Cd, Cu, etc.) and are subject to complex metal-dependent post-translational regulation [49]. AtZIP3/4/7, OsZIP4 and HvZIP3/5/7 have been identified as Zn transporters on the plasma membrane, which are strongly induced by Zn deficiency and mediate the flow of Zn into root cells [50,51,52,53]. It is emphasized that ZmZIP4/5/7 was strongly induced by Zn deficiency, and was highly expressed mainly in shoots, and was significantly inhibited under Zn stress [54]. These reports suggested that the homologous sequence closely related to MszZIP1/3/5 has the function of Zn uptake into root cells and is actively expressed by Zn deficiency and possibly inhibited by Zn excess. Consistently, Zn stress down-regulated the expressions of MsZIP1/3/5 in M. sativa. And BR and GA enhanced the down-regulations. Conversely, ZmZIP2 and HvZIP2 are not induced by Zn deficiency, and ZmZIP2 is substantially induced by Zn excess. This is consistent with MsZIP7. They may be involved in the outflow of Zn from the cell. These results suggested that, to resist Zn stress, plants reduce intracellular Zn accumulation by inhibiting the expressions of genes regulating Zn influx and promoting the expressions of genes regulating Zn outflow. And BR and GA actively regulate this process. In addition, the down-regulation of MsZIP1/5 expressions induced by Zn stress was more extensive in roots than in shoots. This may be because plants already limit Zn uptake and transfer in the roots, so Zn stress in shoots is less intense than in roots.
Cellular vacuoles can store excess Zn in response to Zn poisoning or as a source of Zn deficiency. ZIF1 and MTP1 are involved in the transport of Zn—organic acids complexes into vacuoles. AtMTP1/3 and AtZIF1/2 actively regulate Zn chelation and vacuolar storage to enhance Zn tolerance in Arabidopsis thaliana [55,56,57]. Noticeably, AtMTP1 was strongly expressed in both young leaves and seedling roots, while AtMTP3 was strongly expressed only in roots when Fe deficiency and Zn excess occurred [55]. Consistently, Zn stress upregulated the expressions of MsMTP1 and MsZIF1, and BR and GA promoted the upregulation. There are few reports on the functional characterization of MTP1 and ZIF1 homologous sequences in Zn excess. However, the up-regulation of MsMTP1 and MsZIF1 enhances the tolerance of M. sativa to Zn toxicity by enhancing chelating ability to excess Zn. In addition, up-regulation of MsMTP1 and MsZIF1 in roots may reduce Zn transport to shoots. Notably, BA and GA enhanced the up-regulation of MsMTP1 expression. In summary, BR and GA levels promote Zn detoxification pathways.
NAS regulates niacinamide and heavy metal chelation [58], and HMA and YSL regulate ectopic heavy metal in xylem, phloem or cell to cell [32, 59]. AtHMA2/3/4 can transfer Zn from root cells to xylem cells and are associated with ectopic Zn transfer from roots to shoots. Overexpression of AtHMA3 enhanced the tolerance of Cd and Zn in Arabidopsis thaliana, and increased the accumulation of Zn in shoots and roots [32]. Similarly, the expressions of MsHMA3 in M. sativa shoots and roots was up-regulated when exposed to Zn stressing. This upregulation promotes Zn transport to xylem to alleviate Zn toxicity. Notably, the upregulation was amplified by the application of BR and GA, further mitigating the Zn toxic.
It has been widely reported that NAS and YSL are regulated by Fe levels. Fe deficiency induced up-regulation of ZmNAS1 and ZmNAS2 expressions in maize roots [ 32]. However, ZmNAS3, OsNAS1/2, AtYSL1/2/3, OsYSL2 and ZmYSL1 were induced by excessive Fe [ 60]. In view of this, the alleviating effect of Fe abundance on Zn toxicity has also been reported. AtNAS2, ATYSL1/3 and OSYSL2/16/18 regulated the long-distance transport of Zn in xylem and phloem, and affect the accumulation of Zn in roots [ 53]. Excessive Zn induced up-regulation of MsYSL1 and MsNAS1. These results indicated that the Zn chelating capacity of M. sativa was enhanced and xylem transfer was increased. These processes are beneficial for plants to alleviate excessive Zn toxicity. In addition, chelating substrates (plant ferrite group or niacinamide) also affect the chelation and transport of heavy metals. There was little difference in the expression of MsYSL1 in shoots under different treatments. Whether it was affected by the level of Zn chelating substrate in cells requires more research experiments.
Conclusion
Phytohormone play an important role in plant resistance to stress. Our results suggested that, BR and GA can restore the growth damage caused by zinc poisoning. And BR and GA can also promote plant antioxidant system response and heavy metal detoxification mechanism. Interestingly, the same concentration of BR alleviates plant heavy metal tolerance slightly better than GA. It is well known that plants will adjust the nutrient uptake or heavy metal enrichment efficiency of different tissues such as roots, leaves, stems, buds, flowers and fruits, to cope with heavy metal stress. These processes are involved in phytohormone. Therefore, a more detailed understanding of the detoxification mechanism of heavy metals in plants will be improved by combining the growth status of different tissues and nutrient uptake status. These processes are involved in phytohormone. Therefore, phytohormone changes combined with different tissue growth status and nutrient uptake status will improve the understanding of the detoxification mechanism of heavy metals in plants.
Fundings
This work was supported by the Science and Technology Planning Project of Guangdong Province [Grant number: 201904020022], the Laboratory of Lingnan Modern Agriculture Project [Grant number: NZ2021025], and the National Natural Science Foundation of China [Grant number: 32071639].
Availability of data and materials
The raw data for this article can be accessed in the Supplementary materials.
Abbreviations
- Zn:
-
Zinc
- M. sativa :
-
Medicago sativa L
- Cu:
-
Cuprum
- Pb:
-
Lead
- Cd:
-
Cadmium
- Hg:
-
Mercury
- GA:
-
Gibberellic acid
- VI:
-
Chromium
- BR:
-
Brassinosteroids
- 24-epiBL:
-
24-Epibrassinolide
- Cr:
-
Chromium
- CAT:
-
Catalase
- POD:
-
Peroxidase
- SOD:
-
Superoxide dismutase
- OFR:
-
Superoxide anion
- MDA:
-
Malondialdehyde
- ZIP1 :
-
ZRT-IRT-like protein 1
- MTP1 :
-
Metal tolerance protein 1
- HMA4 :
-
Heavy metal transporters 4
- YSL1 :
-
Yellow stripe-like protein 1
- NAS1 :
-
Nicotianamine synthase
- NJ:
-
Neighbor-joining
References
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173(4):677–702.
Khan MIR, Chopra P, Chhillar H, Ahanger MA, Hussain SJ, Maheshwari C. Regulatory hubs and strategies for improving heavy metal tolerance in plants: chemical messengers, omics and genetic engineering. Plant Physiol Biochem. 2021;164:260–78.
Cherif J, Derbel N, Nakkach M, Hv Bergmann, Jemal F, Lakhdar ZB. Analysis of in vivo chlorophyll fluorescence spectra to monitor physiological state of tomato plants growing under zinc stress. Journal of Photochemistry and Photobiology B: Biology. 2010;101(3):332–9.
Aydin SS, Gökçe E, Büyük I, Aras S. Characterization of stress induced by copper and zinc on cucumber(Cucumis sativus L.) seedlings by means of molecular and population parameters. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2012;746(1):49–55.
Madian AG, Regnier FE. Proteomic identification of carbonylated proteins and their oxidation sites. J Proteome Res. 2010;9(8):3766–80.
Di Baccio D, Tognetti R, Minnocci A, Sebastiani L. Responses of the populus x euramericana clone i-214 to excess zinc: carbon assimilation, structural modifications, metal distribution and cellular localization. Environ Exp Bot. 2009;67(1):153–63.
Kaur H, Garg N. Zinc toxicity in plants: a review. Planta. 2021;253(6):129.
Jones F, Bankiewicz D, Hupa M. Occurrence and sources of zinc in fuels. Fuel. 2014;117:763–75.
Bazihizina N, Taiti C, Marti L, Rodrigo-Moreno A, Spinelli F, Giordano C, Caparrotta S, Gori M, Azzarello E, Mancuso S. Zn 2+-induced changes at the root level account for the increased tolerance of acclimated tobacco plants. J Exp Bot. 2014;65(17):4931–42.
Suwignyo B, Rini EA, Helmiyati S. The profile of tropical alfalfa in Indonesia: a review. Saudi Journal of Biological Sciences. 2023;30(1): 103504.
Kareem HA, Adeel M, Azeem M, Ahmad MA, Shakoor N, Hassan MU, Saleem S, Irshad A, Niu J, Guo Z, Branko Ć, Hołubowicz R, Wang Q. Antagonistic impact on cadmium stress in alfalfa supplemented with nano-zinc oxide and biochar via upregulating metal detoxification. J Hazard Mater. 2023;443: 130309.
Kong F, Ying Y, Lu S. Heavy metal pollution risk of desulfurized steel slag as a soil amendment in cycling use of solid wastes. J Environ Sci. 2023;127:349–60.
Chen F, Wang S, Mou S, Azimuddin I, Zhang D, Pan X, AlMisned FA, Mortuza MG. Physiological responses and accumulation of heavy metals and arsenic of Medicago sativa L growing on acidic copper mine tailings in arid lands. J Geochem Explor. 2015;157:27–35.
Wang Y, Meng Y, Mu S, Yan D, Xu X, Zhang L, Xu B. Changes in phenotype and gene expression under lead stress revealed key genetic responses to lead tolerance in Medicago sativa L. Gene. 2021;791:14571.
Xiong P, He C, Kokyo OH, Chen X, Liang X, Liu X, Cheng X, Wu C, Shi Z. Medicago sativa L. enhances the phytoextraction of cadmium and zinc by ricinus communis L. on contaminated land in situ. Ecol Eng. 2018;116:61–6.
Qu J, Lou C, Yuan X, Wang X, Cong Q, Wang L. The effect of sodium hydrogen phosphate/ citric acid mixtures on phytoremediation by alfalfa & metals availability in soil. J Soil Sci Plant Nutr. 2010;11(2):86–96.
Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A. Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul. 2019;38(2):739–52.
Saini S, Kaur N, Pati PK. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol Environ Saf. 2021;223: 112578.
Hedden P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020;61(11):1832–49.
Mansour MM, Kamel EA. Interactive effect of heavy metals and gibberellic acid on mitotic activity and some metabolic changes of vicia faba L. plants. Cytologia. 2005;70(3):275–82.
Falkowska M, Pietryczuk A, Piotrowska A, Bajguz A, Grygoruk A, Czerpak R. The effect of gibberellic acid (GA3) on growth, metal biosorption and metabolism of the green algae chlorella vulgaris (Chlorophyceae) beijerinck exposed to cadmium and lead stress. Pol J Environ Stud. 2011;20(1):53–9.
Gangwar S, Singh VP, Srivastava PK, Maurya JN. Modification of chromium phytotoxicity by exogenous gibberellic acid application in pisum sativum seedlings. Acta Physiol Plant. 2011;33(4):1385–97.
Zhu X, Jiang T, Wang Z, Lei G, Shi Y, Li G, Zheng S. Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J Hazard Mater. 2012;239–240:302–7.
Kour J, Kohli SK, Khanna K, Bakshi P, Sharma P, Singh AD, Ibrahim M, Devi K, Sharma N, Ohri P, Skalicky M, Brestic M, Bhardwaj R, Landi M, Sharma A. Brassinosteroid signaling, crosstalk and physiological functions in plants under heavy metal stress. Front Plant Sci. 2021;12: 608061.
Sharma I, Sharma A, Pati P, Bhardwaj R. Brassinosteroids reciprocates heavy metals induced oxidative stress in radish by regulating the expression of key antioxidant enzyme genes. Braz Arch Biol Technol. 2018;61: e18160679.
Hasan SA, Hayat S, Ahmad A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere. 2011;84(10):1446–51.
Sharma P, Bhardwaj R. Effects of 24-epibrassinolide on growth and metal uptake in brassica juncea L. under copper metal stress. Acta Physiol Plant. 2007;29(3):259–63.
Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn Circ. 1950;347(2):1–32.
Cardini A, Pellegrino E, White PJ, Mazzolai B, Mascherpa MC, Ercoli L. Transcriptional regulation of genes involved in zinc uptake, sequestration and redistribution following foliar zinc application to Medicago sativa. Plants. 2021;10(3):476.
Shimo H, Ishimaru Y, An G, Yamakawa T, Nakanishi H, Nishizawa NK. Low cadmium (LCD), a novel gene related to cadmium tolerance and accumulation in rice. J Exp Bot. 2011;62(15):5727–34.
Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Kramer U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453(7193):391–5.
Haydon MJ, Cobbett CS. A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in arabidopsis. Plant Physiol. 2007;143(4):1705–19.
Verret F, Gravot A, Auroy P, Leonhardt N, David P, Nussaume L, Vavasseur A, Richaud P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 2004;576(3):306–12.
Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N. Casparian strip diffusion barrier in arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci. 2012;109(25):10101–6.
White PJ, Broadley MR. Physiological limits to zinc biofortification of edible crops. Front Plant Sci. 2011;2:80.
Goodarzi A, Namdjoyan S, Soorki AA. Effects of exogenous melatonin and glutathione on zinc toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicol Environ Saf. 2020;201:110853.
Namdjoyan S, Kermanian H, Soorki AA, Tabatabaei SS, Elyasi N. Interactive effects of salicylic acid and nitric oxide in alleviating zinc toxicity of safflower (Carthamus tinctorius L.). Ecotoxicology. 2017;26(6):752–61.
Bajguz A. An enhancing effect of exogenous brassinolide on the growth and antioxidant activity in Chlorella vulgaris cultures under heavy metals stress. Environ Exp Bot. 2010;68(2):175–9.
Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A. A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ Exp Bot. 2008;62(2):153–9.
Sharma P, Kumar A, Bhardwaj R. Plant steroidal hormone epibrassinolide regulate Heavy metal stress tolerance in oryza sativa L by modulating antioxidant defense expression. Environ Exp Bot. 2016;122:1–9.
Tang Y, Zhang J, Wang L, Wang H, Long H, Yang L, Li G, Guo J, Wang Y, Li Y, Yang Q, Shi W, Shao R. Water deficit aggravated the inhibition of photosynthetic performance of maize under mercury stress but is alleviated by brassinosteroids. J Hazard Mater. 2023;443: 130365.
Mumtazab MA, Hao Y, Mehmood S, Shu H, Zhou Y, Jin W, Chen C, Li L, Altafa MA, Wang Z. Physiological and transcriptomic analysis provide molecular insight into 24-epibrassinolide mediated Cr (VI)-toxicity tolerance in pepper plants. Environ Pollut. 2022;306: 119375.
Gong Q, Li Z, Wang L, Zhou J, Kang Q, Niu D. Gibberellic acid application on biomass, oxidative stress response, and photosynthesis in spinach (Spinacia oleracea L.) seedlings under copper stress. Environ Sci Pollut Res. 2021;28(38):53594–604.
Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MNV. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L◊. Plant Physiol Biochem. 2006;44(1):25–37.
Bukhari SAH, Wang R, Wang W, Ahmed IM, Zheng W, Cao F. Genotype-dependent effect of exogenous 24-epibrassinolide on chromium-induced changes in ultrastructure and physicochemical traits in tobacco seedlings. Environ Sci Pollut Res. 2016;23(18):18229–38.
Ogugua UV, Kanu SA, Ntushelo K. Gibberellic acid improves growth and reduces heavy metal accumulation: A case study in tomato seedlings exposed to acid mine water. Heliyon. 2022;8(12):e12399.
Liu Y, Tao Y, Yang X, Liu Y, Shen R, Zhu X. Gibberellic acid alleviates cadmium toxicity in rice by regulating NO accumulation and cell wall fixation capacity of cadmium. J Hazard Mater. 2022;439: 129597.
Vert G, Barberon M, Zelazny E, Séguéla M, Briat JF, Curie C. Arabidopsis IRT2 cooperates with the high-affinity iron uptake system to maintain iron homeostasis in root epidermal cells. Planta. 2009;229(6):1171–9.
Shin L, Lo J, Chen G, Callis J, Fu H, Yeh K. IRT1 DEGRADATION FACTOR1, a RING E3 Ubiquitin Ligase, Regulates the Degradation of IRON-REGULATED TRANSPORTER1 in arabidopsis. Plant Cell. 2013;25(8):3039–51.
Tiong J, McDonald G, Genc Y, Shirley N, Langridge P, Huang CY. Increased expression of six ZIP family genes by zinc (Zn) deficiency is associated with enhanced uptake and root-to-shoot translocation of Zn in barley (Hordeum vulgare). New Phytol. 2015;207(4):1097–109.
Bashir K, Takahashi R, Nakanishi H, Nishizawa NK. The road to micronutrient biofortification of rice: progress and prospects. Front Plant Sci. 2013;4:15.
Lin Y, Liang H, Yang S, Boch A, Clemens S, Chen C, Wu J, Huang J, Yeh K. Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 2009;182(2):392–404.
Sinclair SA, Krämer U. The zinc homeostasis network of land plants. Biochim Biophys Acta(BBA) Mol Cell Res. 2012;9:1553–67.
Li S, Zhou X, Huang Y, Zhu L, Zhang S, Zhao Y, Guo J, Chen J, Chen R. Identification and characterization of the zinc-regulated transporters, iron-regulated transporter-like protein (ZIP) gene family in maize. BMC Plant Biol. 2013;13:114.
Arrivault S, Senger T, Krämer U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 2006;46(5):861–79.
Desbrosses-Fonrouge A, Voigt K, Schröder A, Arrivault S, Thomine S, Krämer U. Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Lett. 2005;579(19):4165–74.
Olsen LI, Palmgren MG. Many rivers to cross: the journey of zinc from soil to seed Frontiers in Plant. Sci. 2014;5:30.
Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK. Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell. 2003;15(6):1263–80.
Song W, Martinoia E, Lee J, Dongwoo Kim, Doyoung Kim, Vogt E, Shim D, Choi KS, Hwang I, Lee Y. A novel family of Cys-Rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol. 2004;135(2):1027–39.
Mizuno D, Higuchi K, Sakamoto T, Nakanishi H, Mori S, Nishizawa NK. Three nicotianamine synthase genes isolated from maize are differentially regulated by iron nutritional status. Plant Physiol. 2003;132(4):1989–97.
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
Ying Ren: Methodology, Investigation, Validation, Writing – original draft. Xue Li: Investigation, Validation. Jingwei Liang: Investigation, Validation. Sijia Wang: Investigation, Validation. Zhihao Wang: Investigation, Validation. Hui chen: Writing – review & editing. Ming Tang: Conceptualization, Writing – review & editing, Funding acquisition. The author(s) read and approved the final manuscript
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The plant Medicago sativa L. seeds, cultivar Giulia, were purchased from Guangzhou Huisen Forestry Co., LTD. All experimental studies complied with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. The primer sequences of the genes. Table S2. Spearman's Rho among different indexes such as zinc content, biomass and antioxidant enzymes. Table S3. Total variance explained of principal component analysis for all indicators. Table S4. Component Matrixa of principal component analysis for all indicators.
Additional file 2:
Table 1. Effects of different treatments on Medicago sativa L. biomass (Fresh weight, mg/plant). Table 2. Effects of different treatments on Medicago sativa L. Zinc content (μg/g). Table 3. Effects of different treatments on Medicago sativa L. MDA content (nmol/g). Table 4. Effects of different treatments on Medicago sativa L. OFR content (nmol/g). Table 5. Effects of different treatments on Medicago sativa L. OFR generation rate (U/g min). Table 6. Effects of different treatments on Medicago sativa L. SOD acitivity (U/g min). Table 7. Effects of different treatments on Medicago sativa L. POD acitivity (U/g min). Table 8. Effects of different treatments on Medicago sativa L. CAT acitivity (U/g min). Table 9. Effects of different treatments on genes relative expression in Medicago sativa L.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ren, Y., Li, X., Liang, J. et al. Brassinosteroids and gibberellic acid actively regulate the zinc detoxification mechanism of Medicago sativa L. seedlings. BMC Plant Biol 23, 75 (2023). https://doi.org/10.1186/s12870-023-04091-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04091-4