Abstract
Background
Probiotics are live microorganisms that, when administered in adequate amounts, should confer a health benefit to the host. Media sources tend to present probiotics as an appealing health promotion method able to prevent or treat a wide variety of clinical conditions. In obstetrics and gynaecology, Lactobacilli species are mainly used to restore the physiologic vaginal microbiota in order to treat bacterial vaginosis and vulvovaginal candidiasis (VVC) and prevent preterm birth.
Discussion
Several RCTs investigated the potential benefits of probiotics in gynaecological and obstetrics conditions. For all potential indications, recent specific meta-analyses have been published. Considering vulvovaginal candidiasis in non-pregnant women, probiotics slightly improved the short-term clinical and mycological cure, and reduced the 1-month relapse. However, no important impact of probiotic use was observed on long-term clinical or mycological cure. Similarly, the addition of probiotics to metronidazole for the treatment of bacterial vaginosis was not shown to provide any additional benefit. In obstetrics, using probiotics during pregnancy neither decreased nor increased the risk of preterm birth before 34 weeks or before 37 weeks. Similarly, no benefits emerged for gestational diabetes, preterm premature rupture of membrane, and small and large for gestational age infants.
Conclusion
Despite increasing marketing of probiotics for the treatment of vulvovaginal candidiasis and prevention of preterm birth robust evidence demonstrating a beneficial effect is scarce. Moreover, there was considerable heterogeneity among the different studies in terms of route of administration, strain/s of probiotic adopted, and length of probiotic use. Before recommending the systematic use of probiotics to treat bacterial vaginosis and VVC and prevent preterm birth, high-quality research is needed. Professional medical associations should issue recommendations defining if, when, and how probiotics should be used for gynaecological disorders.
Similar content being viewed by others
Background
According to the World Health Organization (WHO), probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit to the host [1]. Probiotics can be ingested with diet or in supplement forms [2]. Their consumption has been proven effective for the management of some gastrointestinal conditions, such as irritable bowel syndrome, and for the prevention of diarrhoea associated with Clostridium difficile infection [3, 4].
In the last decade, many clinical trials have been conducted to assess the effects of probiotics in the prevention and treatment of a broad range of disorders, and the scientific interest in this field is growing. Searching Medline through PubMed for “probiotics”, identifies 14.188 articles published between 2007 and 2017 (accessed 14 January 2018), with an increase of 163% from 2007 to 2017 in the number of articles published per year. In the obstetrical and gynaecological field probiotics, administered both orally and vaginally, have been mainly tested for the prevention and treatment of vaginal infections and for the prevention of preterm birth [2, 5,6,7,8]. The rationale of using oral probiotics in the treatment of gynaecological conditions is related to the ability of these microorganisms to survive through the gastrointestinal system and to ascend to the vaginal tract after their excretion from the rectum; whereas vaginal administration allows a direct and targeted colonization action of the probiotics for restoring unhealthy vaginal microbiota [9].
Also, the use of probiotics is progressively expanding. In particular, women of reproductive age are prone to use these products for gastrointestinal symptoms [10]. Another important catchment area is represented by pregnant women, as it is estimated that up to 1 woman out of 7 in the Netherlands regularly use probiotics during gestation [11].
Media sources tend to present probiotics as an appealing health promotion method able to prevent or treat a wide variety of clinical conditions [12]. Indeed, in 2015 probiotics market exceeded $35 billion and it is estimated to continue to rise in the years to come [13]. An estimate based on a survey conducted for FederSalus (the main Italian referent for institutional and commercial organizations operating in the field of food supplements) on more than 6000 individuals representing the Italian population aged 18 and above, indicated that 32 million Italians have used a nutritional supplement in the last year [14]. In Italy, from November 2016 to October 2017 the market of nutritional supplements reached 2.9 € billions, for a total of almost 212 million packs sold, with an increase in turnover of 7.3% [15]. In particular, in Italy the probiotic market reached € 343 million in 2016, ranking first among the best-selling food supplements [16]. In other Western countries the scenario is similar. In fact, in the North American market (United States and Canada) probiotic represent the category of nutritional supplement with the higher growth in absolute terms (+$725 million) in the period 2009–2014 [17].
Given this background, the time has come to verify whether probiotics really benefits pregnant and non-pregnant women, and whether the magnitude of the effect justifies the expenditure.
Probiotics in non-pregnant women
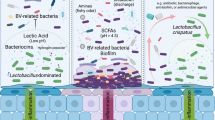
Vaginal infections represent one of the most common reason for gynaecological consultation [7]. It is estimated that approximately seven women out of 10 will experience at least one episode of vulvovaginal candidiasis (VVC) in their lives [18]. Bacterial vaginosis (BV) is another highly prevalent vaginal disorder associated with an increased risk for pelvic inflammatory disease, sexually transmitted infections, HIV transmission, and preterm delivery [19]. Bacterial vaginosis is characterized by a reduction or depletion of lactobacilli and overgrowth of Gardnerella vaginalis, Mycoplasma hominis, Prevotella species, and other pathogenic anaerobic bacteria [18]. Lactobacillus species produce lactic and acetic acid and hydrogen peroxide, maintain the vaginal pH around 4.5 or less, hamper growth of pathogenic bacteria and Candida albicans, and are thus considered protective against VVC and BV [19, 20]. Accordingly, the putative beneficial effect of Lactobacillus species-containing probiotics in restoring and maintaining the physiologic vaginal microbiota, fostered their use for the treatment of both vaginal disorders.
The effectiveness of probiotics for the treatment of VVC in non-pregnant women was recently evaluated in a Cochrane systematic review [7]. A total of 10 RCTs (1656 participants) investigating the effect of probiotics used by the oral and vaginal route as a complementary therapy to conventional antifungal drugs were included. Probiotics slightly improved the short-term clinical and mycological cure rate (risk ratio (RR) 1.14, 95% CI 1.05–1.24, and RR 1.06, 95% CI 1.02–1.10 respectively), and reduced the 1-month relapse rate (RR 0.34, 95% CI 0.17–0.68). However, no important impact of probiotic use was observed on long-term clinical or mycological cure rate (3-month post-treatment evaluation, RR 1.30, 95% CI 1.00–1.70; and RR 1.16, 95% CI 1.00–1.35, respectively). Given the low- or very low-quality of the considered studies, the authors emphasized the need for further and better designed RCTs with larger sample size, standardized methodology for probiotic preparation, and longer follow-up, in order to define also other outcomes that may matter to women, such as time to first relapse, need for repeated or prolonged treatments, patient satisfaction, and cost effectiveness. However, we have to underline that one of the major and unresolved issues related to the treatment of VVC is the high rate of recurrences even after the use of conventional azoles treatment [21, 22]. In addition, treatment of recurrent VVC, defined as four or more symptomatic episodes within 12 months, could be challenging due to the increased presence of azole-drug resistance [23]. Moreover, in complicated forms, all treatments, including antimycotics, are not supportive of long-term beneficial results. In this particular sub-group of patients, the protective role of specific Lactobacillus species-containing probiotics, such as Lactobacillus plantarum P17630, has been proven effective as a potential empirical preventive agent of VVC recurrences [23].
The putative beneficial effect of probiotics supplementation for the treatment of BV has been assessed in various meta-analysis [8, 24, 25]. A 2009 Cochrane review [24] showed promising results derived from the use of oral and vaginal probiotics combined with metronidazole or used alone. In 2013, a systematic review [25] supported the potential beneficial effect of probiotics for the treatment of BV. Huang et al. [25] included in their analysis twelve RCTs published between 1992 and 2012; probiotics were adopted either orally (n = 8) or vaginally (n = 4), with follow-up periods ranging from 4 weeks to 6 months. The pooled result showed that probiotics supplementation was able to significantly improve the cure rate in adult BV patients (RR 1.53, 95% CI 1.19–1.97). Subgroup analyses failed to demonstrate a beneficial effect of probiotics supplementation in terms of long-term (> 1 month) follow-up (RR 1.15, 95% CI 0.89–1.47), and a substantial heterogeneity was shown across different study designs. Finally, in a recent meta-analysis [8], the authors compared the use of metronidazole alone with the combination of this antibiotic plus probiotics. Five RCTs including a total of 1186 participants were selected. An overall risk ratio of 0.98 (95% CI 0.91–1.06; P = 0.57) was observed for the cure rate achieved with combined therapy over metronidazole alone on BV.
At now, given the presence of inconclusive results, some international guidelines do not support the use of probiotics for the treatment of vulvovaginal infections (Table 1).
Probiotics in pregnant women
It has been suggested that probiotics could play a role in the prevention of preterm birth [2, 6]. Preterm birth rates vary across different countries, ranging from 5 to 9% in Europe to 13% in USA [26]. The aetiology of preterm birth is multifactorial, but it has been estimated that about one third of cases is due to intrauterine inflammation [26] caused by ascending vaginal infections. In particular, a pre-existing BV appears to be strongly associated with premature birth [5].
Therefore, here the putative role of probiotics might be associated with their potential ability to displace and kill pathogens. The hypothesized mechanisms include the development of anti-inflammatory cytokines and the reduction of the vaginal pH, so that the vaginal environment becomes again favourable to the growth of healthy bacteria [6, 20]. Moreover, the use of probiotics in pregnancy could improve maternal glucidic metabolism through the modification of gut microbial composition and function, as well as the improvement of insulin sensitivity [27].
To verify these hypotheses, Jarde et al. [2] performed a systematic review and meta-analysis on the risk of preterm birth and other adverse pregnancy outcomes in women with a singleton pregnancy receiving probiotics. In this analysis the authors included also prebiotics, i.e. food ingredients that indirectly induce the growth or activity of beneficial microorganisms. A total of 21 studies (4098 women) were included in the final analysis. Five studies (1017 women) evaluated the risk of preterm birth < 34 weeks of gestation, whereas the risk < 37 weeks was assessed in 11 studies (2484 women). Using probiotics during pregnancy neither decreased nor increased the risk of preterm birth before 34 weeks (RR 1.03; 95% CI:0.29–3.64) or before 37 weeks (RR 1.08; 95% CI: 0.71–1.63). In addition, the authors did not observe a protective effect of probiotics supplementation on most of the secondary outcomes considered, including gestational diabetes, preterm premature rupture of membrane (PPROM), and small and large for gestational age infants. The only statistically significant difference in favour of probiotics supplementation regarded glucose metabolism (Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and Insulin); however, the pooled estimate on gestational diabetes did not show any benefit from probiotics intake (RR 1.25; 95% CI 0.61–2.56).
In contrast with these results, a Greek RCT [28] showed a benefit of probiotic administration in women with PPROM. Patients were allocated to 10-days vaginal probiotic supplementation in combination with antibiotic prophylaxis (n = 59) or to standard antibiotic treatment alone (n = 57). In women treated with the double regimen significantly higher mean gestational age at birth (35.49 vs 32.53 weeks) and latency period (5.60 vs 2.48 weeks) were observed in comparison to control group.
A recent Norwegian population based-prospective cohort study [29] investigated the potential association between the consumption of probiotic milk and the incidence of preterm delivery and preeclampsia. Maternal inflammatory response represents the common background of these two pathologic conditions, and the potential anti-inflammatory effect of probiotics represents the criterion for their use [30, 31]. The authors showed that probiotic milk consumption during late pregnancy, but not before or in early pregnancy, was associated with a reduced risk of preeclampsia (adjusted OR: 0.80; 95% CI, 0.64-0.94). Regarding preterm birth, ingestion of probiotic milk during early pregnancy, but not before or in late pregnancy, was associated with a reduction in risk of preterm delivery (adjusted OR: 0.79; 95% CI, 0.64-0.97). In both cases, no dose-response relationship was found.
The putative role of oral probiotics on vaginal micro-environment in pregnancy has been evaluated by Gille et al. [32] in a randomized, triple-blind, controlled trial (RCT) conducted on 320 women. Participants were allocated to oral probiotic supplementation or placebo. The primary study outcome was the proportion of swabs with normal Nugent score (< 4) after eight weeks of treatment. Oral probiotics did not increase the proportion of normal vaginal microbiota compared to placebo. At post-intervention analysis, the proportion of normal vaginal microbiota decreased from 82.6 to 77.8% in the probiotic group, and from 79.1 to 74.3% in the placebo group, without significant between-group difference (P = 0.29).
An Australian double-blind RCT [33] assessed the impact of oral probiotics on vaginal Group B Streptococcal (GBS) colonization rates in 34 women. Only women with a GBS-positive vaginal swab at 36 weeks were deemed eligible for the study. Patients were assigned to daily oral probiotics plus standard antenatal care (intervention group) or standard antenatal care (control group) for three weeks or until delivery. At the end of the treatment period, no significant between-group difference was observed in GBS infection rate.
Finally, the results of a recent systematic review [34] do not support the treatment of BV-positive pregnant women with probiotics with the objective of reducing the risk of spontaneous preterm delivery.
Probiotics in obstetrics and gynaecology: patient’s health or industry wealth?
From a commercial point of view, it appears that the “golden era” of probiotics has begun. In the words of Arnold [35] “like all good bacteria, probiotics have sprung forth and multiplied. Trillions live in our guts, and even more have begun to occupy grocery store shelves”. However, to be defined a “probiotic”, the strain of bacteria must have demonstrated health benefits [35].
The theoretical benefits deriving from the increase in the number of healthy vaginal bacteria at the expenses of potentially pathogenic micro-organisms appears intuitive. However, in many cases it is unclear whether the alteration of the vaginal microbiota is a consequence of incidental infections or of a systemic endocrine/immunologic/metabolic condition predisposing to lactobacillus extinction. In the former case, re-introducing physiologic bacteria after pharmacological eradication of pathogens seems rational, but in the latter one it would result in trying to cure the consequence rather than the origin of the disorder. The high BV and VVC relapse rate observed after both antibiotic treatment and complementary probiotic use suggests that the second hypothesis might be true. If this is the case, probiotics might reveal no more than an incomplete, temporary, and expensive remedy. Another practical issue is the route of administration. In fact, whether intravaginal insertion of probiotics seems logical [19], aiming at modifying the vaginal microbiota via oral ingestion of physiologic bacteria appears less intuitive, and implies ingested probiotics to reach the rectum, ascend the vagina, and dislodge bacterial pathogens and yeasts [20].
Disappointingly, the widespread use of probiotics to reduce the risk of preterm delivery and to improve the cure rate of BV and VVC does not seem to be justified by the currently available data. In fact, despite increasing marketing and sales of probiotics, the results originated by clinical trials are inconsistent and generally of sub-optimal quality. Moreover, a substantial proportion of these trials have been sponsored by parties with a commercial interest in the outcome [36]. In addition, there was considerable heterogeneity in published studies in term of strain/s of probiotic adopted, route of administration (oral, vaginal), and duration of treatment [36].
The effects of probiotics seem to be strain-specific and dose-dependent, and the lack of a standardized manufacturing process could affect microbial survival, growth, and viability [37]. Along this line, a recent position paper by The European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) Working Group provides evidence on the inadequate quality of commercial probiotic products, in terms of definition of microorganisms, their numbers, functional properties, and presence of contaminating microorganisms. The Working Group suggests the creation of certified laboratories where the quality control of probiotics should be performed using universally shared validated and standardized methodologies [37]. In addition, as it is the case with drugs, adverse events potentially related to the use of probiotics, should be reported to and registered by health authorities [37].
The regulatory aspects related with the production and marketing of these products constitute another reason of concern for both patients/consumers and physicians. The regulation of probiotics differs between countries without a universally shared framework [38]. In general, probiotic products are classified as food or dietary supplements, and their development process has to fulfil considerably less rigorous regulatory criteria compared with drugs [37]. However, if probiotics are to be prescribed to patients with specific disorders, they should be regulated as drugs rather than foods or supplements. Nevertheless, we are not aware of any indication to probiotic use in obstetrics and gynaecology approved by national and supranational regulatory agencies such as Food and Drug Administration (FDA) and European Medical Agency (EMA).
Indeed, the somewhat vague manufacturers claims may generate a sort of nobody’s land where commercial interests may flourish independently of the effect of probiotics for the prevention and treatment of specific disorders [35]. In fact, as in most countries the regulation of probiotics is focused on the legitimacy of any claim rather than on their efficacy, manufacturers are careful not to mention definite medical indications for their products [38]. In this regard, professional medical associations should issue recommendations concerning the role of probiotics in obstetrics and gynaecology, as their uncontrolled implementation might also lead to a potentially harmful decrease in the use of effective standard drug treatments.
The primary aim of probiotics is the re-establishment of a physiological vaginal microbiome. However, there is currently no consensus regarding their use for the treatment of vaginal infections and their sequelae. Thus, further better-quality data are needed to define the real effect size of probiotic use in different obstetrical and gynaecological conditions. At the very least, our duty is to provide complete and quantitative information to patients/consumers, allowing them to decide whether probiotics are worth their cost.
Abbreviations
- BV:
-
Bacterial vaginosis
- EMA:
-
European Medical Agency
- FDA:
-
Food and Drug Administration
- GBS:
-
Group B Streptococcal
- HOMA-IR:
-
Homeostatic Model Assessment of Insulin Resistance
- PPROM:
-
Preterm premature rupture of membranes
- RCT:
-
Randomized controlled trial
- RR:
-
Risk ratio
- VVC:
-
Vulvovaginal candidiasis
- WHO:
-
World Health Organization
References
Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk and live lactic acid bacteria [Internet]. Cordoba, Argentina: Food and Agriculture Organisation (FAO)/World Health organisation (WHO). 2001 : http://www.fao.org/3/a-a0512e.pdf. Accessed 27 Jan 27 2018.
Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18:14.
Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–61.
Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
Kirihara N, Kamitomo M, Tabira T, Hashimoto T, Taniguchi H, Maeda T. Effect of probiotics on perinatal outcome in patients at high risk of preterm birth. J Obstet Gynaecol Res. 2018;44:241–7.
Reid G, Bocking A. The potential for probiotics to prevent bacterial vaginosis and preterm labor. Am J Obstet Gynecol. 2003;189:1202–8.
Xie HY, Feng D, Wei DM, Mei L, Chen H, Wang X, et al. Probiotics for vulvovaginal candidiasis in non-pregnant women. Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst Rev. 2017;11:CD010496.
Tan H, Fu Y, Yang C, Ma J. Effects of metronidazole combined probiotics over metronidazole alone for the treatment of bacterial vaginosis: a meta-analysis of randomized clinical trials. Arch Gynecol Obstet. 2017;295:1331–9.
Homayouni A, Bastani P, Ziyadi S, Mohammad-Alizadeh-Charandabi S, Ghalibaf M, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18:79–86.
Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109:S2–S26.
Rutten N, Van der Gugten A, Uiterwaal C, Vlieger A, Rijkers G, Van der Ent K. Maternal use of probiotics during pregnancy and effects on their offspring’s health in an unselected population. Eur J Pediatr. 2016;175:229–35.
Stanton C, Gardiner G, Meehan H, Collins K, Fitzgerald G, Lynch PB, et al. Market potential for probiotics. Am J Clin Nutr. 2001;73:476S–83S.
Grand View Research. Probiotics Market Analysis By Application (Probiotic Foods & Beverages (Dairy Products, Non-Dairy Products, Cereals, Baked Food, Fermented Meat Products, Dry Food), Probiotic Dietary Supplements (Food Supplements, Nutritional Supplements, Specialty Nutrients, Infant Formula), Animal Feed Probiotics), By End-Use (Human Probiotics, Animal Probiotics) And Segment Forecast To 2024. http://www.grandviewresearch.com/industry-analysis/probiotics-market. Accessed 27 Jan 2018.
Integratori alimentari: nelle abitudini di 32 milioni di italiani attenti al benessere e alla cura di sé. http://www.federsalus.it/drive/File/stampa/CS_Convention%20FederSalus%202017_trade.pdf. Accessed 27 Jan 2018.
FederSalus. Dati di mercato Ottobre 2017. http://www.federsalus.it//news.php?id=1832. Accessed 27 Jan 2018.
Indagine di settore 2016: La filiera italiana degli integratori alimentari. http://www.federsalus.it/drive/File/documenti_news/1485790346Centro%20Studi%20FederSalus%20-%20Presentazione%20II%20indagine%20di%20settore%202016.pdf. Accessed 27 Jan 2018.
Trend globali nel mercato degli integratori alimentari: un confronto tra USA ed Europa. http://www.federsalus.it//news.php?id=1385. Accessed 19 Feb 2018.
Sobel JD. Candidal vulvovaginitis. Lancet. 2007;369:1961–71.
Ma L, Su J, Su Y, Sun W, Zeng Z. Probiotics administered intravaginally as a complementary therapy combined with antibiotics for the treatment of bacterial vaginosis: a systematic review protocol. BMJ Open. 2017;7:e019301.
Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38.
Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15–21.
Blostein F, Levin-Sparenberg E, Wagner J, Foxman B. Recurrent vulvovaginal candidiasis. Ann Epidemiol. 2017;27:575–82.
De Seta F, Parazzini F, De Leo R, Banco R, Maso GP, De Santo D, et al. Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: a retrospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2014;182:136–9.
Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No.: CD006055.
Huang H, Song L, Zhao W. Effects of probiotics for the treatment of bacterial vaginosis in adult women: a meta-analysis of randomized clinical trials. Arch Gynecol Obstet. 2014;289:1225–34.
Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84.
Lindsay KL, Brennan L, Kennelly MA, Maguire OC, Smith T, Curran S, et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. Am J Obstet Gyneco 2015;212:496.e1–11.
Daskalakis GJ, Karambelas AK. Vaginal probiotic Administration in the Management of preterm premature rupture of membranes. Fetal Diagn Ther. 2017;42:92–8.
Nordqvist M, Jacobsson B, Brantsæter AL, Myhre R, Nilsson S, Sengpiel V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: a prospective observational cohort study in Norway. BMJ Open. 2018;8:e018021. https://doi.org/10.1136/bmjopen-2017-018021.
Yeganegi M, Watson CS, Martins A, Kim SO, Reid G, Challis JR, et al. Effect of lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharideinduced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol. 2009;200:532.e1–8.
Bloise E, Torricelli M, Novembri R, Borges LE, Carrarrelli P, Reis FM, et al. Heat-killed lactobacillus rhamnosus GG modulates urocortin and cytokine release in primary trophoblast cells. Placenta. 2010;31:867–72.
Gille C, Böer B, Marschal M, Urschitz MS, Heinecke V, Hund V, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am J Obstet Gynecol. 2016;215:608.e1–7.
Olsen P, Williamson M, Traynor V, Georgiou C. The impact of oral probiotics on vaginal group B streptococcal colonisation rates in pregnant women: a pilot randomised control study. Women Birth. 2018;31:31–7.
Haahr T, Ersbøll AS, Karlsen MA, Svare J, Sneider K, Hee L, et al. Treatment of bacterial vaginosis in pregnancy in order to reduce the risk of spontaneous preterm delivery - a clinical recommendation. Acta Obstet Gynecol Scand. 2017;96:251–2.
Arnold C. The pros and cons of probiotics. Lancet Infect Dis. 2013;13:571–2.
Griffin C. Probiotics in obstetrics and gynaecology. Aust N Z J Obstet Gynaecol. 2015;55:201–9.
Kolaček S, Hojsak I, Berni Canani R, Guarino A, Indrio F, Orel R, et al. Commercial probiotic products: a call for improved quality control. A position paper by the ESPGHAN working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2017;65:117–24.
de Simone C. The unregulated probiotic market. Clin Gastroenterol Hepatol. 2018. https://doi.org/10.1016/j.cgh.2018.01.018 In press.
Sherrard J, Donders G, White D, Jensen JS. European (IUSTI/WHO) guideline on the management of vaginal discharge. Int J STD AIDS. 2011;22:421–9.
Melvin L, Craic J, Abbott M, et al. Management of vaginal discharge in non-genitourinary medicine settings. Faculty of Sexual & repro- ductive Healthcare Clinical Guidance. 2012:1–28.
Mendling W. Guideline: vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses. 2015;58:1–15.
Van Schalkwyk J, Yudin MH, Allen V, et al. Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Can. 2015;37:266–74.
Centers for Disease Control and Prevention. 2015 Sexually Transmitted Diseases Treatment Guidelines. https://www.cdc.gov/std/tg2015/default.htm. Accessed 27 Jan 2018.
Acknowledgements
Not applicable.
Funding
This article was financed by Italian fiscal contribution “5 × 1000” 2012- Ministero dell’Istruzione, dell’Università e della Ricerca - devolved to Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Ca′ Granda Ospedale Maggiore Policlinico, Milano, Italy. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
LB project development, data collection, manuscript writing/editing; ES data collection; AB data collection; PV project development, manuscript writing/editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
LB, AB, and PV declare that they have no competing interests. ES received grants from Ferring and Serono. Serono and Ferring did not support the study. The COI declared by one of the authors refers to grants of research received for other studies not related to the argument of the submitted article. Even if not related, the policy of our Department is to systematically report any COIs with the industry, regardless of the role these fundings could have in the treated argument.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Buggio, L., Somigliana, E., Borghi, A. et al. Probiotics and vaginal microecology: fact or fancy?. BMC Women's Health 19, 25 (2019). https://doi.org/10.1186/s12905-019-0723-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-019-0723-4