Abstract
Background
Psathyrostachys huashanica Keng has long been used as a genetic resource for improving wheat cultivar because of its genes mediating the resistance to various diseases (stripe rust, leaf rust, take-all, and powdery mildew) as well as its desirable agronomic traits. However, a high-resolution fluorescence in situ hybridization (FISH) karyotype of P. huashanica remains unavailable.
Results
To develop chromosome-specific FISH markers for P. huashanica, repetitive sequences, including pSc119.2, pTa535, pTa713, pAs1, (AAC)5, (CTT)12, pSc200, pTa71A-2, and Oligo-44 were used for a FISH analysis. The results indicated that the combination of pSc200, pTa71A-2 and Oligo-44 probes can clearly identify all Ns genomic chromosomes in the two P. huashanica germplasms. The homoeologous relationships between individual P. huashanica chromosomes and common wheat chromosomes were clarified by FISH painting. Marker validation analyses revealed that the combination of pSc200, pTa71A-2, and Oligo-44 for a FISH analysis can distinguish the P. huashanica Ns-genome chromosomes from wheat chromosomes, as well as all chromosomes (except 4Ns) from the chromosomes of diploid wheat relatives carrying St, E, V, I, P and R genomes. Additionally, the probes were applicable for discriminating between the P. huashanica Ns-genome chromosomes in all homologous groups and the corresponding chromosomes in Psathyrostachys juncea and most Leymus species containing the Ns genome. Furthermore, six wheat–P. huashanica chromosome addition lines (i.e., 2Ns, 3Ns, 4Ns, 7Ns chromosomes and chromosomal segments) were characterized using the newly developed FISH markers. Thus, these probes can rapidly and precisely detect P. huashanica alien chromosomes in the wheat background.
Conclusions
The FISH karyotype established in this study lays a solid foundation for the efficient identification of P. huashanica chromosomes in wheat genetic improvement programs.
Similar content being viewed by others
Background
Psathyrostachys huashanica Keng (2n = 2x = 14, NsNs), a diploid, perennial, cross-pollinating species belonging to the genus Psathyrostachys Nevski in the tribe Triticeae, is distributed primarily in the Huashan Mountain region of Shanxi province, China [1]. There has been considerable interest in P. huashanica among wheat breeders because it is a promising wild relative of common wheat [2]. It has many agronomically valuable characteristics, including early maturation, the production of multiple kernels and tillers, drought tolerance, and resistance to biotic factors (e.g., pathogens causing stripe rust, leaf rust, take-all, and powdery mildew) [3]. Moreover, it has been used as a tertiary gene resource for wheat genetic improvement ever since chromosome-mediated gene transfers from P. huashanica into common wheat were initiated in the 1990s [2]. Progeny lines carrying P. huashanica chromosomes or chromosomal segments incorporated into the wheat genome were subsequently developed as lines with chromosomal additions [4,5,6], substitutions [7, 8], and translocations [9, 10]. These lines outperformed their wheat parents in terms of stress resistance and agronomic traits, making them valuable germplasm for wheat breeding. Molecular markers, including random-amplified polymorphic DNA markers targeting the P. huashanica Ns genome [11] and sequence characterized amplified region markers specific for the 1Ns, 2Ns, 3Ns, and 5Ns chromosomes [4, 12,13,14], have been developed for exogenous chromatin tracking.
Fluorescence in situ hybridization (FISH) is a widely used cytogenetic tool for detecting chromosomal aberrations as well as for studying chromosomal behavior and the genomic localization of repetitive DNA sequences [15, 16]. The FISH karyotype established on the basis of informative probe labeling patterns on chromosomes may provide researchers with landmarks useful for identifying chromosomes. In previous FISH studies, repetitive sequence probes, such as pSc119.2, pTa535, pTa713, pAs1, and microsatellites like (AAC)5, enabled the convenient identification of wheat chromosomes according to distinct probe hybridization patterns [17,18,19,20]. Such FISH karyotye has also been conducted in investigations of the diploid Thinopyrun elongatum [21], Dasypyrum villosum [22], Agropyron cristatum [23], Secale cereale [24], Hordeum vulgare [25], and Aegilops species, including Ae. markgrafii, Ae. cylindrica, Ae. triuncialis [26], Ae. comosa, Ae. geniculata, Ae. biuncialis and Ae. umbellulata [27, 28]. To date, few repetitive sequences have been used as FISH probes for analyzing P. huashanica chromosomes. Furthermore, a detailed FISH karyotype based on homoeologous relationships with wheat is not available for the P. huashanica Ns-genome chromosomes. A lack of a standard P. huashanica karyotype has greatly hindered the chromosome-mediated transfer of elite genes from P. huashanica into cultivated wheat.
Although FISH using repetitive sequence probes has been widely used for identifying chromosomes in numerous Triticeae species [19,20,21,22,23,24,25,26,27,28,29], it is not a viable option for determining inter-genera chromosomal homoeologous relationships because of the broad variation in these repetitive elements among diverse genomes [30, 31]. Chromosome painting using chromosome-specific probes, which are designed on the basis of single-copy sequences, is a novel cytological method for diagnosing chromosomal abnormalities, detecting chromosomal rearrangements, investigating chromatin organization, and constructing ancestral karyotypes [32]. Li et al. [33] recently developed seven oligonucleotide pools (Synt1-Synt7) derived from the conserved sequences in the collinear chromosomal regions of each linkage group in wheat and barley. These pools were used to differentiate between the chromosomal homeologous groups in the genera Secale, Aegilops, Thinopyrum, and Dasypyrum. Accordingly, these newly synthesized linkage group-specific bulked oligonucleotide pools are applicable for investigating chromosomal homoeologous relationships among Triticeae species.
In our previous studies, we generated hybrids from a cross between P. huashanica and common wheat without an embryo rescue step and obtained hybrid derivatives by backcrossing and selfing [34]. A detailed FISH karyotype of P. huashanica chromosomes remains unavailable and there are relatively few P. huashanica-specific molecular markers. This has made it difficult to identify wheat–P. huashanica progenies useful for breeding. To optimize the utility of P. huashanica desirable genes for the genetic improvement of wheat via chromosome engineering, FISH-based markers that can efficiently identify P. huashanica chromosomes in the wheat background are urgently needed. Thus, the objectives of this study were to: (1) develop a FISH-based karyotype specific for the P. huashanica Ns-genome chromosomes; (2) validate the P. huashanica chromosome-specific FISH markers in common wheat and wheat-related species; and (3) identify wheat–P. huashanica derivative lines using the developed FISH markers.
Materials and methods
Plant materials
Specific details regarding the plant materials used in this study are provided in Tables 1 and 2. Randomly selected individual plants from two P. huashanica accessions (2n = 2x = 14, NsNs) were used to generate a FISH karyotype. The P. huashanica chromosome-specific FISH markers were validated using the following species: common wheat landrace Chinese Spring (CS; 2n = 6x = 42, AABBDD); the wheat-related species Secale cereale (2n = 2x = 14, RR), Hordeum vulgare (2n = 2x = 14, II), Thinopyrum elongatum (2n = 2x = 14, EE), Agropyron cristatum (2n = 2x = 14, PP), Dasypyrum villosum (2n = 2x = 14, VV), Pseudoroegneria stipifolia (2n = 2x = 14, StSt), Psathyrostachys juncea (2n = 2x = 14, NsNs), Leymus secalinus (2n = 4x = 28, NsNsXmXm), L. pseudoracemosus (2n = 4x = 28, NsNsXmXm), L. multicaulis (2n = 4x = 28, NsNsXmXm), L. racemosus (2n = 4x = 28, NsNsXmXm), L. coreanus (2n = 4x = 28, NsNsXmXm), and L. arenarius (2n = 8x = 56, Ns1Ns1Ns2Ns2XmXmXmXm). Six wheat–P. huashanica derivatives (18-2-6, 19-2-5, 20-1-1, 23-1-1, 26-3-2, and 32-2-5) were obtained via the hybridization between CSph2b and P. huashanica accession ZY3157. Designation of genome formulas were according to Yen and Yang [35,36,37]. Regarding the genomic in situ hybridization (GISH) analysis, the CS genome was used as the blocking DNA, whereas the P. huashanica genome was used as the source of the probe DNA. CS and Secale cereale QL were maintained in our laboratory. Materials with PI numbers were kindly provided by The U.S. National Plant Germplasm System (NPGS), and materials with ZY numbers were collected by Yen. Chi and J. L. Yang (Triticeae Research Institute, Sichuan Agricultural University). Voucher specimens have been deposited in the herbarium of the Triticeae Research Institute, Sichuan Agricultural University, China.
Development of P. huashanica chromosome-specific probes
Actively growing root tips from germinating seeds or growing plants were cut and treated with nitrous oxide for 2.5 h and 70% (v/v) acetic acid for 5 min before being digested with pectinase and cellulase (Yakult Pharmaceutical Industry Co., Ltd., Tokyo, Japan) [19]. Root tips for outcrossing species were taken from randomly selected individual plants or seed of each accession. The FISH and GISH slides were prepared as described by Han et al. [38]. To construct the P. huashanica karyotype, the pSc200, pTa71A-2, and Oligo-44 repetitive sequences were selected for the FISH experiments after screening other oligonucleotides probes, including pSc119.2, pTa535, pTa713, pAs1, (CTT)12, and (AAC)5. Sequence of each probe was listed in Table 3 and all probes were 5’ end-labelled with 6-carboxyfluorescein or 6-carboxytetramethylrhodamine by Sangon (Shanghai, China). The hybridization was completed according to a slightly modified version of the method described by Han et al. [38]. Briefly, 10 µL hybridization solution comprising the probes and buffer [0.5 µL each probe in 2× saline sodium citrate (SSC) and 1× TE buffer] was added to each slide. The samples were denatured at 80 °C for 5 min and then incubated at 37 °C for 2 h, after which they were washed with 2× SSC buffer. The chromosomes were counterstained with a DAPI (4′,6-diamidino-2-phenylindole) solution (Vector Laboratories, Inc., Burlingame, CA, USA) before photomicrographs of the chromosomes were taken using the DP80 CCD camera (Olympus, Tokyo, Japan) installed on the BX-63 microscope (Olympus). The photomicrographs were processed using Photoshop CS5.0 (Adobe Systems Incorporated, San Jose, CA, USA).
Karyotyping of P. huashanica chromosomes
To distinguish the P. huashanica homoeologous chromosomes from the wheat chromosomes, bulked oligonucleotide libraries (Synt1-Synt7) kindly provided by Prof. Z.J. Yang (Center for Informational Biology, School of Life Science and Technology, University of Electronic and Technology of China) were used for FISH painting. The washed slides were used for a FISH involving the oligonucleotide probes pTa71A-2 and Oligo-44. The FISH painting was performed as described by Han et al. [44] and Bi et al. [45]. Slides were washed as described by Komuro et al. [19]. Photomicrographs were taken as described in the FISH protocol. Fifteen well-spread metaphase cells of P. huashanica accession ZY3157 were selected for an analysis of chromosomal parameters. The relative length was calculated by dividing the length of a particular chromosome by the total length of chromosomes in the haploid set. The chromosomal arm ratio was determined by dividing the length of the longer arms by the length of the shorter arms. Data were processed using the Image J software and Microsoft Office Excel 2010.
Validation of the P. huashanica -specific FISH-based markers
To validate the specificity and utility of the developed cytological markers, pSc200, pTa71A-2, and Oligo-44 were used for a FISH analysis of CS, 13 wheat relatives, and wheat–P. huashanica derivatives. A GISH was performed using the washed FISH slides for the derived lines to confirm the presence of P. huashanica chromatin. For both P. huashanica and CS, genomic DNA was extracted from fresh leaves according to the cetyltrimethylammonium bromide method [46]. The P. huashanica (ZY3157) genomic DNA was labeled with Texas Red-12-dUTP in the nick translation mix (Thermo Fisher Scientific, Eugene, OR, USA) and then used as the GISH probe. The CS DNA was used for blocking. The GISH was performed according to a modified version of the method described by Han et al. [38]. Specifically, 10 µL GISH hybridization solution containing 100 ng labeled probe DNA and blocking DNA (1:150 probe DNA:blocking DNA ratio), 2× SSC, 10% (v/v) dextran sulfate, and 50% (v/v) formamide was added to each slide. The samples were denatured at 85 °C for 5 min, incubated at 50 °C overnight, and washed with 2× SSC buffer. The chromosomes were counterstained and photomicrographs were taken as described in the FISH protocol.
Results
Karyotyping of P. huashanica Ns-genome chromosomes
Several DNA probes, including pSc119.2, pTa535, pTa713, pAs1, (AAC)5, (CTT)12, pSc200, pTa71A-2, and Oligo-44, were used for the FISH analysis of P. huashanica chromosomes (Figs. 1 and 2B C). The FISH results indicated that the pTa71A-2 and Oligo-44 probe combination unambiguously discriminated the P. huashanica chromosomes (Fig. 1 F). The probes pTa71A-2 and Oligo-44, which were associated with pSc200, enabled the precise identification of all P. huashanica Ns-genome chromosomes (Fig. 2B C). By combining the oligonucleotide probes for FISH images and the bulked pool probes (Synt1–Synt7) for the seven Triticeae linkage groups (Fig. 3), the FISH patterns of P. huashanica chromosomes 1Ns to 7Ns were established (Fig. 2 A). Details regarding chromosome morphology are summarized in Table 4.
FISH with oligonucleotide probes on chromosomes of P. huashanica accession ZY3156 and ZY3157. A FISH patterns of 1-7Ns chromosomes using probes pSc200 (red), pTa71A-2 (green) and Oligo-44 (yellow). B, C FISH identification in accessions ZY3157 and ZY3156 by probes pSc200 (red), pTa71A-2 (green) and Oligo-44 (yellow). Scale bar: 10 μm
FISH painting using bulked oligo probes and sequential FISH with oligonucleotide probes on chromosomes of P. huashanica accession ZY3157. (A, B, C, D, E, F, G, H) FISH painting using bulked oligo probes Synt1 (red) + Synt2 (green), Synt3 (red) + Synt4 (green), Synt5 (green) + Synt6 (red) and Synt7 (green) with corresponding FISH using probes pTa71A-2(green) + Oligo-44(red). Scale bar: 10 μm
The chromosomes of the two P. huashanica accessions had similar probe hybridization patterns, with some variations in the FISH signals for the 1Ns homologous chromosomes analyzed using probes pTa71A-2 and pSc200, and for 2Ns analyzed using probe pSc200, which hybridizes exclusively to the terminal region of all chromosomes (Fig. 2 A). Chromosome 1Ns was characterized by heteromorphic pTa71A-2 signals, with varying densities at the nucleolar organizing regions (NORs) of the short arms among the homologous chromosomes. Additionally, strong Oligo-44 signals were detected near the centromere region of the long arms. Terminal pSc200 signals were observed at the short arms of chromosome 1Ns. Moreover, pSc200 revealed the heteromorphism at the terminal region of the long arms. Chromosome 2Ns had clear pTa71A-2 signals at the subterminal region of the long arms. The variation at the terminal region of the 2Ns chromosomes detected using pSc200 was identical to that on the 1Ns chromosomes. Chromosome 3Ns was identified on the basis of weak Oligo-44 signals in pericentromeric and strong Oligo-44 signals near the centromere region of the long arms; strong terminal pSc200 signals were detected on both arms. Chromosome 4Ns lacked specific pTa71A-2 and Oligo-44 signals, but strong terminal pSc200 signals were observed on both arms. Clear pTa71A-2 and faint pSc200 signals were simultaneously produced at the terminal of the short arms of 5Ns chromosomes, with strong Oligo-44 signals at the intercalary region of the long arms. Chromosome 6Ns had strong Oligo-44 signals near the centromere region of the long arms and clear terminal pSc200 signals on both arms. Notably, distinct Oligo-44 signals were observed at the middle region of both arms of chromosome 7Ns. Terminal pSc200 signals on chromosome 7Ns were the same as those on chromosomes 3Ns, 4Ns, and 6Ns (Fig. 2 A).
Validation of the FISH markers
To validate the specificity of the FISH markers for P. huashanica Ns-genome chromosomes, probes pTa71A-2, Oligo-44, and pSc200 were used to perform a FISH analysis of the metaphase cells of CS and six diploid wheat relatives containing St, E, V, I, P and R genomes, respectively (Table 1). There was a lack of pSc200 signals on all the chromosomes of wheat, Pse. stipifolia, Th. elongatum, and Das. villosum. However, pSc200 signals were detected on the H. vulgare I-genome chromosomes, but only at the centromeric regions. Therefore, a FISH involving pSc200 can distinguish the P. huashanica Ns-genome chromosomes from the A-, B-, D-, St-, E-, V-, and I-genome chromosomes. Oligo-44 generated signals with varying intensities on a few chromosomes of CS, Th. elongatum, Das. villosum, and S. cereale, whereas pTa71A-2 revealed polymorphic hybridization sites on several chromosomes in all species. Combining pSc200 with Oligo-44 or pTa71A-2 enabled the discrimination of all P. huashanica Ns-genome chromosomes, with the exception of the 4Ns chromosomes, from the P- and R-genome chromosomes, some of which had terminal pSc200 signals that were the same as those on the 4Ns chromosomes (Fig. 4).
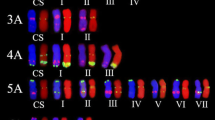
FISH with oligonucleotide probes on chromosomes of P. huashanica accession ZY3157, common wheat Chinese Spring (CS) and diploid wheat relatives. A-G FISH patterns of probes pSc200, pTa71A-2 or Oligo-44 on chromosomes of A, B P. huashanica, C CS, D Pse. stipifolia, E Th. elongatum, F Das. villosum, G H. vulgare, H A. cristatum and I S. cereale, respectively. White arrows indicate the chromosomes which can’t be discriminated from the 4Ns chromosomes of P. huashanica using all the three probes. Scale bar: 10 μm
These probes were also used for a FISH analysis of seven Ns-genome-containing species to assess their utility for distinguishing between P. huashanica chromosomes and other Ns-genome chromosomes in a different genetic background. The pSc200 signals were mainly detected at the terminal region of one or both arms of the Ns-genome chromosomes in most of the examined species. The exceptions were P. juncea, in which faint signals were detected primarily at the subterminal region of several chromosomes, and L. arenarius, in which signals were undetectable. The pSc200 signal distribution patterns were not specific to particular chromosomes or genome types, but the strong terminal signals on all P. huashanica chromosomes except chromosome 5Ns were useful for distinguishing from the Nsj, Nss, Nsp, Nsm, and Nsa chromosomes to some extent. All of the identified Ns-genome chromosomes in the different species had Oligo-44 FISH patterns that were similar to those of P. huashanica chromosomes belonging to homologous groups 1, 3, 5, 6, and 7, but there were obvious interspecific differences in the signal localization for the 1Ns1a and 1Ns2a chromosomes, all 5Ns chromosomes (except for 5Nsm and 5Nsc), chromosome pairs 6Nss, 6Nsp, 7Nsj, 7Nsp, 7Nsm, 7Ns2a, and a single 7Nss and 7Nsc chromosome. The mapping of the pTa71A-2 sites on these Ns-genome chromosomes revealed different 45 S rDNA loci that were the same or similar in size, which provided limited information useful for discriminating between chromosomes (Fig. 5).
Chromosome FISH karyotype of the Ns-genome chromosomes using probes pSc200 (red), pTa71A-2 (green) and Oligo-44 (red) for P. juncea and Leymus species. The arrangement of chromosomes was based on the FISH patterns similarities with P. huashanica (ZY3156) chromosomes and chromosomes morphology including arm ratio and relative length. White arrows indicate the FISH signal variations within homologous chromosomes, red lines indicate the chromosomes which can’t be discriminated from chromosomes of P. huashanica using all the three probes. Scale bar: 10 μm
Using the three probes simultaneously for a FISH analysis unambiguously distinguished chromosome 1Nsh from the homologous chromosomes in all other species. Chromosome 2Nsh could also be distinguished from all other 2Ns chromosomes by the pSc200 and pTa71A-2 probe combination. The pSc200 and Oligo-44 probes were able to differentiate chromosome 3Nsh from the other 3Ns chromosomes. Probes pTa71A-2 and Oligo-44 failed to hybridize to chromosome 4Nsh, whereas pSc200 was able to discriminate this chromosome from chromosomes 4Nsj, 4Nss, 4Nsp, 4Nsm, and 4Nsa. The pSc200, Oligo-44, and pTa71A-2 probe combination helped separate chromosome 5Nsh from all other 5Ns chromosomes. The Nsh and other Ns chromosomes (except for Nsr and Nsc) in homologous groups 6 and 7 could also be clearly distinguished using the pSc200 and Oligo-44 probe combination (Fig. 5).
Utility of the FISH markers for identifying P. huashanica chromosomes in the wheat background
To evaluate the potential utility of pTa71A-2, Oligo-44, and pSc200 for identifying P. huashanica chromosomes in the wheat background, a FISH was performed on the metaphase cells of wheat–P. huashanica hybrid derivatives. Six wheat–P. huashanica chromosome addition lines with diverse chromosomal contents were unambiguously characterized. The FISH results indicated that line 19-2-5 (2n = 44) was a chromosome addition line containing a 2Ns and telosomic chromosome (Fig. 6 A, 6B). Line 32-2-5 (2n = 43) was identified as a monosomic chromosome addition line containing a 3Ns chromosome on the basis of the signal patterns and a larger arm ratio than that of the 6Ns chromosomes (Fig. 6C, D). The probe signals indicated that line 26-3-2 (2n = 44) carried an additional 2Ns and 4Ns chromosome respectively (Fig. 6E F), whereas line 18-2-6 (2n = 44) was a disomic chromosome addition line with a pair of 7Ns chromosomes (Fig. 6G H). Line 20-1-1 (2n = 44) was a 2NsL ditelosomic addition line. Although line 23-1-1 (2n = 44) was also identified as a ditelosomic addition line, it was unclear which group the additional chromosomes belonged to (Fig. 6I L).
Chromosome identification of the wheat-P. huashanica chromosomal addition lines. Images for FISH results using probes pTa71A-2 (green), pSc200 (red) and/or Oligo-44(red/yellow), and the sequential GISH (red) of corresponding spreads. A, B Line 19-2-5 had additional 2Ns and tNs chromosomes. C, D Line 32-2-5 had one 3Ns chromosome. E, F Line 26-3-2 had additional 2Ns and 4Ns chromosome. G, H Line 18-2-6 had a pair of 7Ns chromosomes. I, J Line 20-1-1 had two t2NsL chromosomes. K, L Line 23-1-1 had additional tNs chromosomes. Scale bar: 10 μm
Discussion
The ability to accurately identify alien chromosomes in the wheat background is critical for exploiting alien genetic resources [39]. Accordingly, FISH analyses involving pSc119.2, pTa535, pTa713, pTa794, pAs1, and the microsatellites (AAC)5 and (CTT)12 have been widely conducted to identify chromosomes from wheat [17], S. cereale [47], diploid Th. elongatum [21], Das. villosum [22], and Aegilops species (S-, U-, C-, N-, D-and M-genomes) [26,27,28, 32, 48, 49]. However, the resolution of the results obtained by combining these frequently used FISH probes was insufficient for discriminating the seven pairs of P. huashanica chromosomes (Fig. 1). By conducting a FISH analysis using repetitive sequences, we revealed that all P. huashanica Ns-genome chromosomes can be unambiguously discriminated from each other by pTa71A-2 and Oligo-44 (Fig. 1 F). Moreover, the detailed FISH karyotype constructed using pSc200, pTa71A-2, and Oligo-44 enabled the accurate identification of all P. huashanica chromosomes (Fig. 2). Badaeva et al. [50] developed the FISH karyotypes of 12 diploid Aegilops species and divided the chromosomes into homoeologous groups according to the standard C-banding patterns. Zhang et al. [51] constructed a FISH-based map of H. villosa chromosomes on the basis of the FISH patterns on the V-genome chromosomes from a whole set of wheat–H. villosa disomic chromosome addition lines. Said et al. [23] arranged the P-genome chromosomes by mapping conserved single-copy wheat cDNAs on the homoeologous chromosomes of A. cristatum. Using linkage group-specific bulked oligonucleotide pools, we constructed the P. huashanica karyotype relatively simply and quickly. Notably, FISH using pSc200, Oligo-44, and pTa71A-2 together distinguished the P. huashanica Ns-genome chromosomes from the wheat chromosomes as well as all chromosomes except for 4Ns from the chromosomes of diploid wheat relatives containing St, E V, I, P and R genomes, while also differentiating between the P. huashanica Ns-genome chromosomes in all homologous groups and those of P. juncea and most Leymus species.
A previous study proved that for blotting and FISH analyses, pSc200 from rye telomeric/subtelomeric repetitive sequences does not produce detectable signals in the wheat genome [52]. Hence, it was used to differentiate the R-genome chromosomes of rye from the A-, B-, and D-genome chromosomes of wheat [43]. It has also been used to detect changes in the telomeric/subtelomeric sequences of rye chromosomes in wheat–rye amphidiploids [53]. In this study, the terminal pSc200 signals on all P. huashanica chromosomes enabled the discrimination of the P. huashanica chromosomes from the wheat chromosomes and revealed the variation of telomeric sequences from the Ns-genome chromosomes in P. juncea and Leymus species (Figs. 2 and 4 C, and 5). Probe pTa71A-2, which belongs to the pTa71 family of repeating sequences (45 S rDNA), comprises a 9-kb sequence from the common wheat 18 S–5.8 S–26 S rDNA and intergenic spacers [41, 54]. The 45 S rDNA gene locus is usually linked with NORs in the tribe Triticeae. Previous research indicated that major NORs are preferentially located on the short arms of chromosomes in homoeologous groups 1, 5, and 6 [54, 55]. However, according to the results of this study, pTa71A-2 hybridizes to the short arms of the 1Ns and 5Ns chromosomes while the long arms of the 2Ns chromosomes (Fig. 2 A). The positions of major NORs determined using pTa71A-2 in this study were in accordance with the findings of an earlier study by Kisimi [56]. Thus, the differences in the major 45 S rDNA sites on the long arms of the 2Ns chromosomes in P. huashanica were likely due to a chromosomal non-homologous recombination between 6Ns and 2Ns. Li et al. [33] confirmed the non-homologous chromosomal rearrangements in rye, Ae. uniaristata, Ae. markgrafii, and Ae. umbellulata using linkage group-specific bulked oligo pools. In contrast, we did not detect interchanges involving P. huashanica 2Ns and 6Ns chromosomes because the probe pools mainly hybridized to the non-terminal region of both arms of the P. huashanica chromosomes (Fig. 3 A, C, E, and G), indicative of a relatively low homology between the telomeric regions of wheat and P. huashanica chromosomes. Oligo-44 is a new tandem repeat isolated from the CS genome that has been used to identify specific wheat chromosomal segments [42]. In the current study, abundant Oligo-44 hybridization signals facilitated the identification of P. huashanica chromosomes, and they also helped the detection of several Ns-genome chromosomes in P. juncea and Leymus species (Fig. 5), which contain the Ns genome from the genus Psathyrostachys and the Xm genome from unconfirmed donors [57]. In addition to the pSc200 and pTa71A-2 signals, polymorphic Oligo-44 hybridization patterns elucidated the genetic differences among the Ns-genome chromosomes in diverse genetic backgrounds (Fig. 5), which were previously determined in Southern blots and via molecular studies [58,59,60].
The distribution of Oligo-44 signals provided some insights into the relationship between Ns and Xm genomes. On the basis of the chromosome pairing in intergeneric hybrids and the results of DNA hybridization experiments, the evolutionary distance between the Ns and Xm genomes in Leymus species has been investigated. In earlier studies by Dewey [61] and Wang et al. [62], the trivalent ratio was low during analyses of the configuration of F1 pollen mother cells at metaphase I in Leymus × Psathyrostachys hybrids, suggesting the tetraploid Leymus species are allotetraploids. Zhang et al. [63] subsequently confirmed their findings using GISH and chromosome pairing data. However, FISH analyses involving the mapping of dispersed retrotransposon-like repeats from L. mollis and L. arenarius on the chromosomes of Psathyrostachys and Leymus species indicated that Leymus species should be considered as autopolyploids with the Ns genome or segmental allopolyploids consisting of an altered basic Ns genome [64]. The Oligo-44 signal distribution characteristics among the Ns-genome chromosomes indicated that the corresponding repetitive sequence was conserved during the evolution of the Ns genome (Fig. 5; Table 5). Thus, there are theoretically 2-times and 4-times more chromosomes carrying Oligo-44 signals of assumed autotetraploid and autooctaploid Leymus species, respectively, than in diploid Ns-genome-containing species. In the current study, the number of chromosomes giving Oligo-44 hybridization sites was 10–13 in tetraploid Leymus species and 22 in the octaploid L. arenarius (Table 5), implying that Leymus species are likely not autopolyploids, which is consistent with the available DNA sequence information [65, 66]. Because small-scale analyses may lead to incomplete conclusions, more comprehensive approaches are required for future biosystematics-based investigations of the genus Leymus.
The drawbacks to the conventional methods for detecting P. huashanica chromosomes (e.g., analyses involving Giemsa C-banding technology or molecular and biochemical markers) include the considerable time required to conduct the experiments and the production of low-resolution and inaccurate results. Wang et al. [34] characterized nine wheat–P. huashanica chromosome addition lines, with individual alien chromosomes identified according to Giemsa C-banding, but the distributed bands were unclear, which was detrimental for identifying chromosomes. By combining a GISH, Du et al. [67] identified a complete set of 1Ns–7Ns chromosomal addition lines using EST-SSR and EST-STS markers; however, the process was laborious and time-consuming. Biochemical markers, including HMW-GS, LMW-GS, and gliadin, are useful, but only for tracing the 1Ns and 6Ns chromosomes of P. huashanica [6]. FISH analyses involving suitable probes for identifying alien chromosomes are preferred by researchers because they are relatively simple to perform and inexpensive. For example, Du et al. [68] quickly and easily clarified seven CS–Th. bessarabicum alien chromosome introgressions by completing a FISH using Th. bessarabicum-specific oligo pools. Furthermore, 17 wheat–A. cristatum introgression lines containing different P-genome chromosomes were efficiently identified by using the isolated P-genome-specific DNA sequences for a FISH [69]. Li et al. [70] rapidly determined the chromosomal composition of eight wheat–tetraploid Th. elongatum hybrid derivatives using the pSc119.2, pTa535, pTa71, and pTa713 probe combination and suggested that the E-genome chromosomes of the tetraploid Th. elongatum can be quickly and accurately identified via FISH karyotyping in the wheat background. In our study, we efficiently characterized six wheat–P. huashanica chromosome addition lines in a FISH using pSc200, pTa71A-2, and Oligo-44. Our findings indicate that the P. huashanica Ns-genome chromosomes in the wheat background can be rapidly and precisely identified by a FISH using specific probes. These lines containing monosomic or diverse alien chromosomes have been selfed to obtain stable disomic chromosome addition lines. In future investigations, we will evaluate the agronomic performance and disease resistance of these lines and assess their utility as genetic resources relevant for wheat breeding.
Conclusions
We constructed a FISH karyotype of P. huashanica using oligonucleotide probes pSc200, pTa71A-2 and Oligo-44. The combination of these probes helped distinguish all the Ns-genome chromosomes of P. huashanica (except for 4Ns) from those of wheat, diploid wheat relatives and most Ns-genome-containing species, providing an alternative for sequential GISH and FISH to detected P. huashanica chromosomes in the wheat background directly. Additionally, six wheat-P. huashanica addition lines were characterized and will be evaluated for agronomic performance and disease resistance. The FISH karyotype established here lays a solid foundation for the efficient identification of P. huashanica chromosomes in wheat genetic improvement programs.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
References
Baden C. A taxonomic revision of Psathyrostachys (Poaceae). Nord J Bot. 1991;11(1): 3–26.
Cheng SY, Zhang AJ, Fu J. The hybridization between Triticum aestivum and Psathyrostachys huashanica. Acta Genet Sin. 1991;6:508–512.
Jing JX, Fu J, Yuan HX, Wang MN, Li ZQ. A preliminary study on heredity of the resistance to stripe rust in three wild relatives of wheat. Acta Phytopathol Sin. 1999;29:147–150.
Du WL, Wang J, Pang YH, Wu J, Zhao J, Liu SH, Yang QH, Chen XH. Development and application of PCR markers specific to the 1Ns chromosome of Psathyrostachys huashanica Keng with leaf rust resistance. Euphytica. 2014;200:207–220.
Du WL, Wang J, Lu M, Sun SG, Chen XH, Zhao JX, Yang QH, Wu J. Characterization of a wheat-Psathyrostachys huashanica Keng 4Ns disomic addition line for enhanced tiller numbers and stripe rust resistance. Planta. 2014;239:97–105.
Zhao JX, Ji WQ, Wu J, Chen XH, Cheng XN, Wang JW, Pang YH, Liu SH, Yang QH. Development and identification of a wheat-Psathyrostachys huashanica addition line carrying HMW-GS, LMW-GS and gliadin genes. Genet Resour Crop Evol. 2010;57:387–394.
Li JC, Yao XN, Yang ZJ, Cheng XN, Yuan FP, Liu Y, Wu J, Yang QH, Zhao JX, Chen XH. Molecular cytogenetic characterization of a novel wheat-Psathyrostachys huashanica Keng 5Ns (5D) disomic substitution line with stripe rust resistance. Mol Breed. 2019;39:109.
Bai SS, Yuan FP, Zhang HB, Zhang ZY, Zhao JX, Yang QH, Wu J, Chen XH. Characterization of the wheat-Psathyrostachys huashanica Keng 2Ns/2D substitution line H139: A novel germplasm with enhanced resistance to wheat take-all. Front Plant Sci. 2020;11:233.
Li JC, Zhao L, Cheng XN, Bai GH, Li M, Wu J, Yang QH, Chen XH, Yang ZJ, Zhao JX. Molecular cytogenetic characterization of a novel wheat-Psathyrostachys huashanica Keng T3DS-5NsL•5NsS and T5DL-3DS•3DL dual translocation line with powdery mildew resistance. BMC Plant Biol. 2020;20:163.
Liu YX, Huang SH, Han J, Hou CC, Zheng DS, Zhang ZM, Wu J. Development and molecular cytogenetic identification of a new wheat-Psathyrostachys huashanica Keng translocation line resistant to powdery mildew. Front Plant Sci. 2021;12:689502.
Zhang J, Jiang Y, Guo YL, Li YH, Wang Y, Xuan P. Cloning of Ns genome-specific sequence of Psathyrostachys huashanica and construction of molecular markers. J Agric Biotechnol. 2017;25:1391–1399.
Bai HY. Wheat sharp eyespot resistance evaluation of wheat- Psathyrostachys huashanica derived lines and development of Ns chromosome specific SCAR markers. Northwest A&F University, 2017.
Su JN, Guo J, Wang CJ, Jin F, Zhao JX, Yang QH, Chen XH, Wu J. Specific SCAR markers on chromosome 3Ns of Psathyrostachys huashanica Keng. J Triticeae Crops. 2015;35:1–6.
Wang J, Wang LM, Du WL, Chen LG, Liu SH, Wu J, Zhao JX, Yang QH, Chen XH. Development of 5Ns chromosome-specific SCAR markers for utilization in future wheat breeding programs. Genetika. 2014;50:692–699.
Alkhimova AG., Heslop-Harrison JS, Shchapova AI, Vershinin AV. Rye chromosome variability in wheat-rye addition and substitution lines. Chromosome Res. 1999;7:205–212.
Kato A, Vega JM, Han FP, Lamb JC, Birchler JA. Advances in plant chromosome identification and cytogenetic techniques. Curr Opin Plant Biol. 2005;8:148–154.
Tang, ZX, Yang ZJ, Fu SL. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and PAWRC.1 for FISH analysis. J Appl Genet. 2014;55:313–318.
Mukai Y, Nakahara Y, Yamamoto M. Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome. 1993;36:489–494.
Komuro S, Endo R, Shikata K, Kato A. Genomic and chromosomal distribution patterns of various repeated DNA sequences in wheat revealed by a fluorescence in situ hybridization procedure. Genome. 2013;56:131–137.
Cuadrado A, Jouve N. Chromosomal detection of simple sequence repeats (SSRs) using nondenaturing FISH (ND-FISH). Chromosoma. 2010;119:495–503.
Linc G, Sepsi A, Molnár-Láng M. A FISH karyotype to study chromosome polymorphisms for the Elytrigia elongata E genome. Cytogenet Genome Res. 2012;136:138–144.
Grosso V, Farina A, Gennaro A, Giorgi D, Lucretti S. Flow sorting and molecular cytogenetic identification of individual chromosomes of Dasypyrum villosum L.(H. villosa) by a single DNA probe. PLoS One. 2012;7:e50151.
Said M, Hřibová E, Danilova TV, Karafiátová M, Čížková J, Friebe B, Doleže J, Gill BS, Vrána J. The Agropyron cristatum karyotype, chromosome structure and cross-genome homoeology as revealed by fluorescence in situ hybridization with tandem repeats and wheat single-gene probes. Theor Appl Genet. 2018;131:2213–2227.
Fradkin M, Ferrari MR, Espert SM, Ferreira V, Grassi E, Greizerstein EJ, Poggio L. Differentiation of triticale cultivars through FISH karyotyping of their rye chromosomes. Genome 2013;56:267–272.
Tsujimoto H, Mukai Y, Akagawa K, Nagaki K, Fujigaki J, Yamamoto M, Sasakuma T. Identification of individual barley chromosomes based on repetitive sequences: conservative distribution of Afa-family repetitive sequences on the chromosomes of barley and wheat. Genes Genet Syst. 1997;72:303–309.
Molnár I, Vrána J, Farkas A, Kubaláková M, Cseh A, Molnár-Láng M, Doležel J. Flow sorting of C-genome chromosomes from wild relatives of wheat Aegilops markgrafii, Ae. triuncialis and Ae. cylindrica, and their molecular organization. Ann Bot. 2015;116:189–200.
Molnár I, Cifuentes M, Schneider A, Benavente E, Molnár-Láng M. Association between simple sequence repeat-rich chromosomes regions and intergenomic translocation breakpoints in natural populations of allopolyploid wild wheats. Ann Bot. 2011;107:65–76.
Molnár I, Kubaláková M, Šimková H, Cseh A, Molnár-Láng M, Doležel J. Chromosome isolation by flow sorting in Aegilops umbellulata and Ae. comosa and their allotetraploid hybrids Ae. biuncialis and Ae. geniculate. PLoS One. 2011;6:e27708.
Linc G, Gaál E, Molnár I, Icsó D, Badaeva E, Molnár-Láng M. Molecular cytogenetic (FISH) and genome analysis of diploid wheatgrasses and their phylogenetic relationship. PLoS One. 2017;12:e0173623.
Danilova TV, Friebe B, Gill BS. Development of a wheat single gene FISH map for analyzing homoeologous relationship and chromosomal rearrangements within the Triticeae. Theor Appl Genet. 2014;127:715–730.
Kato A, Lamb JC, Birchler JA. Chromosome painting using repetitive DNA sequences as probes for somatic chromosome identification in maize. Proc Natl Acad Sci USA. 2004;101:13554–9.
Song XY, Song RR, Zhou JW, Yan WK, Zhang T, Sun HJ, Xiao J, Wu YF, Xi ML, Lou QF, Wang HY, Wang XE. Development and application of oligonucleotide-based chromosome painting for chromosome 4D of Triticum aestivum L. Chromosome Res. 2020;28:171–182.
Li GR, Zhang T, Yu ZH, Wang HJ, Yang EN, Yang ZJ. An efficient oligo-FISH painting system for revealing chromosome rearrangements and polyploidization in Triticeae. Plant J. 2020;4:105.
Wang Y, Yu K, Xie Q, Kang H, Lin L, Fan X, Sha L, Zhang HQ, Zhou YH. The 3Ns chromosome of Psathyrostachys huashanica carries the gene(s) underlying wheat stripe rust resistance. Cytogenet Genome Res. 2011;134:136–143.
Yen C, Yang JL. Biosystematics of Triticeae: Volume II. 2nd ed. Beijing: China Agricultural Press; 2013.
Yen C, Yang JL, Baum BR. Biosystematics of Triticeae: Volume III. 2nd ed. Beijing: China Agricultural Press; 2013.
Yen C, Yang JL. Biosystematics of Triticeae: Volume IV. 1st ed. Beijing: China Agricultural Press; 2011.
Han FP, Lamb JC, Birchler JA. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc Natl Acad Sci USA. 2006;103:3238–43.
Zhao LB, Ning SZ, Yu JJ, Hao M, Zhang LQ, Yuan ZW, Zheng YL, Liu DC. Cytological identification of an Aegilops variabilis chromosome carrying stripe rust resistance in wheat. Breed Sci. 2016;66:522–529.
Song ZP, Dai SF, Bao TY, Zuo YY, Xiang Q, Li J, Liu G, Yan ZH. Analysis of structural genomic diversity in Aegilops umbellulata, Ae. markgrafii, Ae. comosa, and Ae. uniaristata by fluorescence in situ hybridization karyotyping. Front Plant Sci. 2020;11:710.
Xiao ZQ, Tang SY, Qiu L, Tang ZX, Fu SL. Oligonucleotides and ND-FISH displaying different arrangements of tandem repeats and identification of Dasypyrum villosum chromosomes in wheat backgrounds. Molecules. 2017;22:973.
Tang SY, Tang ZX, Qiu L, Yang ZJ, Li GR, Lang T, Zhu WQ, Zhang JH, Fu SL. Developing new oligo probes to distinguish specific chromosomal segments and the A, B, D genomes of wheat (Triticum aestivum L.) using ND-FISH. Front Plant Sci. 2018;9:1104.
Fu SL, Chen L, Wang YY, Li M, Yang ZJ, Qiu L, Yan BJ, Ren ZL, Tang ZX. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci Rep. 2015;5:10552.
Han YH, Zhang T, Thammapichai P, Weng YQ, Jiang JM. Chromosome-specific painting in Cucumis species using bulked oligonucleotides. Genetics. 2015;200:771–779.
Bi YF, Zhao QZ, Yan WK, Li MX, Liu YX, Cheng CY, Zhang L, Yu XQ, Li J, Qian CT, Wu YF, Chen JF, Lou QF. Flexible chromosome painting based on multiplex PCR of oligonucleotides and its application for comparative chromosome analyses in Cucumis. Plant J. 2020;102:178–186.
Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc. 2006;1:2320–2325.
Cuadrado A, Vitellozzi F, Jouve V, Ceoloni C. Fluorescence in situ hybridization with multiple repeated DNA probes applied to the analysis of wheat-rye chromosome pairing. Theor Appl Genet. 1997;94:347–55.
Schneider A, Molnár I, Molnár-Láng M. Production and FISH identification of wheat-Aegilops biuncialis addition lines and their use for the selection of U and M genome-specific molecular (SSR) markers. Acta Agron Hung. 2010;58:151–158.
Molnár I, Kubaláková M, Šimková H, Farkas A, Cseh A, Megyeri M, Vrána J, Molnár–Láng M, Doležel J. Flow cytometric chromosome sorting from diploid progenitors of bread wheat, T. urartu, Ae. speltoides and Ae. tauschii. Theor Appl Genet. 2014;127:091–1104.
Badaeva ED, Friebe B, Gill BS. Genome differentiation in Aegilops. 1. Distribution of highly repetitive DNA sequences on chromosomes of diploid species. Genome. 1996;39:293–306.
Zhang W, Zhang RQ, Feng YG, Bie TD, Chen PD. Distribution of highly repeated DNA sequences in Haynaldia villosa and application in the identification of alien chromatin. Chin Sci Bull. 2013;58:890–7.
Vershinin AV, Alkhimova EG, Heslop-Harrison JS. Molecular diversification of tandemly organized DNA sequences and heterochromatic chromosome regions in some Triticeae species. Chromosome Res. 1996;4:517–25.
Tang ZX, Fu SL, Ren ZL, Zhou JP, Yan BJ, Zhang HQ. Variations of tandem repeat, regulatory element, and promoter regions revealed by wheat-rye amphiploids. Genome. 2008;51:399–408.
Gerlach WL, Bedbrook JR. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979;7:1869–85.
Badaeva ED, Friebe B, Gill BS. Genome differentiation in Aegilops. 2. physical mapping of 5S and 18S-26S ribosomal RNA gene families in diploid species. Genome. 1996;39:1150–8.
Kishii MQ, Dou W, Garg M, Tsujimoto H. Production of wheat-Psathyrostachys huashanica chromosome addition lines. Jpn J Genet. 2010;85:281–286.
Wang RR-C, Lu BR. Biosystematics and evolutionary relationships of perennial Triticeae species revealed by genomic analyses. J Syst Evol. 2014;52:697–705.
Orgaard M. Investigation of genome relationships between Leymus Psathyrostachys and Hordeum inferred from genomic DNA: DNA in situ hybridization. Ann Bot London. 1994;73:195–203.
Orgaard M, Heslop-Harrison JS. Relationships between species of Leymus, Psathyrostachys, and Hordeum (Poaceae, Triticeae) inferred from southern hybridization of genomic and cloned DNA probes. Plant Syst Evol. 1994;189:217–231.
Yang RW, Zhou YH, Ding CB, Zheng YL, Zhang L. Relationships among Leymus species assessed by RAPD markers. Biol Plant. 2008;52:237–241.
Dewey DR, The genomic system of classification as a guide to intergeneric hybridization with the perennial Triticeae. In: Gustafson J.P. (eds) Gene Manipulation in Plant Improvement. New York: Plenum; 1984. p. 209–279.
Wang RR-C, Hsiao C. Morphology and cytology of interspecific hybrids of Leymus mollis. J Hered. 1984;6:488–492.
Zhang HQ, Yang RW, Dou QW, Tsujimoto H, Zhou YH. Genome constitutions of Hystrix patula, H. duthiei ssp. duthiei and H. duthiei ssp. longearistata (Poaceae: Triticeae) revealed by meiotic pairing behavior and genomic in-situ hybridization. Chromosome Res. 2006;14:595–604.
Anamthawat-Jónsson K. Molecular cytogenetics of Leymus: Mapping the Ns genome-specific repetitive sequences. J Syst Evol. 2015;52:716–21.
Sha LN, Fan X, Zhang HQ, Kang HY, Wang Y, Wang XL, Yu XF, Zhou YH. Phylogeny and molecular evolution of the DMC1 gene in the polyploidy genus Leymus (Triticeae: Poaceae) and its diploid relatives. J Syst Evol. 2016;54:250–263.
Sha LN, Fan X, Li J, Liao JQ, Zeng J, Wang Y, Kang HY, Zhang HQ, Zheng YL, Zhou YH. Contrasting evolutionary patterns of multiple loci uncover new aspects in the genome origin and evolutionary history of Leymus (Triticeae; Poaceae). Mol Phylogenet Evol. 2017;114:175–188.
Du WL. Molecular cytogenetics characterization of a complete set of wheat- Psathyrostachys huashanica Keng disomic addition lines and development of specific SCAR marker. Northwest A&F University, 2014.
Du P, Zhuang LF, Wang YZ, Yuan L, Wang Q, Wang DR, Dawadondup, Tan LJ, Shen J, Xu HB, Zhao H, Chu CG, Qi ZJ. Development of oligonucleotides and multiplex probes for quick and accurate identification of wheat and Thinopyrum bessarabicum chromosomes. Genome 2017;60:93–103.
Han HM, Liu WH, Zhang JP, Zhou SH, Yang XM, Li XQ, Li LH. Identification of P genome chromosomes in Agropyron cristatum and wheat-A. cristatum derivative lines by FISH. Sci Rep. 2019;9:9712.
Li DY, Li TH, Wu YL, Zhang XH, Zhu W, Wang Y, Zeng J, Xu LL, Fan X, Sha LN, Zhang HQ, Zhou YH, Kang HY. FISH-Based markers enable identification of chromosomes derived from tetraploid Thinopyrum elongatum in hybrid lines. Front Plant Sci. 2018;9:526.
Acknowledgements
We thank Dr. Z J Yang, University of Electronic Science and Technology of China, Chengdu, China, for kindly providing FISH probes and supplying technique guidance of FISH painting, and we thank Dr. S L Fu and Dr. Z X Tang, Sichuan Agricultural University, Chengdu, China, for supplying assistance in FISH protocol.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31771781, 31971883), and Sichuan Science and Technology Programs (2020YJ0348, 2022YFH0069), and the Science and Technology Bureau of Chengdu City (2021-YF05-00681-SN).
Author information
Authors and Affiliations
Contributions
Hao Zhang, Fei Wang, Chunyan Zeng, and Houyang Kang conducted the experiment, analyzed the data, and drafted the manuscript. Wei Zhu, Lili Xu, Yi Wang, and Jian Zeng developed addition lines and evaluated morphological traits. Xing Fan, Lina Sha, Haiqin Zhang, DanDan Wu, Yiran Cheng, and Guoyue Chen provided technique guidance. Yonghong Zhou and Houyang Kang designed the experiment and formulated the questions. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The wheat relatives used in the experiment were supplied by The U.S. National Plant Germplasm System (NPGS) and Triticeae Research Institute of Sichuan Agricultural University, from which authors have obtained permissions to collect these materials. The plant materials are maintained in accordance with the institutional guidelines of Triticeae Research Institute of Sichuan Agricultural University, China. Experimental research on plants, including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation. All experiments and data analyses were conducted in Sichuan Agricultural University.
Consent for publication
Not applicable.
Competing interests
All authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Wang, F., Zeng, C. et al. Development and application of specific FISH probes for karyotyping Psathyrostachys huashanica chromosomes. BMC Genomics 23, 309 (2022). https://doi.org/10.1186/s12864-022-08516-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-08516-6