Abstract
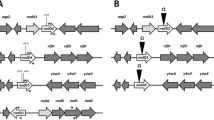
nodD1 of Sinorhizobium fredii HH103, which is identical to that of S. fredii USDA257 and USDA191, repressed its own expression. Spontaneous flavonoid-independent transcription activation (FITA) mutants of S. fredii HH103 M (=HH103 RifR pSym::Tn5-Mob) showing constitutive expression of nod genes were isolated. No differences were found among soybean cultivar Williams plants inoculated with FITA mutants SVQ250 or SVQ253 or with the parental strain HH103M. Soybean plants inoculated with mutant SVQ255 formed more nodules, and those inoculated with mutant SVQ251 had symptoms of nitrogen starvation. Sequence analyses showed that all of the FITA mutants carried a point mutation in their nodD1 coding region. Mutants SVQ251 and SVQ253 carried the same mutation, but only the former was symbiotically impaired, which indicated the presence of an additional mutation elsewhere in the genome of mutant SVQ251. Mutants SVQ251 and SVQ255 were outcompeted by the parental strain for nodulation of soybean cultivar Williams. The symbiotic plasmids of mutants SVQ251 and SVQ255 (pSym251 and pSym255, respectively) and that (pSymHH103M) of the parental strain were transferred to pSym-cured derivatives of S. fredii USDA192 and USDA193 (USDA192C and USDA193C, respectively). Soybean responses to inoculation with S. fredii USDA192C and USDA193C transconjugants carrying pSym251 and pSymHH103M were not significantly different, whereas more nodules were formed after inoculation with transconjugants carrying pSym255. Only transconjugant USDA192C(pSym255) produced a significant increase in soybean dry weight.


Similar content being viewed by others
References
Appelbaum ER, Thompson DV, Idler K, Chartrain N (1988) Bradyrhizobium japonicum USDA191 has two nodD genes that differ in primary structure and function. J Bacteriol 170:12–20
Balatti P, Pueppke SG (1992) Identification of North American soybean lines that form nitrogen-fixing nodules with Rhizobium fredii USDA257. Can J Plant Sci 72:49-55
Becker A, Schmidt M, Jäger W, Pühler A (1995) New gentamicin-resistance and lacZ promoter-probe cassettes suitable for insertion mutagenesis and generation of transcriptional fusions. Gene 162:37–39
Begum AA, Leibovitch S, Migner P, Zhang F (2001) Inoculation of pea (Pisum sativum L.) by Rhizobium leguminosarum bv. viciae preincubated with naringenin and hesperitin or application of naringenin and hesperitin directly into soil increased pea nodulation under short season conditions. Plant and Soil 237:71–80
Beringer JE (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–194
Buendía-Clavería AM, Chamber M, Ruiz-Sainz JE (1989a) A comparative study of the physiological characteristics, plasmid content and symbiotic properties of different Rhizobium fredii strains. System Appl Microbiol 12:203–209
Buendía-Clavería AM, Romero F, Cubo T, Pérez-Silva J, Ruiz-Sainz JE (1989b) Inter and intraspecific transfer of a Rhizobium fredii symbiotic plasmid: expression and incompatibility of symbiotic plasmids. System Appl Microbiol 12:210–215
Buendía-Clavería AM, Moussaid A, Ollero FJ, Vinardell JM, Torres A, Moreno J, Gil-Serrano AM, Rodríguez-Carvajal MA, Tejero-Mateo P, Peart JL, Brewin NJ, Ruiz-Sainz JE (2003) A purL mutant of Sinorhizobium fredii HH103 is simbiotically defective and altered in its lipopolysaccharide. Microbiology 149:1807–1818
Burn JE, Rossen L, Johnston AWB (1987) Four classes of mutations in the nodD gene of Rhizobium leguminosarum biovar viciae that affect its ability to autoregulate and/or activate other nod genes in the presence of flavonoid inducers. Genes Dev 1:456–464
Burn JE, Hamilton WD, Wootton JC, Johnston AWB (1989) Single and multiple mutations affecting properties of the regulatory gene nodD of Rhizobium. Mol Microbiol 3:1567–1577
Chen H, Richardson AE, Gartner E, Djordjevic MA, Roughley RJ, Rolfe BG (1991) Construction of an acid-tolerant Rhizobium leguminosarum biovar trifolii strain with enhanced capacity for nitrogen fixation. Appl Environ Microbiol 57:2005–2011
Chen H, Higgins J, Oresnik IJ, Hynes MF, Natera S, Djorjevic MA, Weinman JJ Rolfe BG (2000) Proteome analysis demonstrates complex replicon and luteolin interactions in pSyma-cured derivatives of Sinorhizobium meliloti strain 2011. Electrophoresis 21:3833–3842
Cullimore JV, Ranjeva R., Bono JJ (2001) Perception of lipo-chitin oligosaccharidic Nod factors in legumes. Trends Plant Sci. 6, 24–30
Cunningham S, Kollmeyer WD, Stacey G (1991) Chemical control of interstrain competition for soybean nodulation by Bradyrhizobium japonicum. Appl Environ Microbiol 57:1886–1892
De Maagd RA, Wijffelman CA, Pees E, Lugtenberg BJ (1988) Detection and subcellular localization of two Sym plasmid-dependent proteins of Rhizobium leguminosarum biovar viciae. J Bacteriol 170:4424–4427
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Espuny MR, Ollero FJ, Bellogín RA, Ruiz-Sainz JE, Perez-Silva J (1987) Transfer of the Rhizobium leguminosarum biovar trifolii plasmid pRtr5a to a strain of Rhizobium sp. that nodulates on Hedysarum coronarium. J Appl Bacteriol 63:13–20
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76:1648–1652
Fisher RF, Long SR (1993) Interactions of NodD at the nod box: NodD binds to two distinct sites on the same face of the helix and induces a bend in the DNA. J Mol Biol 233:336–348
Györgypal Z, Kiss GB, Kondorosi A (1991a) Transduction of plant signal molecules by the Rhizobium NodD proteins. BioEssays 13:575–581
Györgypal Z, Kondorosi E, Kondorosi A (1991b) Diverse signal sensitivity of NodD protein homologs from narrow and broad host range rhizobia. Mol Plant-Microbe Interact 4:356–364
Heron DS, Pueppke SG (1984) Mode of infection, nodulation specificity and indigenous plasmids of 11 fast-growing Rhizobium japonicum strains. J Bacteriol 160:1061–1066
Hooykaas PJJ, Snijdewint FGM, Schilperoort RA (1982) Identification of the Sym plasmid of Rhizobium leguminosarum strain 1001 and its transfer to and expression in other rhizobia and Agrobacterium tumefaciens. Plasmid 8:73–82
Keyser HH, Bohlool BB, Hu TS, Weber DF (1982) Fast-growing rhizobia isolated from root nodules of soybean. Science 215:1631–1632
Knight CD, Rossen L, Robertson JG, Wells B, Downie JA (1986) Nodulation inhibition by Rhizobium leguminosarum multicopy nodABC genes and analysis of early stages of plant infection. J Bacteriol 166:552–558
Kofoid EC, Parkinson JS (1988) Transmitter and receiver modules in bacterial signalling proteins. Proc Natl Acad Sci USA 84:4981–4985
Krishnan HB, Kuo CI, Pueppke SG (1995) Elaboration of flavonoid-induced proteins by the nitrogen-fixing soybean symbiont Rhizobium fredii is regulated by both nodD1 and nodD2, and is dependent on the cultivar-specificity locus nolXWBTUV. Microbiology 141:2245–2251
Lamrabet Y, Bellogín RA, Cubo T, Espuny R, Gil A, Krishnan HB, Megías M, Ollero FJ, Pueppke SG, Ruiz-Sainz JE, Spaink HP, Tejero-Mateo P, Thomas-Oates J, Vinardell JM (1999) Mutation in GDP-fucose synthesis genes of Sinorhizobium fredii alters Nod Factors and significantly decreases competitiveness to nodulate soybeans. Mol Plant-Microbe Interact 12:207–217
Machado D, Pueppke SG, Vinardell JM, Ruiz-Sainz JE, Krishnan HB (1998) Expression of nodD1 and nodD2 in Sinorhizobium fredii, a nitrogen-fixing symbiont of soybean and other legumes. Mol Plant-Microbe Interact 11:375–382
McIver J, Djordjevic MA, Weinman JJ, Bender GL, Rolfe BG (1989) Extension of host range of Rhizobium leguminosarum bv. trifolii caused by point mutations in nodD that result in alterations in regulatory function and recognition of inducer molecules. Mol Plant-Microbe Interact 2:97–106
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Pan B, Smith DL (2000) The effect of application of genistein to Bradyrhizobium japonicum culture and its rooting medium on soyabean growth nodulation and nitrogen assimilation in the presence of nitrate. J Agric Sci 135:19–25
Perret X, Staehelin C, Broughton WJ (2000) Molecular basis of symbiotic promiscuity. Microbiol Mol Biol Rev 64:180–201
Prentki P, Krisch HM (1984) In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303–313
Pueppke SG (1996) The genetic and biochemical basis for nodulation of legumes by rhizobia. Crit Rev Biotechnol 16:1–51
Pueppke SG, Broughton WJ (1999) Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol Plant-Microbe Interact 12:293–318
Ramakrishnan N, Prakash RK, Shantharam S, DuTeau NM, Atherly AG (1986) Molecular cloning and expression of Rhizobium fredii USDA193 nodulation genes: extension of host range for nodulation. J Bacteriol 168:1087–1095
Robleto EA, Scupham AJ, Tripplet EW (2000) Solving the competition problem: genetic and field approaches to enhance the effectiveness of the Rhizobium-legume symbiosis. In: Triplett EW (ed) Prokaryotic nitrogen fixation. Horizon Scientific, Norfolk, pp 251–277
Rodríguez-Navarro DN, Ruiz-Sainz JE, Buendía-Clavería AM, Santamaría C, Balatti PA, Krishnan HB, Pueppke SG (1996) Characterization of fast-growing rhizobia from nodulated soybean [Glycine max (L.) Merr.] in Vietnam. System Appl Microbiol 19:240–248
Romero F (1993) Estudio genético de la nodulación de Rhizobium fredii. Ph.D. Dissertation, University of Seville, Spain
Romero F, Buendía-Clavería A, Ruiz-Sainz JE (1993) Broad host-range effective mutants of Rhizobium fredii strains. J Appl Bacteriol 74, 610–619
Sadowsky M (2000) Competition for nodulation in the soybean/Bradyrhizobium symbiosis. In: Triplett EW (ed) Prokaryotic nitrogen fixation. Horizon Scientific, Norfolk, pp 279–293
Saiki RK (1990) Amplification of genomic DNA. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols. A guide to methods and applications. Academic, San Diego, pp 13–20
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory , Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schell MA (1993) Molecular biology of the LysR family of transcriptional activators. Annu Rev Microbiol 47:597–626
Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Schlaman HRM, Philips DA, Kondorosi E (1998) Genetic organization and transcriptional regulation of rhizobial nodulation genes. In: Spaink HP, Kondorosi A, Hooykaas PJJ (eds) The Rhizobiaceae. Kluwer, Dordrecht, pp 361–386
Simon R (1984) High frequency mobilization of gram-negative bacterial replicons by the in vivo constructed Tn5-Mob transposon. Mol Gen Genet 196:413–420
Spaink HP (2000) Root nodulation and infection factors produced by rhizobial bacteria. Annu Rev Microbiol 54:257–288
Spaink HP, Okker RJH, Wijffelman CA, Pees E, Lugtenberg BJJ (1987) Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol Biol 9:27–39
Spaink HP, Okker RJH, Wijffelman CA, Tak T, Goosen-De Roo L, Pees E, van Brussel AAN, Lugtenberg BJJ (1989) Symbiotic properties of rhizobia containing a flavonoid-independent hybrid nodD product. J Bacteriol 171:4045–4053
Spaink HP, Aarts A, Stacey G, Bloemberg GV, Lugtenberg BJJ, Kennedy EP (1992) Detection and separation of Rhizobium and Bradyrhizobium Nod metabolites using thin-layer chromatography. Mol Plant-Microbe Interact 5:72–80
Spaink HP, Wijfjes AHM, van der Drift KMGM, Haverkamp J, Thomas-Oates JE, Lugtenberg BJJ (1994) Structural identification of metabolites produced by the NodB and NodC proteins of Rhizobium leguminosarum. Mol Microbiol 13:821–831
Van Rhijn P, Vanderleyden J (1995) The Rhizobium-Plant Symbiosis. Microbiol Rev 59:124–142
Vinardell, JM (1997) Estudios fisiológicos y genéticos del gen regulador nodD de Rhizobium fredii HH103. Ph.D. Dissertation, University of Seville, Spain
Vinardell JM, Buendía-Clavería AM, Ruiz-Sainz JE (1993) A method for the positive selection of spontaneous Rhizobium mutants showing transcriptional activation of nodulation genes in the absence of nod-inducers. Mol Plant-Microbe Interact 6:782–785
Vinardell JM, Ollero FJ, Krishnan HB, Espuny MR, Villalobo E, Pueppke SG, Ruiz-Sainz JE (1997) ISRf1, a transposable insertion sequence from Sinorhizobium fredii. Gene 204:63–69
Vincent JM (1970) A manual for the practical study of root nodule bacteria. Blackwell, Oxford
Wood WB (1966) Host specificity of DNA produced by bacterial mutation affecting the restriction and modification. J Mol Biol 16:118–133
Zhang F, Smith DL (1996) Inoculation of soybean (Glycine max (L.) Merr.) with genistein-preincubated Bradyrhizobium japonicum or genistein directly into soil increases soybean protein and dry matter yield under short season conditions. Plant and Soil 179:233–241
Zhang H, Daoust F, Charles TC, Driscoll BT, Prithiviraj B, Smith DL (2002) Bradyrhizobium japonicum mutants allowing improved nodulation and nitrogen fixation of field-grown soybean in a short season area. J Agric Sci 138:293–300
Acknowledgements
This work was supported by the Spanish Ministry of Education and Science, CICYT grant nº BOS 2002-04164-C03-03, and by FEDER grant 1FD97-1430-C04-02. J.M. Vinardell was supported by PFPI fellowship from the Andalusian Government and by E.C. return fellowship HPMF-CT-1999-00222.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vinardell, J.M., López-Baena, F.J., Hidalgo, A. et al. The effect of FITA mutations on the symbiotic properties of Sinorhizobium fredii varies in a chromosomal-background-dependent manner. Arch Microbiol 181, 144–154 (2004). https://doi.org/10.1007/s00203-003-0635-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-003-0635-3