Abstract
Background
Probiotics are important tools in therapies against vaginal infections and can assist traditional antibiotic therapies in restoring healthy microbiota. Recent research has shown that microorganisms belonging to the genus Lactobacillus have probiotic potential. Thus, this study evaluated the potential in vitro probiotic properties of three strains of Lactiplantibacillus plantarum, isolated during the fermentation of high-quality cocoa, against Gardnerella vaginalis and Neisseria gonorrhoeae. Strains were evaluated for their physiological, safety, and antimicrobial characteristics.
Results
The hydrophobicity of L. plantarum strains varied from 26.67 to 91.67%, and their autoaggregation varied from 18.10 to 30.64%. The co-aggregation of L. plantarum strains with G. vaginalis ranged from 14.73 to 16.31%, and from 29.14 to 45.76% with N. gonorrhoeae. All L. plantarum strains could moderately or strongly produce biofilms. L. plantarum strains did not show haemolytic activity and were generally sensitive to the tested antimicrobials. All lactobacillus strains were tolerant to heat and pH resistance tests. All three strains of L. plantarum showed antimicrobial activity against the tested pathogens. The coincubation of L. plantarum strains with pathogens showed that the culture pH remained below 4.5 after 24 h. All cell-free culture supernatants (CFCS) demonstrated activity against the two pathogens tested, and all L. plantarum strains produced hydrogen peroxide. CFCS characterisation in conjunction with gas chromatography revealed that organic acids, especially lactic acid, were responsible for the antimicrobial activity against the pathogens evaluated.
Conclusion
The three strains of L. plantarum presented significant probiotic characteristics against the two pathogens of clinical importance. In vitro screening identified strong probiotic candidates for in vivo studies for the treatment of vaginal infections.
Similar content being viewed by others
Background
The misuse of antibiotics and the unavailability of newer drugs have been considered the main reasons for the current antimicrobial resistance crisis. In response to this, new approaches such as probiotics have shown promising results in trials, suggesting the role of alternative treatments as preventive or adjunct therapies in the future [1]. The Food and Agriculture Organization (FAO) defines probiotics as live microorganisms that confer a health benefit to the host when administered in adequate amounts [2]. In recent years, probiotic microorganisms have been investigated for their beneficial effects in many clinical studies on the treatment and prevention of various pathogens responsible for gastrointestinal disorders and vaginal infections in humans [3].
Vaginal infections are one of the main causes for gynaecological consultations [4]. In this study, two vaginal conditions were considered: bacterial vaginosis (BV) and gonorrhoea. BV is a dysbiosis common in adult women of reproductive age, characterised by the replacement of the resident microbiota composed of lactobacilli by various anaerobic bacteria, of which G. vaginalis is the most prevalent [5, 6]. With this dysbiosis, inflammation of the mucosa presents several symptoms, including vaginal discharge, itching, and burning associated with the lack of leukocytic exudate and redness. Other health complications related to BV include greater susceptibility to HIV infection, infections by specific microorganisms, pelvic inflammatory diseases, and premature births [7].
Gonorrhoea is a classic sexually transmitted infection caused by N. gonorrhoeae, a gram-negative intracellular diplococci bacterium [8]. Gonococcal infections can result in severe complications and sequelae, including pelvic inflammatory disease, infertility, ectopic pregnancy, first trimester abortion, and neonatal conjunctivitis, leading to blindness [9]. Within this panorama, it is clear that as gonorrhoea causes great morbidity and has significant socioeconomic consequences, the emergence of therapies to reduce gonococcal infections without the use of antibiotics would be beneficial for public health worldwide [10].
In this context, it is understood why research on probiotics has been successful in therapies for women's health. Probiotic properties have been observed in many genera of bacteria and fungi, but the most commonly used probiotics belong to the genus Lactobacillus, particularly to the species L. plantarum. Historically, lactobacilli have been generally recognised as safe (GRAS) for consumption and therapeutic applications [11].
L. plantarum has been widely used as a model species for ecological, metabolic, and genetic studies. Furthermore, L. plantarum is of commercial importance as a starter culture for multiple food fermentations, and is used as a probiotic culture [12].
Many lactobacilli strains are able to colonise and produce antimicrobials that prevent the growth of pathogenic microorganisms [13]. From this perspective, the search for new probiotics is motivated by the knowledge that each lactobacillus strain possesses different properties and could have unique effects on human health. A few studies have reported on lactobacilli strains isolated from non-human sources that have shown promising probiotic effects [14,15,16]. In the present study, we evaluated the in vitro probiotic potential of three strains of L. plantarum, isolated during the fermentation of high-quality cocoa, against two vaginal pathogens, G. vaginalis and N. gonorrhoeae.
Results
Cell surface properties of Lactobacillus
Results of hydrophobicity (H%), autoaggregation (AA%), co-aggregation (CA%), and biofilm formation of the Lactobacillus strains are shown in Table 1. The hydrophobicity of the Lactobacillus strains ranged from 26.67 to 91.67%. Among the three Lactobacillus strains, only the Lp289 strain showed a significant increase in hydrophobicity compared to the Lp291 and Lp03 strains (P < 0.05). Regarding the first classification that considers the microbial adhesion to hydrocarbons, the Lp03 strain showed moderate hydrophobicity, Lp289 strain showed high hydrophobicity, and Lp291 strain showed low hydrophobicity. According to the second classification that considers microbial adhesion to solvents, Lp03 and Lp291 strains were considered hydrophilic, while L. plantarum Lp289 was considered hydrophobic.
An autoaggregation assay was performed to evaluate the ability of Lactobacillus to aggregate with strains of the same species. Autoaggregation rates obtained for Lactobacillus ranged from 18.10 to 30.64% after 5 h of incubation. The highest level of autoaggregation was observed for L. plantarum Lp291 (30.64%), which differed significantly from the Lp289 and Lp03 strains (P < 0.05).
Similarly, a co-aggregation assay was performed to evaluate whether Lactobacillus strains interact directly with genital pathogens. All Lactobacillus strains co-aggregated with the applied vaginal pathogens, with distinct levels of interaction, and exhibited a higher percentage of co-aggregation with N. gonorrhoeae compared to G. vaginalis (P < 0.05). However, there was no significant difference in the co-aggregation of Lactobacillus strains with G. vaginalis or with N. gonorrhoeae (P > 0.05). In addition, our data demonstrated that the lactobacilli strains could adhere and form biofilms under the conditions tested. L. plantarum Lp03 was strongly adherent, and Lp298 and 291 strains were moderately adherent.
Evaluation of haemolytic activity and antibiotic susceptibility of Lactobacillus strains
In the present study, none of the Lactobacillus strains showed haemolytic activity. Results described in Table 1 demonstrate that the Lp03, Lp289, and Lp291 strains were considered γ-haemolytic. Together with these data, results in Table 2 demonstrate the susceptibility profile of Lactobacillus strains to antibiotics based on the disk diffusion method. Nine antibiotics belonging to different classes were used in this study, including cell wall, protein, and nucleic acid synthesis inhibitors, and urinary tract antiseptics. Results of this trial revealed that the three strains of L. plantarum demonstrated resistance to vancomycin only. The data also showed that L. plantarum Lp03 and Lp289 were susceptible to increased exposure to ciprofloxacin. All strains were classified as sensitive to other antibiotics. The Lp03 and Lp289 strains demonstrated identical antimicrobial susceptibility profiles.
Evaluation of the resilience of lactobacilli strains to thermal and pH stress
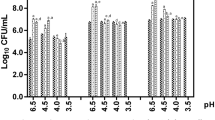
Concerning thermal resistance of Lactobacillus strains, there was a significant reduction (P < 0.05) in the viable cell counts of the three strains after thermal shock, as can be seen in Fig. 1. Regardless, all lactobacilli strains remained viable after treatment. L. plantarum tested were also evaluated for their ability to grow in different pH ranges, ranging from acidic to basic environments. Results of this assay are shown in Fig. 2. Our data showed that all Lactobacillus strains grew at all pH values, except at pH 3. The same pH tolerance assay was applied to the pathogens to characterise their growth profiles. Similar to Lactobacillus strains, the G. vaginalis strain grew at all pH values, except at pH 3. For the N. gonorrhoeae strain, the pathogen was able to grow only in media with a pH above 5.
Heat resistance standard of Lactobacillus strains isolated from cocoa fermentation. (Lp03) L. plantarum Lp03; (Lp289) L. plantarum Lp289; (Lp291) L. plantarum Lp291. (**) Statistically significant differences compared to control (P < 0.01); (***) Statistically significant differences compared to control (P < 0.001). Presented values represent the mean and standard deviation from triplicate determinations
Inhibition of pathogen growth by lactobacilli strains
Co-incubation experiments were performed to quantitate growth inhibition of G. vaginalis and N. gonorrhoeae to evaluate the possible inhibition caused by a direct interaction between Lactobacillus strains and pathogen cells. The inhibitory effects of the lactobacilli strains against the tested pathogens are shown in Fig. 3. Our data showed that all Lactobacillus strains inhibited both G. vaginalis and N. gonorrhoeae when co-incubated. After 24 h of co-incubation, results showed that all Lactobacillus strains were able to significantly reduce the viability of G. vaginalis (P < 0.05) and N. gonorrhoeae (P < 0.001) with different levels of effectiveness. There was a significant reduction in the CFU mL-1 count of pathogens compared to monoculture (106), with counts of approximately 102 CFU mL-1 and 105 CFU mL-1 for N. gonorrhoeae and G. vaginalis, respectively.
Growth inhibition of pathogens by Lactobacillus strains after 24 h of culture. The growth of pathogenic microorganism is expressed as log10 CFU mL−1. Control: (Gv) G. vaginalis or (Ng) N. gonorrhoeae. The different Lactobacillus strain isolates are represented by their respective numbers. a represents coculture of G. vaginalis with Lactobacillus sp.; b represents coculture of N. gonorrhoeae with Lactobacillus sp. (*) Statistically significant differences compared to control (P < 0.05); (***) Statistically significant differences compared to control (P < 0.001). Presented values represent the mean and standard deviation from triplicate determinations
Evaluation of pH reduction by lactobacilli in cultures with or without pathogen
The pH of the bacterial growth medium was measured in isolated cultures of Lactobacillus strains and in co-culture of lactobacilli with pathogens (Table 3). The lowest pH recorded was from the isolated culture of the Lp03 strain (3.76), and the highest pH was observed in the co-culture of the Lp291 strain with N. gonorrhoeae (4.47). There were no significant differences (P > 0.05) between the pH of the growth media of the Lactobacillus strains growing alone or in combination with the pathogens. This demonstrated that the pH did not change in the presence of the pathogens tested. We also observed that the three Lactobacillus strains were able to reduce the pH of the culture medium from an initial pH of 6.5, to values below 4.5, in all culture conditions.
Identification and characterization of the antimicrobial activity of CFCS against pathogens
Tables 3 and 4 show the effects of Lactobacillus strains and their CFCS on G. vaginalis and N. gonorrhoeae, and the effect of different physical and chemical treatments of CFCS on antimicrobial activity, respectively. The inhibitory activity of the Lactobacillus strains against vaginal pathogens was assessed through two different in vitro experiments. Our data demonstrated that bioactive compounds produced by Lactobacillus strains inhibited the growth of N. gonorrhoeae and G. vaginalis, inducing the formation of an inhibition halo around the colony. All strains had greater inhibition halos for N. gonorrhoeae than G. vaginalis.
Results of the microdiffusion assay showed that the bioactive compounds inhibited the growth of pathogens, showing inhibition by contact or forming halos of moderate inhibition. Contact inhibition refers only to the area of the agar within the PVC cylinder, which maintained direct contact with CFCS and did not show growth of pathogens. Only the Lp298 strain was able to inhibit both pathogens with similarly sized halos.
The Lp03 strain was able to inhibit N. gonorrhoeae more effectively than G. vaginalis, and the Lp291 strain was able to inhibit G. vaginalis more effectively than N. gonorrhoeae.
After this verification, a characterisation test of the antimicrobial substances present in CFCS was carried out. Results presented in Table 4 reveal the inhibitory activity of the treated and untreated CFCS of Lactobacillus strains against G. vaginalis and N. gonorrhoeae. Treatments applied to the CFCS of each Lactobacillus strain were established to identify which substances were responsible for the inhibition of pathogens in the previous tests. Treatments of CFCS included neutralisation of organic acids, boiling, and inactivation of possible bacteriocins through the enzymatic action of trypsin or proteinase K. Our data demonstrated that both pathogens had the same inhibition response with respect to CFCS treated or not in this trial. The CFCS boiled or treated with trypsin or proteinase K did not affect their inhibitory activities against the two pathogens tested.
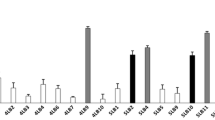
In addition to these compounds, we identified that the Lactobacillus strains were hydrogen peroxide producers; all strains were able to produce hydrogen peroxide at concentrations varying between and 1-5 μM, as shown in Fig. 4. The Lp03 strain showed higher H202 production than the Lp289 strain (P < 0.01), and there was no significant difference in hydrogen peroxide production in the Lp291 strain compared to the other two strains (P > 0.05).
Hydrogen peroxide levels present in CFCS of Lactobacillus strains isolated from cocoa fermentation. The standard curve (R2 = 0.9927) was performed together with the experimental samples in a controlled environment protected from light. Presented values represent the mean and standard deviation from triplicate determinations. (**) Statistically significant differences (P < 0.01)
CFCS metabolome profile
The metabolome of Lactobacillus strains proved to be diverse, with variability of substances such as organic acids, alcohols, sugars, and other organic compounds. The present study specifically evaluated the metabolome of Lactobacillus strains for organic acid production, as shown in Table 5. In this regard, we observed that lactic acid was the most abundant organic acid produced by all Lactobacillus strains, constituting more than 50% of the CFCS sample. The Lp03 strain had the highest percentage of lactic acid (68.16%) among all samples, followed by phosphoric acid (7.18%) and 1,2,3-propanetricarboxylic acid (4.27%). Following the same pattern, the main organic acids produced by the Lp289 strain were lactic acid (56.35%), phosphoric acid (8.32 %), 1,2,3-propanetricarboxylic acid (4.33%) and butyric acid (1.53%). The Lp291 analysis of the CFCS strain revealed the prevalence of lactic acid (67.34%), phosphoric acid (7.92%), acetic acid (6.52%), and 1,2,3-propanetricarboxylic acid (3.34%).
In addition, trace amounts of other organic acids, including 3-hydroxybutyric, α-hydroxyvaleric, 4-methyl-2-hydroxypentanoic, 3-methyl-2-hydroxypentanoic, butyric, 2.4-dihydroxybutanoic, malic, 2-pyrrolidone-5-carboxylic, and benzenepropanoic acids. Although acid production is strain-specific, we observed that γ-amino butyric, glutamic, aspartic, 2.3-dihydroxypropylphosphoric, D-ribo-hexonic, and 2-keto-D-gluconic acids were detected only in samples of the Lp289 strain. In the present study, we observed that the prominent presence of phosphoric acid in the samples was due to the MRS medium.
Discussion
Cell surface hydrophobicity is an important characteristic of potential probiotics as it indicates whether Lactobacillus strains can bind to the mucosa. This mechanism is one of the major factors by which probiotic bacteria are believed to exert beneficial effects in the host [18]. It is known that a large variety of surface glycoproteins are inserted into the hydrophobic cell wall of some microorganisms and they are responsible for increasing the likelihood of adhesion to cell receptors or proteins anchored in the cell wall [19,20,21]. Based on literature, we observed that strain 289 had a potential common to probiotics because of its high hydrophobicity. In addition, as the Lactobacillus strains were isolated from cocoa fermentation and that the cocoa pulp is made up of 82 to 87% of water, this could explain the low hydrophobicity of the Lp03 and Lp291 strains [22].
In addition to hydrophobicity, autoaggregation is another probiotic criterion allowing the colonisation and adherence of bacteria to epithelial cells, leading to the prevention of colonisation by pathogens, one of the main defence mechanisms against infection of the urogenital tract [23]. Our data revealed that the three strains of L. plantarum had a low percentage of autoaggregation. Lactobacillus strains usually show an autoaggregation capacity ranging from low to moderate [24], indicating that results are within the expected range, as there is great variation in autoaggregation among strains of both human vaginal microbiota and nonhuman origin [17]. Other studies have demonstrated that the values of autoaggregation are quite variable within the same species or genus Lactobacillus, which does not preclude the use of lactobacilli with low autoaggregation as probiotics [25,26,27,28].
Another important tool that demonstrates Lactobacillus strains use to eliminate bacteria is the ability to aggregate pathogens. Co-aggregation is one of the mechanisms exerted by probiotics to create a competitive microenvironment around the pathogen [29]. In our study, all strains co-aggregated more effectively with N. gonorrhoeae than with G. vaginalis. Similarly, Vielfort et al. [30] reported that their lactobacilli strains exhibited the ability to interact and aggregate with N. gonorrhoeae, configuring this process as an important mechanism to neutralise gonococci viability. This close interaction permits the Lactobacillus strains to create an unfavourable microenvironment for pathogens, where antimicrobial substances produced by lactobacilli in a localised manner harm epithelial colonisation by pathogens [31].
Biofilm formation by lactobacilli can be considered a determinant element for a probiotic microorganism because it is important to promote colonisation and persistence of Lactobacillus strains on vaginal epithelium, and to exert their protective role by interfering with the growth and adhesion of pathogens [32]. Kaur et al. [33] reported that the maturation of biofilms is strongly dependent on the autoaggregation properties of the probiotic microorganism, as it helps the bacteria to form micro-colonies. However, this relationship was not observed in the present study. All Lactobacillus strains were able to adhere to the abiotic polystyrene device, and the Lp03 strain stood out as a strong biofilm producer. The methodology used in our study showed that all strains tested were able to moderately or strongly form biofilms on polystyrene surfaces [34].
The absence of haemolytic activities presented by our lactobacilli strains is a recommended safety characteristic in probiotic selection [31, 32]. Regarding the phenotypical vancomycin resistance, it is noteworthy that most of Lactobacillus sp. are intrinsically resistant to this antibiotic [26, 35]. The gene responsible for this resistance is chromosomal, and therefore cannot be transferred by mobile genetic elements to other bacteria. Probiotics with this type of antimicrobial resistance are already used to restore the microbiota after treatment with antibiotics without posing a risk to human health [36, 37].
In addition to the safety characteristics of the strains, it is interesting that lactobacilli have been approved for resilience tests. It is known that during different industrial and biotechnological processes, probiotic bacteria must respond rapidly to stress to survive, and heat is among the most destructive stress conditions. Exposure to high temperatures destabilises macromolecules such as RNA and ribosomes, leading to denaturation of proteins and alterations in membrane fluidity, which have also been reported [38]. As our strains were isolated from cocoa fermentation, we propose that the tested strains are similarly adapted to the relatively high temperature stress found during spontaneous cocoa fermentation which can reach 50°C [39].
Under the influence of oestrogen, glycogen is deposited in the human vagina, and the Lactobacillus strains use this glycogen to produce lactic acid. Thus, acidification of the vagina (pH ≤ 4.5) results in growth inhibition of other bacteria [40, 41]. Since vaginal pathogens colonise the vagina and raise the pH (4.5 to 6.0), these Lactobacillus strains can be used under these situations to acidify the mucosa and displace the pathogens, assisting other types of interventions such as antibiotic therapy [42, 43].
As observed in our work, many authors have reported that some Lactobacillus strains have the ability to inhibit G. vaginalis and N. gonorrhoeae growth [44,45,46]. The co-culture assay is able to assess the influence of one microorganism on the growth of another when both are incubated together, simulating what actually happens in the vaginal environment [47]. The growth-inhibiting activity of lactobacilli has generally been attributed to its lowering of pH, and production of lactic acid, hydrogen peroxide, and antibacterial compounds [48]. It is interesting to note that in each co-culture of Lactobacillus strains with pathogenic strains, the pH remained below 4.5, reinforcing both the presence of organic acids produced by L. plantarum strains and an acidic environment common to the healthy vagina [34].
Literature often correlates the probiotic activity of Lactobacillus strains against vaginal pathogens with the production of metabolites such as hydrogen peroxide [49]. Bacterial cell membranes are known to be semipermeable to H2O2, a reactive oxygen species, and act intracellularly, forming free radicals that cause widespread damage to DNA, membranes, enzymes, and proteins [50]. However, although vaginal colonisation by hydrogen peroxide-producing Lactobacillus strains is associated with lower rates of bacterial vaginosis, some authors suggest that the presence of these lactobacilli alone is not able to suppress BV-associated infection [51], and that lactic acid is the true effector molecule against uropathogens [52, 53].
The identification and characterisation of the antimicrobial substances produced by Lactobacillus strains demonstrated that organic acid was the key molecule in inhibiting both pathogens. In accordance with our data, Shokryazdan et al. [54] observed that the antimicrobial activity present in the CFCS of Lactobacillus strains was due to organic acids and antibacterial substances which can inhibit microbial growth by lowering the pH [55]. The physiological importance of lactic acid has been well documented in a review by Tachedjian et al. [56], which reported that lactic acid at physiological concentrations (110 mM), even at pH 4.5, mediates a potent 106-fold decrease in the viability of 17 different BV-associated microorganisms, but does not affect the viability of four vaginal lactobacilli in vitro. Lactic acid also acts not only on pathogens, but also interferes with vaginal immunomodulation, by directly inhibiting pro-inflammatory responses by IL-6, IL-8, and IL-1RA and inducing the Th17 lymphocyte pathway via IL-23 [57], promoting vaginal tissue homeostasis.
Conclusion
This study showed the potential probiotic characteristics of L. plantarum 03, L. plantarum 289, and L. plantarum 291 against G. vaginalis and N. gonorrhoeae. L. plantarum strains isolated from cocoa fermentation are safe and have probiotic properties, including biofilm formation, tolerance to heat and pH, direct competition with pathogens, and production of lactic acid and hydrogen peroxide. Our results indicate that these three lactobacilli strains have desirable properties for the development of future therapeutic agents; however, in vivo studies are necessary to confirm their properties against the tested vaginal pathogens.
Methods
Microorganisms and growth conditions
Three strains of Lactiplantibacillus plantarum (Lp03, Lp289, and Lp291) were isolated from spontaneous cocoa (Theobroma cacao) from the region of Ilhéus and Itabuna, BA, Brazil, and donated by the Laboratory of Applied Microbiology from the State University of Santa Cruz, Ilhéus, BA, Brazil [22]. Lactobacilli strains were grown in de Man, Rogosa, and Sharpe (MRS) agar or broth (Acumedia, Lansing, USA) for 18 to 24 h, at 37°C under microaerophilic conditions (5% CO2 atmosphere).
Gardnerella vaginalis ATCC 49154 was grown on 5% blood agar (HiMedia, Mumbai, India) or brain and heart infusion (BHI) (HiMedia) for 18 to 24 h at 37°C under microaerophilic conditions (5% CO2 atmosphere).
Neisseria gonorrhoeae (clinical isolate) was grown on chocolate agar (HiMedia) or BHI for 18 to 24 h, at 37°C under microaerophilic conditions (5% CO2 atmosphere).
Preparation of cell-free culture supernatant
The assay for obtaining CFCS was adapted from Pessoa et al. [47]. After overnight cultures of Lactobacillus strains were centrifuged (15 min, 3000 ×g), supernatants were discarded, and cell pellets (lactobacilli) were washed twice with sterile saline (0.9% NaCl) and resuspended to a final cell density (108 CFU mL-1). Suspensions (1,5 mL) of each Lactobacillus strain were then inoculated (10%, v/v) in sterile MRS broth (15 mL). After incubation (24 h, 37°C, 5% CO2 atmosphere), cultures were centrifuged (15 min, 3000 ×g) and the supernatants were aspirated using sterile syringes and sterilised by filtration (0.22 μm nitrocellulose filter; Merck, Darmstadt, Germany) to obtain CFCS.
Hydrophobicity assay
The hydrophobicity assay was adapted from Rodríguez et al. [58]. Suspensions (108 CFU mL-1) of each strain were obtained as previously described, and the optical density (660 nm) was measured. The solvent (xylene, 0.4 mL) was added to each bacterial suspension (1 mL) and the mixtures were vortexed vigorously and incubated for 2 h at 37°C. Then, the lower aqueous phase was removed with subsequent measurement of the optical density. The percentage of hydrophobicity (H%) was calculated as follows: H% = ((A0 – A2) / A0) × 100, where A0 indicates the absorbance at time 0 h, and A2 is the absorbance after 2 h. Hydrophobicity can be presented as microbial adhesion to solvents (MATS), which are classified as hydrophilic (MATS ≤ 44.99%), amphiphilic (45.00% ≤ MATS ≤ 54.99%), or hydrophobic (MATS ≥ 55.00%) [59]. In the second classification, hydrophobicity can also be presented as microbial adhesion to hydrocarbons (MATH), which can be classified as high (MATH > 66%), medium (33% < MATH < 66%), or low (MATH < 33%) [17].
Autoaggregation and co-aggregation assays
Autoaggregation and co-aggregation assays were adapted from Kos et al. [60]. For the autoaggregation assay, L. plantarum suspensions (108 CFU mL-1) were obtained as previously described. Then, these suspensions were vortexed (10 s) and incubated for 5 h at room temperature (25°C). Absorbance (660 nm) was measured at time 0 h (A0) and after 5 h (A5). The percentage of autoaggregation (AA%) was calculated using the following formula: AA% = ((A0 – A5) / A0) × 100.
For the co-aggregation assay, L. plantarum and pathogen strains were grown (MRS or BHI broth, 24 h, 37°C, 5% CO2 atmosphere), centrifuged (15 min, 3000 ×g), washed twice with sterile saline (0.9% NaCl), and resuspended to a final cell density (108 CFU mL-1). Cell suspensions with mixed suspensions containing equal volumes (1 mL) of each Lactobacillus strain and pathogen strain were vortexed (10 s) and incubated for 4 h at 37°C. Absorbance (660 nm) was measured before and after the incubation. The percentage of co-aggregation (CA%) was calculated using the following formula: CA% = [(ALAC + APAT) / 2 – AMIX] / [(ALAC + APAT)] / 2], where ALAC indicates the absorbance of the Lactobacillus strain, APAT indicates the absorbance of the pathogen strain, and AMIX indicates the absorbance of the mixtures.
Biofilm formation assay
The biofilm formation assay was adapted from Ouarabi et al. [61]. Initially, suspensions (108 CFU mL-1) of L. plantarum strains were obtained as previously described. An aliquot (10 μL) of each Lactobacillus strain was inoculated separately in trypticase soy broth (TSB, Acumedia) (200 μL) supplemented with peptone (20 g mL-1) in a 96-well polystyrene plate and then incubated overnight. After incubation, plates were washed twice with sterile saline to remove non-adherent cells. Cells were fixed with 96% ethanol (200 μL) and incubated for 15 min at room temperature (25°C). Plates were emptied and then filled with violet crystals (200 μL, 0.1%) and incubated for 15 min at room temperature (25°C). The plates were then washed twice with sterile saline, and the wells were resuspended in 96% ethanol (200 μL); absorbance (650 nm) was immediately measured and used as an indication of biofilm formation. Sterile medium was included as a negative control to ensure that the influence on biofilm formation was not attributed to a non-specific binding effect to crystal violet. Based on the optical densities of the isolates (ODI) and the negative control (ODC), the formation of biofilms by Lactobacillus strains was classified according to their adherence: non-adherent, ODI ≤ ODC; weakly adherent, ODC < ODI ≤ (2 × ODC), moderately adherent: (2 × ODC) < ODI ≤ (4 × ODC); strongly adherent: (4 × ODC) < ODI.
Haemolytic activity assay
The haemolytic activity assay was adapted from Abouloifa et al. [15]. Initially, suspensions (108 CFU mL-1) of L. plantarum strains were obtained as previously described and were spot-inoculated (10 μL) on 5% human blood agar. After incubating the plates (48 h, 37°C, 5% CO2 atmosphere), haemolytic activity was detected by observing a clear zone of hydrolysis around the colonies (β-haemolysis), partial hydrolysis with green-hued zones around colonies (α-haemolysis), or no zone around colonies (γ-haemolysis). γ-Haemolysis was considered negative haemolysis.
Antibiotic susceptibility assay
The susceptibility of Lactobacillus strains to antimicrobials was determined by the modified disk-diffusion method of the Clinical and Laboratory Standards Institute (CLSI). Overnight cultures of Lactobacillus strains were adjusted to 0.5 McFarland standards. An aliquot of this suspension was then swabbed onto MRS agar plates, followed by the arrangement of antibiotic disks. The antimicrobials (Laborclin, Pinhais, SP, Brazil) tested were ampicillin (10 μg), ceftriaxone (30 μg), ciprofloxacin (5 μg), clindamycin (2 μg), chloramphenicol (30 μg), erythromycin (15 μg), nitrofurantoin (300 μg), penicillin (10 μg) and vancomycin (30 μg). Plates were incubated overnight, and the diameters of the halos were measured and classified as sensitive (S), susceptible, increased exposure (SIE), and resistant (R), according to Charteris et al. [62]. Staphylococcus aureus ATCC 25923 was used as the positive control.
Heat tolerance assay
The heat resistance of Lactobacillus strains was evaluated according to Paéz et al. [35], with modifications. Initially, suspensions (108 CFU mL-1) of L. plantarum were obtained as previously described. An aliquot (100 μL) was resuspended in volume (500 μL) of 10% skim milk (Nestlé, Araçatuba, SP, Brazil). Then, each cell suspension was incubated in a water bath (60°C, 5 min), followed by cooling in an ice bath. Aliquots (10 μL) of each strain were plated on MRS agar, and after incubation (48 h, 37°C, 5% CO2 atmosphere) colonies were counted and enumerated considering CFU mL-1. As a control, aliquots (10 μL) of the same samples were plated under the same conditions before exposure to heat.
pH tolerance assay
Analysis of bacterial growth under various pH conditions was adapted from Melo et al. [16]. MRS and BHI broth solutions of pH 3-8 were prepared by addition of 1 mol L-1 of hydrochloric acid or sodium hydroxide. Before the assay, suspensions (108 CFU mL-1) of each strain (Lactobacillus and pathogens) were obtained as previously described. Trials were performed in 96-well microplates, where 180 μL of MRS or BHI broth at each pH was inoculated with 20 μL of active culture or saline as a control. The microplate was incubated overnight, and the optical density (600 nm) was determined at 8 h-intervals using a spectrophotometer (Tp-reader, Thermoplate, USA).
Coculture inhibition assay
The antimicrobial activity of Lactobacillus strains against pathogens was tested using a co-culture assay adapted from Hütt et al. [63]. Initially, suspensions (108 CFU mL-1) of Lactobacillus and pathogens were obtained as previously described. Activated cultures of pathogens and Lactobacillus were inoculated together (1%, v/v) in mixed growth medium (0.5 mL BHI broth + 0.5 mL MRS broth) and incubated (18 to 24 h, 37°C, 5% CO2 atmosphere). Serial dilutions were performed and aliquots (10 μL) were seeded on blood or chocolate agar followed by the reincubation (24 h, 37°C, 5% CO2 atmosphere) of plates. Cultures performed with the pathogen alone were used as negative controls. The growth of the pathogen with each Lactobacillus strain was compared with the growth of the control.
Evaluation of pH modulation by Lactobacillus strains
The ability of Lactobacillus strains to modulate the pH of the growth medium with or without pathogens was evaluated according to Melgaço et al. [64], with modifications. This test separately evaluated the modulation of pH with cultures isolated from lactobacilli strains in MRS medium, the modulation of pH with mixed cultures of each Lactobacillus strain with G. vaginalis in MRS + BHI medium, and the modulation of pH with mixed cultures from each Lactobacillus strain with N. gonorrhoeae. Initially, microorganism suspensions (108 CFU mL-1) were obtained as previously described and the pH of the MRS or MRS + BHI (v/v) broths was measured and adjusted to 6.5. Then, an aliquot of each Lactobacillus strain was added separately to the broth (10%, v/v) and the same volume of pathogen strains was added separately to the MRS + BHI broth. After incubation (24 h, 37°C, 5% CO2 atmosphere), cultures were centrifuged (15 min, 3000 ×g), the bacterial pellet was separated from the supernatant, and the pH of the bacterial cultures was measured (HMMPB-210, Highmed, Tatuapé, SP, Brazil).
Determination of CFCS antimicrobial activity: Deferred inhibition assay and microdiffusion assay on semi-solid agar
Antimicrobial activity was evaluated by the deferred inhibition assay according to Nardi et al. [65]. Initially, an aliquot (5 μL) of each lactobacillus strain suspension (108 CFU mL-1) was pipetted into the centre of the plate with MRS agar. After incubation for 48 h at 37°C in a 5% CO2 atmosphere, colony cells were killed by exposure to chloroform (30 min, 1 mL). Residual chloroform was evaporated and the Petri dish was overlaid with BHI semi-solid agar (3.5 mL, 0.75%, w/v), previously inoculated with pathogens (1%, v/v, 108 CFU mL-1). After overnight incubation, there was an inhibition halo. After incubation (18-24 h, 37°C, 5% CO2 atmosphere), the presence or absence of inhibition halos was observed, followed by measuring the inhibition halos (mm). Sterile MRS broth was used as the negative control.
The presence of diffusible inhibitory substances was also evaluated by a microdiffusion assay on semi-solid agar adapted from Rodrigues et al. [66]. Initially, suspensions of pathogens (108 CFU mL-1) were added (1%, v/v) on semi-solid BHI agar (0.75%, w/v) and plated. After solidification, sterile polyvinyl chloride (PVC) cylinders (8 mm) were placed centrally on the plates and aliquots (100 μL) of CFCS from each Lactobacillus were added. After incubation (18-24 h, 37°C, 5% CO2 atmosphere), the presence or absence of inhibition halos was observed, followed by measurement of the inhibition halos (mm). Sterile MRS broth was used as the negative control.
Detection of organic acids, thermotolerant antimicrobial substances, and bacteriocins in the CFCS
The lactobacilli strains were assayed for the production of organic acids, thermotolerant antimicrobial substances, and bacteriocins using the agar-well diffusion technique described by Touré et al. [67], with modifications. Initially, G. vaginalis suspension (108 CFU mL-1) was swabbed onto 5% blood agar plates, and N. gonorrhoeae suspension (108 CFU mL-1) was swabbed onto chocolate agar plates. Plates were then incubated for 30 min at room temperature (25°C). Concomitantly, the CFCS aliquots were distributed in fractions for treatment. For the organic acid assay, the CFCS was adjusted to pH 6.5 ± 0.1, using 1 mol · L-1 sodium hydroxide; for the thermotolerant substance assay the CFCS was incubated at high temperature (5 min, 100°C), and for bacteriocin assay the CSCF was treated with trypsin (1%, v/v; Gibco, Mississauga, Canada) or proteinase K (1%, v/v, Invitrogen, Darmstadt, Germany). Aliquots (100 μL) of treated and untreated CFCS were added to the wells (8 mm diameter) previously made on chocolate and 5% blood agar plates. Plates were incubated overnight and the diameters of the inhibition zones (including the 8 mm well diameter) were measured.
Amplex red hydrogen peroxide assay
Hydrogen peroxide levels present in CFCS were measured using the Amplex Red Hydrogen Peroxide/Peroxidase kit according to the manufacturer’s recommendations (Thermo Fisher Scientific, Waltham, MA, USA). After preparing the kit stock solutions, aliquots (50 μL) of the standard curve samples, controls, and experimental samples were added to individual wells on a microplate. The Amplex Red reagent/HRP working solution (50 μL) was added to the wells previously plotted. After incubation (30 min, room temperature - 25°C, protected from light), the absorbance was measured in a microplate reader (550 nm) to construct the standard curve and measure the H2O2 concentration (μM) of the CFCS.
Analysis of the CFCS metabolome by GC-MS
The CFCS metabolome was analysed by gas chromatography-mass spectrometry (GC-MS) according to the method described by Rodrigues et al. [66]. Initially, the CFCS of each Lactobacillus strain was previously conditioned (48 h, -18°C), then the frozen samples were inserted in a freeze dryer (Alpha 1-2 LDplus, CHRIST, Osterode, Germany) and subjected to the sublimation drying process (48 h) in two distinct phases. The primary drying phase consisted of removing free water (-20°C; 1.0 mbar) and the secondary drying phase consisted of partial removal of bound water (-30°C; 0.34 mbar). At the end of the process, the lyophilized CFCS was derivatized by silylation, and lyophilized samples (3 mg) were diluted in a mixture of 100 μL N, O-bis (trimethylsilyl)trifluoroacetamide (BSTFA) containing 1% trimethylchlorosilane (Sigma-Aldrich, Merck, Darmstadt, Germany) with pyridine (60 μL). During this reaction, mixtures were incubated for 30 min at 70°C in a water bath for better dilution. Samples were then injected (1 μL) separately into the chromatograph (QP2010SE-GC2010 Plus, Shimadzu, Kyoto, Japan) for metabolome screening. The hardware and software configurations of the equipment are described below: Chromatograph with Rtx-5MS (0.25 μm film, 30 m, and 0.25 mm internal diameter); helium gas as carrier gas; temperature of 290°C used in the injector, in the detector, and in the GC-MS system interface; initial temperature of 80°C (5 min); final temperature of 285°C (20 min); gradual increase from initial temperature to final temperature of 4°C min-1; the sweep mass operated from 30 to 600 Da; and the mass detector operated with electron impact ionisation (70 eV). GC-MS identified the substances present in the CFCS when comparing the mass spectra existing in the equipment database (WILEY8, NIST 08, and FFNSC1.3) with the mass spectra of the CFCS samples. This chromatography analysis did not require the use of positive or negative controls.
Statistical analysis
GraphPad Prism 6.0 software (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Quantitative data are presented by means and standard deviations. Normality was tested by D'Agostino & Pearson, Shapiro-Wilk and KS tests. The statistical differences between mean values were determined by the t test, Mann-Whitney test or Kruskal-Wallis test with Dunn's post-test. Data were considered statistically significant when: * = P < 0.05, ** P < 0.01, *** = P < 0.001, **** = P < 0.0001. Except for CFCS metabolome, all assays were performed in triplicate.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AA%:
-
Percentage of autoaggregation
- ATCC:
-
American Type Culture Collection
- BHI:
-
brain and heart infusion
- BSTFA:
-
N,O-Bis(trimethylsilyl)trifluoroacetamide
- BV:
-
bacterial vaginosis
- CA%:
-
percentage of co-aggregation
- CFCS:
-
cell-free culture supernatants
- CLSI:
-
Clinical and Laboratory Standards Institute
- FAO:
-
Food and Agriculture Organization
- GC-MS:
-
Gas chromatography-mass spectrometry
- GRAS:
-
Generally recognised as safe
- H%:
-
Percentage of hydrophobicity
- Lp03:
-
Lactiplantibacillus plantarum 03
- Lp289:
-
Lactiplantibacillus plantarum 289
- Lp291:
-
Lactiplantibacillus plantarum 291
- MATH:
-
Microbial adhesion to hydrocarbons
- MATS:
-
Microbial adhesion to solvents
- MRS:
-
Man, Rogosa, and Sharpe
- OD:
-
Optical density
- PVC:
-
Polyvinyl chloride
- TSB:
-
Trypticase soy broth
References
Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–58. https://doi.org/10.2147/IDR.S173867.
Azad MAK, Sarker M, Li T, Yin J. Probiotic species in the modulation of gut microbiota: an overview. BioMed Res Int. 2018;2018:9478630. https://doi.org/10.1155/2018/9478630.
Buggio L, Somigliana E, Borghi A, Vercellini P. Probiotics and vaginal microecology: fact or fancy? BMC Womens Health. 2019;19(1):25. https://doi.org/10.1186/s12905-019-0723-4.
Xie HY, Feng D, Wei DM, Mei L, Chen H, Wang X, et al. Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst Rev. 2017;11(11):CD010496. https://doi.org/10.1002/14651858.
Wang Z, He Y, Zheng Y. Probiotics for the treatment of bacterial vaginosis: a meta-analysis. Int J Environ Res Public Health. 2019;16(20):3859. https://doi.org/10.3390/ijerph16203859.
Algburi A, Volski A, Chikindas ML. Natural antimicrobials subtilosin and lauramide arginine ethyl ester synergize with conventional antibiotics clindamycin and metronidazole against biofilms of Gardnerella vaginalis but not against biofilms of healthy vaginal lactobacilli. Pathog Dis. 2015;73(5):ftv018. https://doi.org/10.1093/femspd/ftv018.
Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29(2):223–38. https://doi.org/10.1128/CMR.00075-15.
Ali S, Sewunet T, Sahlemariam Z, Kibru G. Neisseria gonorrhoeae among suspects of sexually transmitted infection in Gambella hospital, Ethiopia: risk factors and drug resistance. BMC Res Notes. 2016;9(1):439. https://doi.org/10.1186/s13104-016-2247-4.
Unemo M. Current and future antimicrobial treatment of gonorrhoea - the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis. 2015;15:364. https://doi.org/10.1186/s12879-015-1029-2.
Foschi C, Salvo M, Cevenini R, Parolin C, Vitali B, Marangoni A. Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: an in vitro study. Front Cell Infect Microbiol. 2017;7:502. https://doi.org/10.3389/fcimb.2017.00502.
Zawistowska-Rojek A, Tyski S. Are probiotic really safe for humans? Pol J Microbiol. 2018;67(3):251–8. https://doi.org/10.21307/pjm-2018-044.
Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782–858. https://doi.org/10.1099/ijsem.0.004107.
Vicariotto F, Mogna L, Del Piano M. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: a pilot study. J Clin Gastroenterol. 2014;48(Suppl 1):S106–12. https://doi.org/10.1097/MCG.0000000000000226.
Todorov SD, Wachsman MB, Knoetze H, Meincken M, Dicks LM. An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soya beans. Int J Antimicrob Agents. 2005;25(6):508–13. https://doi.org/10.1016/j.ijantimicag.2005.02.005.
Abouloifa H, Rokni Y, Bellaouchi R, Ghabbour N, Karboune S, Brasca M, et al. Characterization of probiotic properties of antifungal Lactobacillus strains isolated from traditional fermenting green olives. Probiotics Antimicrob Proteins. 2020;12(2):683–96. https://doi.org/10.1007/s12602-019-09543-8.
Melo TA, Dos Santos TF, Pereira LR, Passos HM, Rezende RP, Romano CC. Functional profile evaluation of Lactobacillus fermentum TCUESC01: a new potential probiotic strain isolated during cocoa fermentation. BioMed Res Int. 2017;2017:5165916. https://doi.org/10.1155/2017/5165916.
Bouchard DS, Seridan B, Saraoui T, Rault L, Germon P, Gonzalez-Moreno C, et al. Lactic acid bacteria isolated from bovine mammary microbiota: Potential allies against bovine mastitis. PLOS ONE. 2015;10(12):e0144831. https://doi.org/10.1371/journal.pone.0144831.
Huys G, Botteldoorn N, Delvigne F, De Vuyst L, Heyndrickx M, Pot B, et al. Microbial characterization of probiotics--advisory report of the working group “8651 probiotics” of the Belgian Superior Health Council (SHC). Mol Nutr Food Res. 2013;57(8):1479–504. https://doi.org/10.1002/mnfr.201300065.
Jose NM, Bunt CR, Hussain MA. Comparison of microbiological and probiotic characteristics of lactobacilli isolates from dairy food products and animal rumen contents. Microorganisms. 2015;3(2):198–212. https://doi.org/10.3390/microorganisms3020198.
do Carmo MS, Noronha FM, Arruda MO, Costa ÊP, Bomfim MR, Monteiro AS, et al. Lactobacillus fermentum ATCC 23271 displays in vitro inhibitory activities against Candida spp. Front Microbiol. 2016;7:1722. https://doi.org/10.3389/fmicb.2016.01722.
Polak-Berecka M, Waśko A, Paduch R, Skrzypek T, Sroka-Bartnicka A. The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus. Antonie Leeuwenhoek. 2014;106(4):751–62. https://doi.org/10.1007/s10482-014-0245-x.
Teles Santos T, Santos Ornellas RM, Borges Arcucio L, Messias Oliveira M, Nicoli JR, Villela Dias C, et al. Characterization of lactobacilli strains derived from cocoa fermentation in the south of Bahia for the development of probiotic cultures. LWT. 2016;73:259–66. https://doi.org/10.1016/j.lwt.2016.06.003.
Zhang B, Zuo F, Yu R, Zeng Z, Ma H, Chen S. Comparative genome-based identification of a cell wall-anchored protein from Lactobacillus plantarum increases adhesion of Lactococcus lactis to human epithelial cells. Sci Rep. 2015;5:14109. https://doi.org/10.1038/srep14109.
Anandharaj M, Sivasankari B, Santhanakaruppu R, Manimaran M, Rani RP, Sivakumar S. Determining the probiotic potential of cholesterol-reducing Lactobacillus and Weissella strains isolated from gherkins (fermented cucumber) and south Indian fermented koozh. Res Microbiol. 2015;166(5):428–39. https://doi.org/10.1016/j.resmic.2015.03.002.
Bouridane H, Sifour M, Idoui T, Annick L, Thonard P. Technological and probiotic traits of the lactobacilli isolated from vaginal tract of the healthy women for probiotic use. Iran J Biotechnol. 2016;14(3):192–201. https://doi.org/10.15171/ijb.1432.
Kang CH, Han SH, Kim Y, Paek NS, So JS. In vitro probiotic properties of Lactobacillus salivarius MG242 isolated from human vagina. Probiotics Antimicrob Proteins. 2018;10(2):343–9. https://doi.org/10.1007/s12602-017-9323-5.
Prabhurajeshwar C, Chandrakanth RK. Probiotic potential of lactobacilli with antagonistic activity against pathogenic strains: an in vitro validation for the production of inhibitory substances. Biomed J. 2017;40(5):270–83. https://doi.org/10.1016/j.bj.2017.06.008.
Pino A, Bartolo E, Caggia C, Cianci A, Randazzo CL. Detection of vaginal lactobacilli as probiotic candidates. Sci Rep. 2019;9(1):3355. https://doi.org/10.1038/s41598-019-40304-3.
Sabbatini S, Monari C, Ballet N, Mosci P, Decherf AC, Pélerin F, et al. Saccharomyces cerevisiae-based probiotic as novel anti-microbial agent for therapy of bacterial vaginosis. Virulence. 2018;9(1):954–66. https://doi.org/10.1080/21505594.2018.1464362.
Vielfort K, Sjölinder H, Roos S, Jonsson H, Aro H. Adherence of clinically isolated lactobacilli to human cervical cells in competition with Neisseria gonorrhoeae. Microbes Infect. 2008;10(12-13):1325–34. https://doi.org/10.1016/j.micinf.2008.07.032.
Arena MP, Capozzi V, Russo P, Drider D, Spano G, Fiocco D. Immunobiosis and probiosis: antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl MicrobiolBiotechnol. 2018;102(23):9949–58. https://doi.org/10.1007/s00253-018-9403-9.
Strus M, Kucharska A, Kukla G, Brzychczy-Włoch M, Maresz K, Heczko PB. The in vitro activity of vaginal Lactobacillus with probiotic properties against Candida. Infect Dis Obstet Gynecol. 2005;13(2):69–75. https://doi.org/10.1080/10647440400028136.
Kaur S, Sharma P, Kalia N, Singh J, Kaur S. Anti-biofilm properties of the fecal probiotic lactobacilli against Vibrio spp. Front Cell Infect Microbiol. 2018;8:120. https://doi.org/10.3389/fcimb.2018.00120.
Leccese Terraf MC, Mendoza LM, Juárez Tomás MS, Silva C, Nader-Macías ME. Phenotypic surface properties (aggregation, adhesion and biofilm formation) and presence of related genes in beneficial vaginal lactobacilli. J Appl Microbiol. 2014;17(6):1761–72. https://doi.org/10.1111/jam.12642.
Paéz R, Lavari L, Vinderola G, Audero G, Cuatrin A, Zaritzky N, et al. Effect of heat treatment and spray drying on lactobacilli viability and resistance to simulated gastrointestinal digestion. Food Res Int. 2012;48(2):748–54. https://doi.org/10.1016/j.foodres.2012.06.018.
Sharma P, Tomar SK, Sangwan V, Goswami P, Singh R. Antibiotic resistance of Lactobacillus sp. Isolated from commercial probiotic preparations. J Food Saf. 2016;36(1):38–51. https://doi.org/10.1111/jfs.12211.
Al Kassaa I, Hamze M, Hober D, Chihib NE, Drider D. Identification of vaginal lactobacilli with potential probiotic properties isolated from women in North Lebanon. Microb Ecol. 2014;67(3):722–34. https://doi.org/10.1007/s00248-014-0384-7.
Zommiti M, Connil N, Hamida JB, Ferchichi M. Probiotic characteristics of Lactobacillus curvatus DN317, a strain isolated from chicken ceca. Probiotics Antimicrob Proteins. 2017;9(4):415–24. https://doi.org/10.1007/s12602-017-9301-y.
Aakko J, Sánchez B, Gueimonde M, Salminen S. Assessment of stress tolerance acquisition in the heat-tolerant derivative strains of Bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus rhamnosus GG. J Appl Microbiol. 2014;117(1):239–48. https://doi.org/10.1111/jam.12520.
Schwan RF, Wheals AE. The microbiology of cocoa fermentation and its role in chocolate quality. Crit Rev Food Sci Nutr. 2004;44(4):205–21. https://doi.org/10.1080/10408690490464104.
Witkin SS, Linhares IM. Why do lactobacilli dominate the human vaginal microbiota? BJOG. 2017;124(4):606–11. https://doi.org/10.1111/1471-0528.14390.
Spear GT, French AL, Gilbert D, Zariffard MR, Mirmonsef P, Sullivan TH, et al. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis. 2014;210(7):1019–28. https://doi.org/10.1093/infdis/jiu231.
Bradshaw CS, Sobel JD. Current treatment of bacterial vaginosis-limitations and need for innovation. J Infect Dis. 2016;214(Suppl 1):S14–20. https://doi.org/10.1093/infdis/jiw159.
Bertuccini L, Russo R, Iosi F, Superti F. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int J Immunopathol Pharmacol. 2017;30(2):163–7. https://doi.org/10.1177/0394632017697987.
Breshears LM, Edwards VL, Ravel J, Peterson ML. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015;15:276. https://doi.org/10.1186/s12866-015-0608-0.
Santos CMA, Pires MCV, Leão TL, Hernández ZP, Rodriguez ML, Martins AKS, et al. Selection of Lactobacillus strains as potential probiotics for vaginitis treatment. Microbiology (Reading). 2016;162(7):1195–207. https://doi.org/10.1099/mic.0.000302.
Pessoa WFB, Melgaço ACC, de Almeida ME, Ramos LP, Rezende RP, Romano CC. In vitro activity of lactobacilli with probiotic potential isolated from cocoa fermentation against Gardnerella vaginalis. BioMed Res Int. 2017;2017:3264194. https://doi.org/10.1155/2017/3264194.
Graver MA, Wade JJ. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob. 2011;10:8. https://doi.org/10.1186/1476-0711-10-8.
Atassi F, Brassart D, Grob P, Graf F, Servin AL. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol Med Microbiol. 2006;48(3):424–32. https://doi.org/10.1111/j.1574-695X.2006.00162.x.
Quillin SJ, Hockenberry AJ, Jewett MC, Seifert HS. Neisseria gonorrhoeae exposed to sublethal levels of hydrogen peroxide mounts a complex transcriptional response. mSystems. 2018;3(5):e00156–18. https://doi.org/10.1128/mSystems.00156-18.
Mitchell C, Fredricks D, Agnew K, Hitti J. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Dis. 2015;42(7):358–63. https://doi.org/10.1097/OLQ.0000000000000298.
O’Hanlon DE, Lanier BR, Moench TR, Cone RA. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect Dis. 2010;10:120. https://doi.org/10.1186/1471-2334-10-120.
Rose WA 2nd, McGowin CL, Spagnuolo RA, Eaves-Pyles TD, Popov VL, Pyles RB. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLOS ONE. 2012;7(3):e32728. https://doi.org/10.1371/journal.pone.0032728.
Shokryazdan P, Sieo CC, Kalavathy R, Liang JB, Alitheen NB, Faseleh Jahromi M, et al. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res Int. 2014;2014:927268. https://doi.org/10.1155/2014/927268.
Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol. 2015;6:164. https://doi.org/10.3389/fphys.2015.00164.
Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017;168(9–10):782–92. https://doi.org/10.1016/j.resmic.2017.04.001.
Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595(2):451–63. https://doi.org/10.1113/JP271694.
Rodríguez C, Cofré JV, Sánchez M, Fernández P, Boggiano G, Castro E. Lactobacilli isolated from vaginal vault of dairy and meat cows during progesteronic stage of estrous cycle. Anaerobe. 2011;17(1):15–8. https://doi.org/10.1016/j.anaerobe.2010.12.001.
Sandes S, Alvim L, Silva B, Acurcio L, Santos C, Campos M, et al. Selection of new lactic acid bacteria strains bearing probiotic features from mucosal microbiota of healthy calves: Looking for immunobiotics through in vitro and in vivo approaches for immunoprophylaxis applications. Microbiol Res. 2017;200:1–13. https://doi.org/10.1016/j.micres.2017.03.008.
Kos B, Susković J, Vuković S, Simpraga M, Frece J, Matosić S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol. 2003;94(6):981–7. https://doi.org/10.1046/j.1365-2672.2003.01915.x.
Ouarabi L, Chait YA, Seddik HA, Drider D, Bendali F. Newly isolated lactobacilli strains from Algerian human vaginal microbiota: Lactobacillus fermentum strains relevant probiotic’s candidates. Probiotics Antimicrob Proteins. 2019;11(1):43–54. https://doi.org/10.1007/s12602-017-9360-0.
Charteris WP, Kelly PM, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot. 1998;61(12):1636–43. https://doi.org/10.4315/0362-028x-61.12.1636.
Hütt P, Shchepetova J, Lõivukene K, Kullisaar T, Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol. 2006;100(6):1324–32. https://doi.org/10.1111/j.1365-2672.2006.02857.x.
Melgaço ACC, Blohem Pessoa WF, Freire HP, Evangelista de Almeida M, Santos Barbosa M, Passos Rezende R, et al. Potential of maintaining a healthy vaginal environment by two Lactobacillus strains isolated from cocoa fermentation. BioMed Res Int. 2018;2018:7571954. https://doi.org/10.1155/2018/7571954.
Nardi RD, Santos ARM, Carvalho MAR, Farias LM, Benchetrit LC, Nicoli JR. Antagonism against anaerobic and facultative bacteria through a diffusible inhibitory compound produced by a Lactobacillus sp. isolated from the rat fecal microbiota. Anaerobe. 1999;5(3-4):409–11. https://doi.org/10.1006/anae.1999.0217.
Rodrigues JZS, Passos MR, Silva de Macêdo Neres N, Almeida RS, Pita LS, Santos IA, et al. Antimicrobial activity of Lactobacillus fermentum TcUESC01 against Streptococcus mutans UA159. Microb Pathog. 2020;142:104063. https://doi.org/10.1016/j.micpath.2020.104063.
Touré R, Kheadr E, Lacroix C, Moroni O, Fliss I. Production of antibacterial substances by bifidobacterial isolates from infant stool active against Listeria monocytogenes. J Appl Microbiol. 2003;95(5):1058–69. https://doi.org/10.1046/j.1365-2672.2003.02085.x.
Acknowledgements
The authors are grateful to the AcademicEnglishSolutions.com for revising the English language.
Funding
Experiments were performed at the Federal University of Bahia, Campus Anísio Teixeira, and were funded by Fundação de Amparo a Pesquisa do Estado da Bahia (FAPESB - RED0016/2014) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Code 001).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: NNS, GBC, JT, APTU, RY and LMM; Cell surface properties of lactobacilli assays: NNS, HBMO, HFL, YBA, BAS and LMM; Safety assessment of lactobacilli strains for use as a probiotic assays: NNS, HBMO, HFL, YBA, BAS and LMM; Analysis of the technological characteristics lactobacilli assays: NNS, HBMO, HFL, YBA, BAS and LMM; Inhibition of pathogen growth by lactobacilli assays: NNS, HBMO, HFL, YBA, BAS, CCR, RPR and LMM; Evaluation of acid production assays: NNS, CFA, LSCP, MMR, YBA, BAS and LMM; Identification and characterization of antimicrobial substances assays: NNS, HBMO, TMLC, LFB, TLSB, AMC and LMM; CFCS metabolome profile assay: NNS, MPC, RY, AMC and LMM.
Analysed the data: NNS, GBC, RY, APTU, JT, and LMM; Contributed reagents/materials/analysis tools: JT, RY, APTU, and LMM. Wrote the paper: NNS, GBC, RY, APTU, and LMM. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
das Neves Selis, N., de Oliveira, H.B.M., Leão, H.F. et al. Lactiplantibacillus plantarum strains isolated from spontaneously fermented cocoa exhibit potential probiotic properties against Gardnerella vaginalis and Neisseria gonorrhoeae. BMC Microbiol 21, 198 (2021). https://doi.org/10.1186/s12866-021-02264-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-021-02264-5