Abstract
Background
H2O2 produced by vaginal lactobacilli is believed to protect against infection, and H2O2-producing lactobacilli inactivate pathogens in vitro in protein-free salt solution. However, cervicovaginal fluid (CVF) and semen have significant H2O2-blocking activity.
Methods
We measured the H2O2 concentration of CVF and the H2O2-blocking activity of CVF and semen using fluorescence and in vitro bacterial-exposure experiments.
Results
The mean H2O2 measured in fully aerobic CVF was 23 ± 5 μM; however, 50 μM H2O2 in salt solution showed no in vitro inactivation of HSV-2, Neisseria gonorrhoeae, Hemophilus ducreyii, or any of six BV-associated bacteria. CVF reduced 1 mM added H2O2 to an undetectable level, while semen reduced 10 mM added H2O2 to undetectable. Moreover, the addition of just 1% CVF supernatant abolished in vitro pathogen-inactivation by H2O2-producing lactobacilli.
Conclusions
Given the H2O2-blocking activity of CVF and semen, it is implausible that H2O2-production by vaginal lactobacilli is a significant mechanism of protection in vivo.
Similar content being viewed by others
Background
The health of the female genital tract depends significantly upon the composition of the vaginal microflora. Bacterial vaginosis (BV) is a common microfloral disturbance: the lactobacilli that dominate a healthy vaginal microflora are replaced by a high-density, polymicrobial mix of other bacteria [1, 2]. BV is associated with increased rates of many different genital tract infections, suggesting that vaginal lactobacilli provide broad-spectrum protection against pathogens.
Hydrogen peroxide is a broad-spectrum disinfectant, and cervicovaginal fluid (CVF) contains myeloperoxidase (MPO) that enhances pathogen-inactivation by H2O2 [3, 4]. Epidemiological studies suggest that women with H2O2-producing lactobacilli are less likely to be infected with HIV-1, HSV-2, Trichomonas vaginalis, Gardnerella vaginalis, and gram-negative anaerobes associated with BV [5–7]. Several studies have reported that BV and H2O2-producing lactobacilli are strongly negatively associated: women with BV are between three and twelve times less likely to have H2O2-producing lactobacilli than women without BV [8–11].
Hydrogen peroxide-producing lactobacilli have also been shown to inactivate HIV-1 virions, and BV-associated bacteria when tested in protein-free salt solutions, giving support to the hypothesis that H2O2-production by vaginal lactobacilli is protective [12, 13]. However, CVF and semen contain proteins, glycoproteins, polysaccharides, lipids, and other molecules with the potential to react with and inactivate H2O2. Additionally, the vagina is hypoxic most of the time, though the concentration of oxygen in the vagina increases following the insertion of a contraceptive diaphragm, during sexual arousal, and presumably during sexual intercourse [14–16]. Lactobacilli require oxygen to produce hydrogen peroxide: H2O2 concentration is undetectable during anaerobic culture, reaches 29-450 μM during aerobic culture, and 1.0-1.8 mM with vigorous aeration [17–19].
The primary aim of this study was to measure the H2O2 concentration of CVF from women with H2O2-producing lactobacilli microflora, and the H2O2-blocking activities of CVF and semen, to assess the likelihood that H2O2 produced by vaginal lactobacilli provides significant protection in vivo. Additionally, we tested whether H2O2 in simple salt solution at somewhat more than the concentration found in our CVF samples could inactivate vaginal pathogens, and whether CVF has the ability to block pathogen-inactivation by H2O2-producing lactobacilli.
Methods
All materials and reagents were supplied by Sigma-Aldrich Inc. (St. Louis MO) unless otherwise specified; all microorganisms were supplied by the American Type Culture Collection (Manassas VA).
Cervico-vaginal fluid and semen donors
The study was carried out at the Johns Hopkins University Homewood campus; participants were recruited primarily from among students and staff at the university. Our research conforms to the requirements of the Declaration of Helsinki, and the relevant federal and state laws; each participant gave written informed consent under a protocol approved by the Homewood Institutional Review Board on the Use of Human Subjects at Johns Hopkins University. Participants were required to be between 18 and 45 years old, and in good general health; female participants were at least three days past the most recent menstruation or unprotected penile-vaginal intercourse, at least three weeks past the most recent use of vaginal or systemic antimicrobials, and free from vaginal symptoms (discharge, odour, itching, or pain). Results from samples donated by six male and twenty-two female participants are reported here; the group comprised roughly equal numbers of non-Hispanic whites, blacks, and Asians, aged between 18 and 44 years old (mean age 26 ± 5 years).
Collection of cervicovaginal fluid and semen samples
The non-absorbent disposable Instead® menstrual cup (Instead Inc., La Jolla CA) was used to sample non-menstrual CVF. Unlike the more common collection methods of lavage, tampon, swab, or filter paper, the Instead® cup collects a relatively large sample of CVF (a mix of cervical mucus, other secretions, and transudate) from a large area of the vagina without the use a speculum or dilution of the sample [20–22]. CVF adheres to the rim and both sides of the dome of the Instead® cup, and is removed from the device by centrifugation.
The Instead® cup was vaginally inserted, removed, and placed in a conical tube that was immediately transferred to a glove-box mimicking the hypoxia that generally prevails in the vagina: partial pressure of oxygen in the glove-box was 6.0 mm Hg ± 0.7 mm Hg, as measured with an MI-730 oxygen electrode (Microelectrodes Inc., Bedford NH), similar to the 4-14 mm Hg reported for the vagina [23]. Anaerotest® indicator strips (manufacturer's estimated threshold of sensitivity 7 mm Hg, EMD Chemicals Inc., Gibbstown NJ) were used to ensure that the glove-box maintained hypoxic conditions throughout experiments. The tube containing the Instead® was sparged with nitrogen and sealed. In all cases, the time elapsed between inserting the Instead® into the vagina, sparging and sealing the tube in the glove-box was less than one minute. The sealed tube was removed from the glove-box, centrifuged for 1 minute at 1000 rpm (500g), and returned to the glove-box before being opened.
Semen samples were obtained by masturbation, maintained aerobically at room temperature, and used within 45 minutes of collection. Neither CVF nor semen samples were pooled; each experiment was performed several times (see results for n values) using samples from different donors.
Evaluation of the samples
A smear from each sample of CVF was gram-stained and scored using the Nugent standardized system [24]. Samples for use in the study were restricted to those with Nugent scores ≤3 (indicating healthy vaginal microflora) and absence of vaginal leucorrhea (mean polymorphonuclear leukocyte per high powered field [PMNL/hpf] <10) [25]. A total of twenty-four female participants donated CVF; two samples were discarded due to Nugent scores >3, and no samples were excluded due to leucorrhea (mean PMNL/hpf of the included samples was 2.1). The low rate of discarded samples is consistent with the participant exclusion criteria (i.e., presence of any vaginal symptoms), and the study population's low-risk composition (generally young, affluent, with a high rate of condom use, and low rates of smoking, vaginal cleansing, and sexually transmitted infections).
H2O2-production in each CVF sample was assessed by growth on TMB-plus agar [26]; all samples met the criteria for H2O2-producing microflora [27], consistent with the characteristics of the study population [28, 29]. An aliquot from each sample of semen was viability-stained, and the number of live sperm, dead sperm, and leukocytes present were scored [30]. All semen samples collected met WHO criteria for normal quality [31].
Measuring hydrogen peroxide concentration in cervicovaginal fluid
Amplex Red® (Invitrogen, Eugene OR) substrate in combination with horseradish peroxidase is a sensitive fluorescence assay for H2O2 [32]. Following the manufacturer's protocol, the assay reactions contained 50 μM Amplex Red®, 1 U/mL horseradish peroxidase, and 100 μL of CVF or semen in a 200 uL final reaction volume; however, the concentration of the reaction salt solution was increased from 50 mM to 250 mM to maintain an optimal reaction pH in the presence of CVF. Control experiments showed the change in salt solution concentration did not interfere with the ability of the assay to detect and quantify H2O2 (data not shown). The assay was carried out hypoxically or aerobically as indicated for the individual experiments. Control experiments showed that deoxygenating with nitrogen and incubating hypoxically did not interfere with the sensitivity or accuracy of the assay (data not shown).
Measuring hydrogen peroxide-blocking activity of cervicovaginal fluid and semen
The oxidant-blocking activity in a sample differs with respect to different oxidant species [33, 34]; we therefore tested the ability of CVF and semen to block exogenous H2O2. Aliquots of CVF or semen were diluted with an equal volume of 250 mM Na2HPO4 containing H2O2 at concentrations between 20 μM and 2 M, giving final H2O2 concentrations between 10 μM and 1 M. These aliquots were stirred for five seconds, and H2O2 concentration was measured aerobically using the Amplex Red® assay.
Measuring effect of aerobic exposure on hydrogen peroxide in cervicovaginal fluid
To estimate the amount of H2O2 produced in the generally hypoxic environment of the vagina, the H2O2 of CVF was measured immediately after sample collection and transfer to the hypoxic glove-box (i.e., after the approximately one minute aerobic exposure necessitated by the collection method). Some CVF samples were then maintained hypoxically at 37°C for one and a half hours, with an aliquot withdrawn every fifteen minutes and assayed hypoxically for H2O2 content.
To estimate the amount of H2O2 produced during periods of increased vaginal oxygen (as during sexual intercourse, when sexually transmitted pathogens might be introduced), CVF samples were first hypoxically equilibrated at 37°C for four hours, then exposed to air for one minute, fifteen minutes, or four hours and assayed for H2O2 content.
Measuring effect of CVF supernatant on pathogen-inactivation by H2O2-producing lactobacilli
The production of H2O2 by Lactobacillus crispatus ATCC® 33820™ was confirmed by growth on TMB-plus agar. We replicated the protocol used by Klebanoff et al [35] to test in vitro pathogen-inactivation by H2O2-producing lactobacilli: L. crispatus was grown anaerobically in Difco™ Lactobacilli MRS broth (Becton, Dickinson and Co., Sparks MD) for approximately 24 hours; six hours before use in an experiment, the lactobacilli were transferred to peptone-yeast extract broth and grown aerobically with vigorous agitation. Gardnerella vaginalis ATCC® 14018™ was grown anaerobically in NYC III broth for approximately 24 hours; three hours before use in an experiment, the bacteria were transferred to peptone-starch-dextrose broth and grown aerobically with vigorous agitation. Prevotella bivia ATCC® 29303™ was grown anaerobically in chopped meat broth for approximately 24 hours. Immediately before an experiment, the cultures of all three bacterial species were washed twice in 100 mM Na2SO4, and re-suspended in Na2SO4 containing 100 mM NaCl and 48 mU/mL human MPO. A 250 μL aliquot of re-suspended G. vaginalis or P. bivia was mixed with an equal volume of re-suspended L. crispatus.
To avoid conflating endogenous vaginal bacteria with the cultured L. crispatus, G. vaginalis, and P. bivia used in these experiments, bacteria-depleted supernatants of CVF were prepared: each collected sample was diluted with a half-volume of 0.9% saline, mixed thoroughly, centrifuged at 1000 g for three minutes, and the supernatant drawn off for immediate use in an experiment [36]. Control experiments showed this centrifugation reduced bacterial concentrations in the diluted CVF from a mean of 5.6 × 107 cfu/mL to a mean of 4.0 × 101 cfu/mL (data not shown). The CVF supernatant (or an equal volume of saline as a negative control) was added to the washed and re-suspended G. vaginalis or P. bivia immediately before the addition of the L. crispatus. G. vaginalis mixtures were incubated aerobically at 37°C for 1 hour, then serially diluted with 100 mM Na2SO4 and plated onto blood agar that was incubated aerobically. P. bivia mixtures were incubated aerobically at 37°C for 30 minutes, then serially diluted with 0.9% saline containing 0.02% dithiothreitol, and duplicate-plated onto two sets of brucella agar plates; one set was incubated aerobically and the other anaerobically. After approximately 36 hours, plated colonies were counted: as in Klebanoff's experiments, G. vaginalis was distinguished from L. crispatus by colony morphology, growth habit, and presence of haemolysis on blood agar; P. bivia was distinguished from L. crispatus by colony morphology, growth habit, and the failure of P. bivia to grow on the aerobically incubated plates.
Measuring pathogen-inactivation by 50 μM exogenous hydrogen peroxide
In these experiments, we replaced the H2O2-producing L. crispatus with exogenous H2O2 at a concentration above the concentration we measured in CVF samples, and expanded the list of target pathogens. Mycoplasma hominis ATCC® 14268™, Mobiluncus curtsii ATCC® 35241™, Mobiluncus mulieris ATCC® 35239™, Peptostreptococcus anaerobius ATCC® 27337™, Hemophilus ducreyii ATCC® 33940™, G. vaginalis and P. bivia were grown anaerobically, washed, and re-suspended as described for P. bivia, above. Neisseria gonorrhoeae ATCC® 19424™ was grown aerobically in ATCC® medium #814, washed and re-suspended in the same way as P. bivia.
Aliquots of each individual bacterial culture were mixed with an equal volume of 100 mM Na2SO4 containing either 100 μM H2O2 with 100 mU/mL MPO, or 100 μM H2O2 without MPO, or plain salt solution as a negative control. The aliquots were incubated aerobically at 37°C for 1 hour, then serially diluted with 100 mM Na2SO4 and plated onto blood agar; plates were incubated for approximately 36 hours and colonies counted.
Aliquots of HSV-2 cell-free virus (ATCC® VR-734™) were mixed with an equal volume of 100 μM H2O2 and 100 mU/mL MPO, or 100 μM H2O2 without MPO, or plain 100 mM Na2SO4, and incubated aerobically at 37°C for 30 minutes. The aliquots were then serially diluted with DMEM cell-growth medium supplemented with 10% fetal bovine serum (SAFC Biosciences, Lenexa KS) and plated onto ELVIS HSV-2 indicator cells (Diagnostic Hybrids, Athens OH), which were incubated, fixed, stained, and enumerated according to the manufacturer's instructions.
Statistical analysis
Results are reported as means ± SDs of at least four independently repeated experiments. Difference between three or more means was tested using an ANOVA one-way analysis of variance; difference between two means was tested using a two-tailed Student's t test (comparisons are paired unless otherwise indicated in the results). Statistical analysis was performed using Microsoft Excel PHSTAT.
Results
Hydrogen peroxide in cervicovaginal fluid
Hydrogen peroxide was only detectable after aerobic exposure: CVF samples (n = 6) incubated hypoxically for one hour had no detectable H2O2 remaining (Figure 1). Samples equilibrated hypoxically for four hours produced H2O2 following even brief exposures to air (Figure 2, open circles). H2O2 concentration was 15 μM ± 4 μM (n = 4) after 1 minute of aerobic exposure, and rose to 22 μM ± 4 μM after 15 minutes (n = 4), but H2O2 concentration did not continue to rise with further aerobic exposure. H2O2 concentration was 23 μM ± 5 μM after 4 hours in air (n = 8), not significantly increased (P = .36) compared to the concentration after 15 minutes (Figure 2, open circles).
The mean H2O2 content of CVF samples (n = 16) assayed immediately after sample collection and transfer to the hypoxic glove-box (i.e., after approximately one minute of aerobic exposure) was 12 μM ± 5 μM. There was no significant difference (unpaired comparison, P = .77) in H2O2 content between this group of CVF samples assayed immediately after collection, and the samples assayed after four hours hypoxic incubation followed by one minute of aerobic exposure (Figure 2, closed circles versus open circles), supporting our conclusion that 4 hours hypoxia had not affected the samples' ability to produce H2O2 when exposed to air.
In vitro pathogen-inactivation by 50 μM exogenous hydrogen peroxide
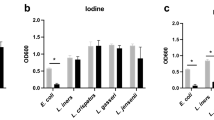
Exogenous H2O2 at 50 μM (more than the maximum concentration we found in fully aerobically exposed CVF) had no effect on HSV-2, N. gonorrhoeae, H. ducreyii, or any of six BV-associated bacteria, when tested under in vitro conditions designed to maximize H2O2 pathogen-inactivation (i.e., with addition of MPO and NaCl, in a protein-free salt solution (Table 1).
Blocking of exogenous hydrogen peroxide by cervicovaginal fluid and semen
In all CVF samples tested (n = 8), addition of exogenous H2O2 to 1 mM produced no significant increase (P = .53) in the concentration of H2O2 detected (Figure 3, closed squares); in semen (n = 6), addition of H2O2 to 10 mM produced no significant increase (P = .72) (Figure 3, open squares).
Blocking by CVF supernatant of pathogen-inactivation by H2O2-producing lactobacilli
Consistent with the report by Klebanoff et al of pathogen-inactivation by H2O2-producing lactobacilli, we found that G. vaginalis aerobically exposed for 1 hour to H2O2-producing L. crispatus in a salt solution containing MPO and NaCl showed complete inactivation; P. bivia exposed for 30 minutes to L. crispatus in salt solution containing MPO and NaCl also showed complete inactivation (Figure 4). However, the addition of CVF supernatant to a final CVF concentration of just 1% w/v abolished all inactivation; there was no significant reduction (paired comparisons, individual p values given in Table 1) in cfu/mL of G. vaginalis or P. bivia exposed for one hour to MPO, NaCl, 1% CVF, and H2O2-producing L. crispatus, compared to controls exposed to MPO, NaCl, 1% CVF, and no L. crispatus. As little as 0.1% CVF resulted in significant reduction in inactivation of G. vaginalis and P. bivia (P = 0.05).
Discussion
Hydrogen peroxide production by vaginal lactobacilli has been widely emphasized as a mechanism of protection against genital tract pathogens. This view is based on epidemiological association, plausibility of mechanism, and in vitro studies of pathogen inactivation by H2O2-producing lactobacilli in protein-free salt solution, but CVF and semen contain proteins, glycoproteins, polysaccharides, lipids, and other molecules with H2O2-blocking activities. Indeed, a previous investigator noted that the toxicity of an in vitro H2O2-peroxidase-halide system against Escherichia coli was compromised by even trace amounts of protein [37]. Additionally, previous in vitro experiments were done under aerobic conditions that maximize H2O2-production, whereas the vagina is generally hypoxic.
Our hypoxic glove-box mimicked the hypoxic condition of the vagina. The H2O2 content of CVF samples decreased during incubation in the glove-box and became undetectable (<1 μM) within one hour, suggesting that H2O2 would generally be undetectable in vivo. The H2O2 of CVF samples collected with brief (~1 minute) aerobic exposure and assayed immediately under hypoxic conditions was not significantly different from that measured in samples equilibrated under hypoxic conditions (i.e., to the point where they contained no detectable H2O2) and then exposed to air for one minute. We conclude that the H2O2 detected in the CVF samples immediately following collection is attributable to the brief aerobic exposure.
The oxygen concentration in the vagina increases substantially during sexual intercourse. However, the mean H2O2 content in CVF samples after four hours of full exposure to air was still only 23 ± 5 μM, one hundred times lower than maximal aerobic in vitro production (~2 mM). We attribute this lower H2O2 content to the H2O2-blocking activity of CVF: even if H2O2-production reached millimolar concentrations, CVF had sufficient H2O2-blocking activity to leave only micromolar amounts available to the assay reaction (and presumably to pathogen-inactivating reactions in vivo). This interpretation is supported by our finding that CVF samples spiked with millimolar concentrations of exogenous H2O2 still only contained micromolar concentrations of detectable H2O2. It may seem surprising, given the copious H2O2-blocking activity of CVF, that any H2O2 could be detected at all. We attribute this to the continuing production of H2O2 by the lactobacilli in our CVF samples during and after aerobic exposure: when the Amplex Red® substrate was added to the sample, it competed with the endogenous H2O2-blocking activity for the newly forming H2O2. Our hypothesis is supported by our finding that the H2O2 concentration decreased during aerobic incubation of bacterially-depleted CVF supernatant (n = 6), becoming undetectable after 45 minutes (Figure 1).
H2O2 at 50 μM (more than the maximum concentration we found in CVF) produced no inactivation of HSV-2, N. gonorrhoeae, H. ducreyii, or six major BV-associated bacteria, even in protein-free salt solution supplemented with MPO to enhance H2O2 toxicity. (P. bivia and P. anaerobius did show significant loss of viability (P = 0.01) irrespective of whether H2O2 was present or not, attributable to the effect of aerobic exposure on these strict anaerobes.) Additionally, the in vitro inactivation of G. vaginalis and P. bivia by H2O2-producing L. crispatus was completely abolished by the addition of only 1% CVF.
Our study has the following limitations: firstly, the concentration of H2O2 may be substantially higher in the immediate vicinity of H2O2-producing lactobacilli, and underestimated by our measurements in bulk CVF. A high local concentration of H2O2 might inactivate pathogens in close proximity to lactobacilli, even though the dispersed H2O2 concentration had no pathogen-inactivating activity (Table 1). Against this hypothesis is the fact that co-culture with H2O2-producing lactobacilli in the presence of CVF supernatant failed to inactivate both G. vaginalis and P. bivia, Second, the bacteria used in these experiments are established laboratory strains; primary isolates might be more sensitive to H2O2, although it is unclear how laboratory passage would provide selective pressure toward greater H2O2 resistance.
Conclusions
Given the H2O2-blocking activity of CVF and semen, we conclude that H2O2-production by vaginal lactobacilli is implausible as a mechanism of direct protection against genital tract pathogens generally, and sexually-transmitted pathogens in particular. There remains the strong inverse association between H2O2-producing lactobacilli and BV. We hypothesize that lactobacilli strains that produce H2O2 may also produce more of other microbicidal factors such as lactic acid or bacteriocins, or alternatively, that H2O2-producing lactobacilli may be more susceptible to inhibitory factors elaborated by BV-associated organisms. We are currently investigating these possibilities.
References
Livengood CH: Bacterial vaginosis: an overview for 2009. Rev Obstet Gynecol. 2009, 2: 28-37.
Allsworth JE, Peipert JF: Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007, 109: 114-20. 10.1097/01.AOG.0000247627.84791.91.
Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM: Control of the microbial flora of the vagina by H2O2-generating lactobacilli. JID. 1991, 164: 94-100.
Klebanoff SJ: MPO: friend and foe. J Leukoc Biol. 2005, 77: 598-625. 10.1189/jlb.1204697.
Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS: Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008, 22: 1493-501. 10.1097/QAD.0b013e3283021a37.
Baeten J, Hassan W, Chohan V, Richardson B, Mandaliya K, Ndinya-Achola J, Jaoko W, McClelland RS: Prospective study of correlates of vaginal Lactobacillus colonization among high-risk HIV-1 seronegative women. Sex Transm Infect. 2009
Demba E, Morison L, Loeff van der MS, et al: Bacterial vaginosis, vaginal flora patterns and vaginal hygiene practices in patients presenting with vaginal discharge syndrome in The Gambia, West Africa. BMC Infect Dis. 2005, 5: 12-10.1186/1471-2334-5-12.
Eschenbach DA, Davick PR, Williams BL, et al: Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989, 27: 251-6.
Al-Mushrif S, Jones BM: A study of the prevalence of hydrogen peroxide generating Lactobacilli in bacterial vaginosis: the determination of H2O2 concentrations generated in vitro by isolated strains and the levels found in vaginal secretions of women with and without infection. J Obstet Gynaecol. 1998, 18: 63-7. 10.1080/01443619868325.
Mijac VD, Dukic SV, Opavski NZ, Dukic MK, Ranin LT: Hydrogen peroxide producing lactobacilli in women with vaginal infections. Eur J Obstet Gynecol Reprod Biol. 2006, 129: 69-76. 10.1016/j.ejogrb.2005.11.036.
Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA: A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive Herpes Simplex Virus Type 2 serology. Sex Trans Dis. 2008, 35: 78-83. 10.1097/OLQ.0b013e318156a5d0.
Klebanoff SJ, Coombs RW: Viricidal effect of Lactobacillus acidophilus on Human Immunodeficiency Virus type 1: possible role in heterosexual transmission. J Exp Med. 1991, 174: 289-92. 10.1084/jem.174.1.289.
Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM: Control of the microbial flora of the vagina by H2O2-generating lactobacilli. JID. 1991, 164: 94-100.
Sommer F, Caspers HP, Esders K, Klotz T, Engelman U: Measurement of vaginal and minor labial oxygen tension for the evaluation of female sexual function. J Urol. 2001, 166: 2324-25. 10.1016/S0022-5347(05)65578-4.
Wagner G, Levin RJ, Bohr L: Diaphragm insertion increases human vaginal oxygen tension. Am J Obstet Gynecol. 1988, 158: 1040-43.
Wagner G, Levin R: Oxygen tension of the vaginal surface during sexual stimulation in the human. Fertil Steril. 1978, 30: 50-
Whittenbury R: Hydrogen peroxide formation and catalase activity in the lactic acid bacteria. J Gen Microbiol. 1964, 35: 13-26.
Aslim B, Kilic E: Some probiotic properties of vaginal lactobacilli isolated from healthy women. Jpn J Infect Dis. 2006, 59: 249-53.
Strus M, Brzychczy-Wloch M, Gosiewski T, Kochan P, Heczko PB: The in vitro effect of hydrogen peroxide on vaginal microbial communities. FEMS Immunol Med Microbiol. 2006, 48: 56-63. 10.1111/j.1574-695X.2006.00120.x.
Shaw JL, Smith CR, Diamandis EP: Proteomic analysis of human cervico-vaginal fluid. J Proteome Res. 2007, 6: 2859-65. 10.1021/pr0701658.
Klein LL, Jonscher KR, Heerwagen MJ, Gibbs RS, McManaman JL: Shotgun proteomic analysis of cervico-vaginal fluid from women in late pregnancy. Reprod Sci. 2008, 15: 263-73. 10.1177/1933719107311189.
Boskey ER, Moench TR, Hees PS, Cone RA: A self-sampling method to obtain large volumes of undiluted cervicovaginal mucus. Sex Trans Dis. 2003, 30: 107-9. 10.1097/00007435-200302000-00002.
Hill DR, Brunner ME, Schmitz DC, Davis CC, Flood JA, Schlievert PM, Wang-Weigand SZ, Osborn TW: In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J Appl Physiol. 2005, 99: 1582-91. 10.1152/japplphysiol.01422.2004.
Nugent RP, Krohn MA, Hillier SL: Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991, 29: 297-301.
Lusk MJ, Konecny P: Cervicitis: a review. Curr Opin Infect Dis. 2008, 21: 49-55.
Rabe LK, Hillier SL: Optimization of media for detection of hydrogen peroxide production by Lactobacillus species. J Clin Microbiol. 2003, 41: 3260-4. 10.1128/JCM.41.7.3260-3264.2003.
Martin HL, Richardson BA, Nyange PM, et al: Vaginal lactobacilli, microbial flora, and risk of Human Immunodeficiency Virus Type 1 and sexually transmitted disease acquisition. JID. 1999, 180: 1863-1868. 10.1086/315127.
Antonio MA, Hawes SE, Hillier SL: The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999, 180: 1950-6. 10.1086/315109.
Beigi RH, Wiesenfeld HC, Hillier SL, Straw T, Krohn MA: Factors associated with absence of H2O2-producing Lactobacillus among women with bacterial vaginosis. J Infect Dis. 2005, 191: 924-9. 10.1086/428288.
Eliasson R, Treich L: Supervital staining of human spermatozoa. Fertil Steril. 1971, 22: 134-7.
WHO Laboratory manual for the examination of Human semen and Sperm-Cervical mucus interaction. 1992, Cambridge University Press, 3
Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP: A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997, 253: 162-8. 10.1006/abio.1997.2391.
Huang D, Ou B, Prior RL: The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005, 53: 1841-56. 10.1021/jf030723c.
Magalhães LM, Segundo MA, Reis S, Lima JL: Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta. 2008, 613: 1-19. 10.1016/j.aca.2008.02.047.
Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM: Control of the microbial flora of the vagina by H2O2-generating lactobacilli. JID. 1991, 164: 94-100.
Dimitonova SP, Danova ST, Serkedjieva JP, Bakalov BV: Antimicrobial activity and protective properties of vaginal lactobacilli from healthy Bulgarian women. Anaerobe. 2007, 13: 178-84. 10.1016/j.anaerobe.2007.08.003.
Klebanoff SJ, Waltersdorph AM, Rosen H: Antimicrobial activity of MPO. Methods Enzymol. 1984, 105: 399-403. full_text.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/10/120/prepub
Acknowledgements
The work was supported by NIH grants AI45967, AI60598, and AI66726.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DEOH designed the study, collected data, analyzed the data, and prepared the manuscript. BL designed the study and collected data. TM participated in data analysis and the preparation of the manuscript. RC participated in data analysis and the preparation of the manuscript. All of the authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
O'Hanlon, D.E., Lanier, B.R., Moench, T.R. et al. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect Dis 10, 120 (2010). https://doi.org/10.1186/1471-2334-10-120
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-10-120