Abstract
Background
Grain length (GL) that is directly associated with appearance quality is a key target of selection in rice breeding. Although abundant quantitative trait locus (QTL) associated with GL have been identified, it was still relatively weak to identify QTL for GL from japonica genetic background, as the shortage of japonica germplasms with long grains. We performed QTLs analysis for GL using a recombinant inbred lines (RILs) population derived from the cross between japonica variety GY8 (short grains) and LX1 (long grains) in four environments.
Results
A total of 197 RILs were genotyped with 285 polymorphic SNP markers. Three QTLs qGL5.3, qGL6.1 and qGL11 were detected to control GL by individual environmental analyses and multi-environment joint analysis. Of these, a major-effect and stable QTL qGL6.1 was identified to be a novel QTL, and its LX1 allele had a positive effect on GL. For fine-mapping qGL6.1, a BC1F2 population consisting of 2,487 individuals was developed from a backcross between GY8 and R176, one line with long grain. Eight key informative recombinants were identified by nine kompetitive allele specific PCR (KASP) markers. By analyzing key recombinants, the qGL6.1 locus was narrowed down to a 40.41 kb genomic interval on chromosome 6. One candidate gene LOC_Os06g43304.1 encoding cytochrome P450 (CYP71D55) was finally selected based on the difference in the transcriptional expression and variations in its upstream and downstream region.
Conclusions
Three QTLs qGL5.3, qGL6.1 and qGL11 were identified to control grain length in rice. One novel QTL qGL6.1 was fine mapped within 40.41 kb region, and LOC_Os06g43304.1 encoding cytochrome P450 (CYP71D55) may be its candidate gene. We propose that the further cloning of the qGL6.1 will facilitate improving appearance quality in japonica varieties.
Similar content being viewed by others
Introduction
Grain length (GL) is the main factor that determines appearance in rice, and affect milling, cooking and eating quality, and is therefore an important agronomic traits in rice (Oryza sativa L.) breeding. (Fitzgerald et al. 2009). The indica varieties generally exhibit long grains, while the japonica varieties have short grains. However, with the wide popularity of some japonica varieties with long grains, such as Daohuaxiang, among the consumers, increasing GL has become an important target in japonica breeding recently (Huang and Qian 2017).
As rice GL is quantitatively inherited (McKenzie and Rutger 1983), it is difficult for breeders to efficiently improve GL using conventional selection methods. Therefore, it should be particularly beneficial to enhance breeding efficiency to use markers closely related to genes or major quantitative trait loci (QTLs) for GL, while the target genotypes could be screened directly in early generations (Wan et al. 2006). A lot of QTLs associated with rice GL have been detected in the previous studies, and some major or major-effect QTLs as well as their candidate genes have been identified, including GS3 (Fan et al. 2006), GS2 (Hu et al. 2015), GL7 (Wang et al. 2015), GLW7 (Si et al. 2016), TGW6 (Ishimaru et al. 2013), qGL3 (Gao et al. 2019), GL6 (Wang et al. 2019) and GW6a (Song et al. 2015; Gao et al. 2021). Importantly, most of these QTLs or genes were detected from the indica varieties with long grains, such as Minghui63 and Kasalath, which were widely used as the long grain parents to cross with the japonica to develop the mapping populations. Recently only few QTLs for GL, such as qGL3.1 and qGL3.3, have been identified from japonica rice (Hu et al. 2018; Qi et al. 2012; Zhang et al. 2012; Ying et al. 2018; Xia et al. 2018). However, it was still relatively weak to identify QTLs or genes associated with GL from japonica genetic background.
To improve GL in japonica breeding, it was an efficient way by introgression of alleles from indica to japonica via subspecies cross. For example, the major QTL GS3 for grain size was identified from indica (Fan et al. 2006), and its long-grain-associated gs3 allele has been introgressed in japonica gene pool by genetic improvement (Sun et al. 2012). Whereas, most of dense and erect panicle japonica varieties, released as high-yielding varieties and had extensive production areas in China, did not showed long grains, even though they carried the gs3 allele. The main reason was that the dense and erect panicle varieties carried the high-yield-associated dep1 allele, which showed negative effect on GL (Li et al. 2019; Zhao et al. 2019). Both GS3 and DEP1 encodes Gγ protein containing a C-terminal cysteine-rich domain (Botella 2012). The gs3 allele could enhance the interaction between Gγ subunits DEP1 and Gβ, resulting in a long and slender grain, while the dep1 allele could reduce the interaction between DEP1 and Gβ, resulting in not only dense and erect panicles but also smaller grains (Sun et al. 2018). Recently, a major QTL qLGY3 has been identified from American japonica variety L-204, which could simultaneously improve both the grain yield and GL by combining with dep1 and gs3 alleles (Liu et al. 2018). These results indicated that discovering novel genes associated with GL in japonica germplasms would be of great importance to improve grain size of erect panicle varieties.
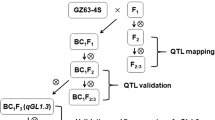
In our previous studies, to improve the GL of erect panicle varieties, the japonica variety Shennong9017 with long grains was used as parent to cross with dense and erect panicle variety Liaojing454 to thus develop a japonica super rice variety Liaoxing1 (LX1). In addition to dense and erect panicles and more grains per panicle, one of the most significant morphological features of LX1 is their long grains, which were higher than those of other dense and erect panicle varieties. The objective of present study was (1) to detect the QTLs for GL by using a recombinant inbred lines (RILs) population derived from a cross between Gangyuan8 (GY8) and LX1 in four environments, (2) to localize the major-effect QTL qGL6.1 to a narrow genomic region by using the BC1F2 population, and (3) to identify the candidate gene of qGL6.1. Our results will provide important information to unearth the QTL for rice GL and help to understand how to achieve high yield and long grains in the LX1.
Results
Phenotypic Variation of Grain Length in the RIL Population
There was significant genetic variation in GL among each RIL line and two parents in all environments. LX1 and GY8 gave averaged GL of 7.6 mm and 6.7 mm, respectively (Fig. 1A). GL of the RILs ranged from 6.55 mm to 8.02 mm in each environment and exhibited approximately normal distributions (Fig. 1B), indicating that they controlled by multiple genes. Pearson’s correlation coefficients among the four environments and BLUP ranged from 0.596 to 0.906 (P < 0.01), indicating that the GL was consistent across environments (Additional file 1: Table S1). The ANOVAs showed significant phenotypic variation in GL among genotypes, environments and genotype × environment interactions, and the variation among replications within experiments was no significant. More than 79% heritabilities were estimated for GL in four environments (Additional file 1: Table S2), indicating that the QTL with large effect for GL could be detected in this study.
QTL Analysis in Individual Environments and Their BLUP Values
The phenotypic values of GL in four environments and its BLUP values were determined using the ICIM-ADD mapping method in BIP module for QTL mapping. A total of 20 QTLs that controlled GL was detected in the individual environment, which included two QTL in 2018DG, four QTLs in 2019DG, four QTLs in 2019PJ, four QTLs in 2019SY and six QTLs in BLUP values, explaining 30.03%, 32.26%, 36.94%, 38.69% and 50.31% of the phenotypic variation, respectively (Table 1). We identified eight single QTL with phenotypic contributions of 10%-20%. Fourteen favorable QTL alleles were from the long grain parent LX1, whereas 6 favorable QTL alleles emerged from GY8.
QTL qGL6.1 detected simultaneously by BLUP data and was consistent across four environments, was flanked by AX-95928617 and AX-95962924 on chromosomes 6. Two QTL qGL5.3 and qGL11 detected simultaneously by BLUP data and were consistent across two environments, were flanked by AX-95952779 and AX-154326915 on chromosomes 5 and AX-115836788 and AX-115753113 on chromosomes 11, respectively. qGL6.1, qGL5.3 and qGL11 had positive effect on GL, explaining 13.53–21.68%, 4.87–7.63% and 10.15–12.05% of the total phenotypic variation, respectively, and the positive allele came from the same donor LX1.
Joint Analysis in Multiple Environments
A total of 15 QTLs that controlled GL was detected based on joint analysis in multiple environments and showed in the physical map (Fig. 2). Three stable QTL qGL5.3, qGL6.1 and qGL11 detected in individual environment analyses were all mapped to the same marker intervals to those detected by the joint analysis (Table 2). By using the ICIM-ADD mapping method in the MET module, the qGL5.3 and qGL11 showed significant additive effect (LOD(A) > 2.5) as well as QTL × environment interaction effects (LOD(AbyE) > 2.5), but these additive contribution rates were higher than those between the additive effect and environment interaction, indicating that these additive effects were the main contributors to phenotypic variation. The qGL6.1 had significant additive effect (LOD(A) > 2.5) rather than additive-environment interaction (LOD(AbyE) < 2.5), which were considered to be expressed independently. These three QTL qGL5.3, qGL6.1 and qGL11 were detected by the joint analysis with phenotypic contribution of 2.86%, 17.51% and 5.45%, respectively.
Fine Mapping of qGL6.1 and Analyzing Candidate Genes
By individual environmental analyses and multi-environment joint analysis, the major-effect QTL qGL6.1 for GL was detected in an approximate 97.46-kb interval on chromosomes 6 (Fig. 3A). To obtain a candidate genomic region for the positioning of qGL6.1, the line R176 with long grains (7.62 mm) to backcrossed with recipient parent GY8, as they carried different allele in qGL6.1 locus but had 68% similar in genetic background by 8 K-SNP chip analysis. A total of 2, 487 BC1F2 plants were detected by nine KASP markers across the qGL6.1 target region to identify eight key informative recombinants (Fig. 3B). Among them, the heterozygous recombinants RT2, RT4, RT5, RT6 and RT7 showed longer grains compared with the others. The progeny test was further conducted in the BC1F3 populations derived from the heterozygous recombinants RT1, RT3, RT5 and RT8. Only in the population derived from RT5, the homozygotes with LX1 allele had longer grains than those with GY8 allele (Fig. 3C). The difference of GL between these progenies with LX1 and GY8 allele at qGL6.1 locus was more than 1.4 mm, because the phenotypic variation was affected by other QTLs, such as qGL11, qGL5.3, qGL5.1, qGL5.2, qGL6.2, qGL7.2 and qGL10 (Additional file 2: Fig. S1). To eliminate the interference of these QTLs, we found two type of plants (RT5-13 and RT5-14) carried same alleles at qGL11, qGL5.3, qGL5.1, qGL5.2, qGL6.2, qGL7.2 and qGL10 rather than qGL6.1 locus. The RT5-14 with LX1 allele at qGL6.1 locus showed an increase of 0.29 mm in GL than RT5-13 with GY8 allele (Fig. 3D). Finally, the qGL6.1 locus was narrowed down to a 40.41-kb genomic interval with flanking markers SNP5 (Chr6: 26,011,771) and SNP7 (Chr6: 26,052,181) (Fig. 3E). According to the Rice Annotation Project annotation (http://rapdb.dna.affrc.go.jp/), this region contains four annotated genes, LOC_Os06g43290.1, LOC_Os06g43304.1, LOC_Os06g43320.1 and LOC_Os06g43330.1. The LOC_Os06g43290.1 and LOC_Os06g43330.1 encodes putative Ty1-copia subclass retrotransposon protein, and LOC_Os06g43304.1 and LOC_Os06g43320.1 encodes cytochrome P450, respectively.
Fine mapping of qGL6.1 and and analyzing candidate genes. a The physical map of qGL6.1 region on chromosome 6. b Fine mapping of qGL6.1 with eight recombinants. The black bar with markers denotes the recombinant sites. The white, black and gray boxes stand for genotypes of Gangyuan8, R176 and heterozygote, respectively. Vertical dotted lines denote the target region. c The progeny test in the BC1F3 populations derived from the heterozygous recombinants R1, R3, R5 and R8. d Two BC1F3 plants derived from heterozygous recombinants R5 carried different alleles at qGL6.1 locus without the interference of other QTLs. e Four candidate genes in the fine mapping region. Arrows indicate direction of gene orientation. f The expression level of four candidate genes by qPCR analysis. g Variations in the upstream, intron and downstream region of LOC_Os06g43304.1 among Nipponbare, Gangyuan8 (GY8) and Liaoxing1 (LX1)
By qPCR analysis in the inflorescence meristem, only the LOC_Os06g43304.1 differentially expressed between GY8 and R176 among these four annotated genes (Fig. 3F). The expression of LOC_Os06g43304.1 was down-regulated in R176 as compared to GY8. We also found 10 SNP and 5 InDel in upstream region, 11 SNP and 2 InDel in intron region, as well as 9 SNP and 1 InDel in downstream region, respectively, in the DNA sequence of LOC_Os06g43304.1 between GY8 and LX1 (Fig. 3G). Through gene prediction and sequencing, the LOC_Os06g43304.1 encoding cytochrome P450 in rice, was identified as a candidate gene of qGL6.1.
Discussion
In this study, we sought to identify novel genes that control GL in rice by using the RIL population in four environments to dissect the genetic basis of the long grains japonica variety. As we known, the QTL detected in multiple environments could be more stable than QTL with high effect values detected in a single environment and have a more useful value for MAS breeding (Fulton et al. 1997). We simultaneously detected three QTL qGL5.3, qGL6.1 and qGL11 associated with GL regulation in the japonica variety LX1 by individual environmental analyses and multi-environment joint analysis, while their additive effects had higher phenotypic contributions than QTL × environment interactive effects. Among them, qGL6.1 were identified by BLUP data and were consistent across four environments, so it can be considered as stable QTL for GL. Importantly, the qGL6.1 that explained 13.53–21.68% and 17.51% of the phenotypic variation by individual environment analyses and joint analysis, respectively, could be identified as the major-effect QTL for GL.
By using the RIL population, the qGL6.1 was detected in an approximate 97.46 kb interval on chromosome 6 between AX-95928617 and AX-95962924. The qGL6.1 was compared to QTL that had been previously reported for the same or related traits by physical positions of the respective interval markers. The position of qGL6.1 differed slightly from those of known QTL cloned as single genes related to GL on chromosome 6, like TGW6 (Ishimaru et al. 2013), GL6 (Wang et al. 2019), SDT (Zhao et al. 2015), qGL-6 (Zhang et al. 2020) and GW6a (Song et al. 2015; Gao et al. 2021). The DNA sequences analysis showed none polymorphism in the promoter or coding region of GW6a (LOC_Os06g44100), SDT (LOC_Os06g44034) and qGL-6 (LOC_Os06g48950) between the GY8 and LX1 (Additional file 3: Fig. S2A–C). Although six nucleotide substitutions were found in coding regions of TWG6 (LOC_Os06g41850) between GY8 and LX1, they not expected to affect the protein (Additional file 3: Fig. S2D). In previous study, the premature stop codon by a nucleotide substitutions was found in GL6 (LOC_Os06g45540), which cause reduction or loss of function of GL6, thus affecting rice grain length (Wang et al. 2019). However, the long grains japonica variety LX1 only showed a loss of Arg by deletion of nucleotide GAC in the coding region of GL6 (Additional file 3: Fig.S2E). Inportantly, there were non-significant different in the expression levels of SDT, qGL-6, GW6a, TGW6 and GL6 between GY8 and LX1 (Additional file 4: Fig. S3), suggesting that these genes could not explain the variation of GL in this study. Interestingly, the interval of qGL6.1 was within qGN6 and qBNS6.2, controlling grain number per panicle and number of secondary branches, respectively, which were co-localized in a 0.94 Mb interval on chromosome 6 between AX-95955496 and AX-115755704 in our previous study (Zhao et al. 2020). However, it was unknown whether a cluster of QTLs or a true pleiotropy is the cause of multiple phenotypic variations. We suggested that qGL6.1 as a novel QTL could be considered as important candidate loci for fine-mapping to identify the molecular regulatory mechanism for GL as well as other panicle components.
For fine mapping of qGL6.1, in this study, the line R176 was used as parent to develop the BC1F2 population, which had 68% similar in genetic background to GY8. Moreover, KASP as the uniplex SNP genotyping platform offers cost-effective and scalable flexibility in QTL fine mapping that require small to moderate numbers of markers (Semagn et al. 2014). By nine KASP markers developed in the target region, eight key informative recombinants were identified in the BC1F2 population. Although these recombinants shared similar genetic backgrounds with heterozygous rather than homozygous genotypes at the QTL region, they also showed significant difference in GL. We further found significant difference in GL between the homozygotes with LX1 and GY8 allele in a BC1F3 population. However, the difference of GL was more than 1.4 mm, which almost equals to the range of phenotypic variation in the RILs (Fig. 1B), indicated that the phenotypic difference includes effect of other QTLs. By eliminating the background noise, we found the GL was increased of approximately 0.29 mm by LX1 allele at qGL6.1 (Additional file 2: Fig. S1), which was consistent with the results of QTLs analysis in single environment (Table 1). Thus, the qGL6.1 locus was narrowed down to an interval between KASP markers SNP5 (Chr6: 26,011,771) and SNP7 (Chr6: 26,052,181), covering a 40.41-kb region in the Nipponbare genome.
In qGL6.1 interval, only the LOC_Os06g43304.1 encoding a cytochrome P450 (CYP71D55) was significantly differentially expressed in the inflorescence meristem at the stage of primary and secondary rachis branch formation between GY8 and R176, which was most likely caused by the variations in its upstream and downstream region. As we known, the cytochrome P450 monooxygenases catalyze numerous monooxygenation/hydroxylation reactions, play an important role in different biochemical pathways, plant metabolism, cell proliferation, and expansion (Nelson et al. 2008; Schuler and Werck-Reichhart 2003). The CYP71D55 as the member of cytochrome P450 family was found to catalyze the successive regio-selective oxidation at the carbon atom C-2 position of premnaspirodiene to yield solavetivone (Banerjee and Hamberger 2018). Although the function of CYP71D55 in rice was unknown, it was reported that other homologous members regulated the grain size in Arabidopsis, wheat and rice. In Arabidopsis, several cytochrome P450s, including CYP78A5, CYP78A6, CYP78A7and CYP78A9, have been characterized as the positive regulators of seed size, which acts through a non-cell-autonomous signal to promotes organ growth (Adamski et al. 2009; Stransfeld et al. 2010; Sotelo-Silveira et al. 2013). In rice, the global identification, structural analysis and expression characterization of cytochrome P450 superfamily have been systematically studied, which provided a clue to understanding biological function of cytochrome P450 in development regulation and drought stress response (Wei and Chen 2018). The d11 gene, encodes CYP724B1, was implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced GL in rice (Tanabe et al. 2005). The BG2 and GL3.2 encodes CYP78A13 are also responsible for grain size and variations in the exon regions of these genes determined the difference in grain yield (Xu et al. 2015). By referring the function of its homologous genes, therefore, we suggested that the LOC_Os06g43304.1 encoding CYP71D55 in rice might be the candidate gene for GL in the QTL, qGL6.1.
Conclusions
In this study, using the RIL population, three QTL qGL5.3, qGL6.1 and qGL11 were detected to control GL by individual environmental analyses and multi-environment joint analysis. The positive alleles of these QTLs came from the long grain parent LX1. With the BC1F2 population, a major-effect and stable QTL qGL6.1 was fine mapped within 40.41 kb physical interval on chromosome 6. One candidate gene LOC_Os06g43304.1 encoding cytochrome P450 (CYP71D55) was finally selected based on the difference in the transcriptional expression. The cloning and genetic mechanism study of the qGL6.1 will facilitate improving appearance quality in japonica, especially in erect panicle varieties.
Materials and Methods
Plant Materials
The parental lines in this study were the long grains japonica variety LX1 and short grains japonica variety GY8. The mapping population consisted of 197 F6:9 and F6:10 RILs developed from a cross between GY8 × LX1.
Field Trials and Trait Measurement
All 197 RILs and parents were grown at Donggang (39°87′ N, 124°15′ E), Shenyang (41°48′ N, 123°25′ E) and Panjin (41°12′ N, 122°07′ E) in Liaoning Province in 2018 and 2019 cropping seasons (hereafter referred as 2018DG and 2019DG, 2019SY, and 2019PJ) for evaluation of GL. Each RIL and parents were sown in a seedling nursery on April and with one seedling being transplanted per hill on May 24 ~ 30. A completely random block design was repeated three times in each environment. Each plot area was 2.5 m2, and the seedlings were transplanted at a spacing of 30 cm between rows and 13 cm between plants. Protection lines were set up everywhere, and field management followed local standards of production for rice.
At maturity, the panicles of 10 plants of each line, and their parents were harvested from each plot. The distribution of primary branch number per panicle was firstly measured for each plot. Ten panicles with the largest distribution proportion of primary branch number in each plot were selected to collect the fully filled grains. After the grains were dried for 72 h at 50℃, the lengths of twenty randomly chosen grains from each plot were estimated as the lengthwise distance between opposite tips using a vernier caliper, and the values were averaged and used as the measurements for the line. In 2018DG, the GL of only 142 RILs were measured because of serious lodging of some lines, while that of all RILs were measured in the other three environments.
Phenotypic Data Analyses
The phenotypic values of RILs in each environment were analyzed. SPSS 19.0 was used for correlation analysis, and AOV function in QTL IciMapping (version 4.2) was used for variance analysis, with default parameters. The information in the ANOVA table was used to calculate broad sense heritability (H2) of each trait: H2 = σg2/(σg2 + σge2 /e + σε2/re), where σg2 is (MSf − MSfe)/re, σge2 is (MSfe − MSe)/r and σε2 is MSe; σg2 = genetic variance, σge2 = genotype × environment interaction variance, σε2 = error variance, MSf = mean square of genotypes, MSfe = mean square of genotype × environment interaction, MSe = mean square of error, r = number of replications, and e = number of environments.
The best linear unbiased prediction (BLUP) of each parameter is calculated in the mixed linear model by using the lmer function in package lme4 of R software (Bates et al. 2012) (http://www.R-project.org/). Genotype, environment, genotype × environment interaction, and nested repeats in the environment are all considered random factors.
Genotyping
Genomic DNA was extracted from the fresh leaves of each line using the cetyl trimethyl ammonium bromide method (Murray and Thompson 1980). The whole genome resequencing of parents GY8 and LX1 was conducted by Biomarker Technologies. Each RIL (n = 197 genotypes) along with their parents were genotyped using the 8 K-SNP chip by Beijing Golden Marker Biotechnology Co., Ltd (Beijing, China). The SNP markers were screened as follows: ambiguous SNP calling, simplex and poor quality SNP loci with > 10% missing values, or minor allele frequencies < 0.05 were removed in further analysis.
Linkage Mapping and QTL Analysis
After removing the redundant markers, QTL IciMapping 4.2 software was used to generate an input file for genetic map construction (Meng et al. 2015). In this genetic linkage maps, a total of 559 SNPs covering the 12 chromosomes were polymorphic between Gangyuan8 and Liaoxing1 (Zhao et al. 2020). In an individual environmental analysis, the inclusive composite interval mapping (ICIM) method was conducted using the BIP function to detect the additive QTL for phenotypic value and BLUP value of panicle traits (Li et al. 2007). A 1000-permutation test was conducted at a 95% confidence level, and an LOD threshold of 2.5 was used. In addition, the multi-environmental joint analysis utilized the ICIM method in the MET functional module to detect the QTL additive and QTL × environment interaction effects (Li et al. 2015).
QTL were named using the abbreviated English name of trait with “q” before it, followed by the chromosome number or chromosome number plus an ordered number designating one of multiple QTL in a single chromosome. The QTL detected in this study were compared to the Q-TARO database (Yonemaru et al. 2010).
Fine-Mapping by Using BC1F2 Population
After phenotypic and genotypic data analysis, one line with long grains, carrying the LX1 allele in major-effect QTL for GL but having similar genetic background to GY8, was selected in RIL population. For QTL fine-mapping, this line was backcrossed with GY8 to generate the BC1F2 population. A total of 2, 487 BC1F2 plants were grown at SY in 2021 cropping seasons for evaluation of GL. DNA was isolated from the two parental lines and the BC1F2 population. According to the SNPs in the QTL region obtained from deep re-sequencing of two parental lines, the informative KASP markers (Additional file 1: Table S3) were developed to genotype each plant of the BC1F2 population. A total of 753 BC1F3 plants derived from the heterozygous recombinants were further identified at Hainan Province, China, in the winter of 2021. To eliminate the interference of other QTLs, the informative KASP markers (Additional file 1: Table S4) were also developed to genotype the plants of the BC1F3 population. KASP assays were conducted in a 1536-well plate format using the protocol of LGC Genomics (LGC, Middlesex, UK). The Synergy H1 fullfunction microplate reader (FLUO star Omega, BMG Labtech, Germany) was used to read the fluorescence signal. Then the linkage relationship between markers and the qGL6.1 locus was analysed for fine-mapping.
RNA Isolation and qPCR Analysis
To identify the expression of candidate genes, at the stage of primary and secondary rachis branch formation, the inflorescence meristem of GY8, LX1 and R176 were sampled for RNA extraction and qPCR analysis. Total RNA was extracted with TriZol reagent (Invitrogen, Germany) according to the manufacturer’s instructions. The first strand of cDNAs were synthesized from DNaseI-treated total RNA using a Primer Script RT reagent Kit with gDNA Eraser (Takara, Japan). Reverse-transcribed RNA was used as PCR template for gene-specific primers (Additional file 1: Table S5). Each reaction contained 3.0 μl of first-strand cDNAs, 2 μl of 200 μM gene-specific primers, and 12.5 μl of 2 × SYBR Green Master Mix reagent (Applied Biosystems) in a final volume of 25 μl. The qPCR analysis was performed in a real-time PCR system (BIO-RAD). The qRT-PCR analysis was performed for each cDNA sample with four replications. Relative expression levels were calculated by 2−△△CT (Livak and Schmittgen 2001).
PCR and Sequencing
To identify candidate gene, the upstream region (2000 bp), exon and intron region, as well as downstream region (1000 bp) of related genes were amplified from genomic DNA using KOD plus Neo FX (Toyobo, Japan). The primer used to sequence these locus was listed in Additional file 1: Table S6. The sequencing of the PCR production was performed at Quintara Biosciences (Wuhan, China). The genomic sequences were assembled by ContigExpress and aligned by DNAStar.
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- QTL:
-
Quantitative trait loci
- GL:
-
Grain length
- RILs:
-
Recombined inbred lines
- KASP:
-
Kompetitive allele specific PCR
- DG:
-
Donggang
- SY:
-
Shenyang
- PJ:
-
Panjin
References
Adamski NM, Anastasiou E, Eriksson S, O’Neill CM, Lenhard M (2009) Local maternal control of seed size by KLUH/CYP78A5-dependent growth signaling. P Natl Acad Sci USA 106:20115–20120
Banerjee A, Hamberger B (2018) P450s controlling metabolic bifurcations in plant terpene specialized metabolism. Phytochem Rev 17:81–111
Bates D, Maechler M, Bolker B (2012) lme4: linear mixed-effects models using S4 classes (R package version 0999999-0), http://cran.r-projectorg/web/packages/lme4/index.html
Botella JR (2012) Can heterotrimeric G proteins help to feed the world? Trends Plant Sci 17:563–568
Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112:1164–1171
Fitzgerald Ma McCouch SR, Hall RD (2009) Not just a grain of rice: the quest for quality. Trends Plant Sci 14:133–139
Fulton TM, Beck-Bunn T, Emmatty D, Eshed Y, Lopez J, Petiard V, Uhlig J, Zamir D, Tanksley SD (1997) QTL analysis of an advanced backcross of Lycopersicon peruvianum to the cultivated tomato and comparisons with QTLs found in other wild species. Theor Appl Genet 95:881–894
Gao X, Zhang JQ, Zhang X, Zhou J, Jiang Z, Huang P, Tang Z, Bao Y, Cheng J, Tang H, Zhang W, Zhang H, Huang J (2019) Rice qGL3/OsPPKL1 functions with the GSK3/SHAGGY-like kinase OsGSK3 to modulate brassinosteroid signaling. Plant Cell 31:1077–1093
Gao Q, Zhang N, Wang WQ, Shen SY, Bai C, Song XJ (2021) The ubiquitin–interacting motif–type ubiquitin receptor HDR3 interacts with and stabilizes the histone acetyltransferase GW6a to control the grain size in rice. Plant Cell 33:3331–3347
Hu J, Wang Y, Fang Y, Zeng L, Xu J, Yu H, Shi Z, Pan J, Zhang D, Kang S, Zhu L, Dong G, Guo L, Zeng D, Zhang G, Xie L, Xiong G, Li J, Qian Q (2015) A rare allele of GS2 enhances grain size and grain yield in rice. Mol Plant 8:1455–1465
Hu Z, Lu SJ, Wang M, He H, Sun L, Wang H, Liu X, Jiang L, Sun J, Xin X, Kong W, Chu C, Xue H, Yang J, Luo X, Liu J (2018) A novel QTL qTGW3 encodes the GSK3/SHAGGY-like kinase OsGSK5/OsSK41 that interacts with OsARF4 to negatively regulate grain size and weight in rice. Mol Plant 11:736–749
Huang HX, Qian Q (2017) Progress in genetic research of rice grain shape and breeding achievements of long-grain shape and good quality japonica rice. Chin J Rice Sci 31(6):665–672
Ishimaru K, Hirotsu N, Madoka Y, Murakami N, Hara N, Onodera H, Kashiwagi T, Ujiie K, Shimizu B, Onishi A, Miyagawa H, Katoh E (2013) Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat Genet 45:707–711
Li H, Ye G, Wang J (2007) A modified algorithm for the improvement of composite interval mapping. Genetics 175:361–374
Li S, Wang J, Zhang L (2015) Inclusive Composite Interval Mapping (ICIM) of QTL by environment interactions in bi-parental populations. PLoS One. https://doi.org/10.1534/genetics.106.066811
Li X, Tao Q, Miao J, Yang Z, Gu M, Liang G, Zhou Y (2019) Evaluation of differential qPE9-1/DEP1 protein domains in rice grain length and weight variation. Rice 12:1–10
Liu Q, Han R, Wu K, Zhang J, Ye Y, Wang S, Chen J, Pan Y, Li Q, Xu X, Zhou J, Tao D, Wu Y, Fu X (2018) G-protein βγ subunits determine grain size through interaction with MADS–domain transcription factors in rice. Nat Commun 9:1–12
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real–time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
McKenzie KS, Rutger JN (1983) Genetic analysis of amylose content, alkali spreading score, and grain dimensions in rice. Crop Sci 23:306–313
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3:269–283
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Nelson DR, Ming R, Alam M, Schuler MA (2008) Comparison of cytochrome P450 genes from six plant genomes. Trop Plant Biol 1:216–235
Qi P, Lin YS, Song XJ, Shen JB, Huang W, Shan JX, Zhu MZ, Jiang L, Gao JP, Lin HX (2012) The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3. Cell Res 22:1666–1680
Schuler MA, Werck-Reichhart D (2003) Functional genomics of P450s. Annu Rev Plant Biol 54:629–667
Semagn K, Babu R, Hearne S, Olsen M (2014) Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): overview of the technology and its application in crop improvement. Mol Breeding 33:1–14
Si L, Chen J, Huang X, Gong H, Luo J, Hou Q, Zhou T, Lu T, Zhu J, Shangguan Y, Chen E, Gong C, Zhao Q, Jing Y, Zhao Y, Li Y, Cui L, Fan D, Lu Y, Weng Q, Wang Y, Zhan Q, Liu K, Wei X, An E, An G, Han B (2016) OsSPL13 controls grain size in cultivated rice. Nat Genet 48:447–456
Song XJ, Kuroha T, Ayano M, Furuta T, Nagai K, Komeda N, Segami S, Miura K, Ogawa D, Kamura T, Suzuki T, Higashiyama T, Yamasaki M, Mori H, Inukai Y, Wu J, Kitano H, Sakakibara H, Jacobsen SE, Ashikari M (2015) Rare allele of a previously unidentified histone H4 acetyltransferase enhances grain weight, yield, and plant biomass in rice. Proc Natl Acad Sci 112:76–81
Sotelo-Silveira M, Cucinotta M, Chauvin AL, Chavez Montes RA, Colombo L, Marsch-Martínez N, de Folter S (2013) Cytochrome P450 CYP78A9 is involved in Arabidopsis reproductive development. Plant Physiol 162:779–799
Stransfeld L, Eriksson S, Adamski NM, Breuninger H, Lenhard M (2010) KLUH/CYP78A5 promotes organ growth without affecting the size of the early primordium. Plant Signal Behav 5:982–984
Sun J, Liu D, Wang JY, Ma DR, Tang L, Gao H, Xu ZJ, Chen WF (2012) The contribution of intersubspecific hybridization to the breeding of super-high-yielding japonica rice in northeast China. Theor Appl Genet 125:1149–1157
Sun S, Wang L, Mao H, Shao L, Li X, Xiao J, Ouyang Y, Zhang Q (2018) A G-protein pathway determines grain size in rice. Nat Commun 9:1–11
Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, Kato H, Iwasaki Y (2005) A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17:776–790
Wan XY, Wan JM, Jiang L, Wang JK, Zhai HQ, Weng JF, Wang HL, Lei CL, Wang JL, Zhang X, Cheng ZJ, Guo XP (2006) QTL analysis for rice grain length and fine mapping of an identified QTL with stable and major effects. Theor Appl Genet 112:1258–1270
Wang Y, Xiong G, Hu J, Jiang L, Yu H, Xu J, Fang Y, Zeng L, Xu E, Xu J, Ye W, Meng X, Liu R, Chen H, Jing Y, Wang Y, Zhu X, Li J, Qian Q (2015) Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat Genet 47:944–948
Wang A, Hou Q, Si L, Huang X, Luo J, Lu D, Zhu J, Shangguan Y, Miao J, Xie Y, Wang Y, Zhao Q, Feng Q, Zhou C, Li Y, Fan D, Lu Y, Tian Q, Wang Z, Han B (2019) The PLATZ transcription factor GL6 affects grain length and number in rice. Plant Physiol 180:2077–2090
Wei K, Chen H (2018) Global identification, structural analysis and expression characterization of cytochrome P450 monooxygenase superfamily in rice. BMC Genomics 19:1–18
Xia D, Zhou H, Liu R, Dan W, Li P, Wu B, Chen J, Wang L, Gao G, Zhang Q, He Y (2018) GL3.3, a novel QTL encoding a GSK3/SHAGGY-like kinase, epistatically interacts with GS3 to produce extra-long grains in rice. Mol Plant 11:754–756
Xu F, Fang J, Ou S, Gao S, Zhang F, Du L, Xiao Y, Wang H, Sun X, Chu J, Wang G, Chu C (2015) Variations in CYP78A13 coding region influence grain size and yield in rice. Plant Cell Enviro 38:800–811
Ying JZ, Ma M, Bai C, Huang XH, Liu JL, Fan YY, Song XJ (2018) TGW3, a major QTL that negatively modulates grain length and weight in rice. Mol Plant 11:750–753
Yonemaru JI, Yamamoto T, Fukuoka S, Uga Y, Hori K, Yano M (2010) Q-taro: QTL annotation rice online database. Rice 3:194–203
Zhang X, Wang J, Huang J, Lan H, Wang C, Yin C, Wu Y, Tang H, Qian Q, Li J, Zhang H (2012) Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. P Natl Acad Sci USA 109:21534–21539
Zhang T, Wang S, Sun S, Zhang Y, Li J, You J, Su T, Chen W, Ling Y, He G, Zhao F (2020) Analysis of QTL for grain size in a rice chromosome segment substitution line Z1392 with long grains and fine mapping of qGL-6. Rice 13:1–11
Zhao M, Liu B, Wu K, Ye Y, Huang S, Wang S, Wang Y, Han R, Liu Q, Fu X, Wu Y (2015) Regulation of OsmiR156h through alternative polyadenylation improves grain yield in rice. PLoS One 10:e0126154
Zhao M, Ma Z, Wang L, Tang Z, Mao T, Liang C, Gao H, Zhang L, He N, Fu L, Wang C, Sui G, Zheng W (2020) SNP–based QTL mapping for panicle traits in the japonica super rice cultivar Liaoxing 1. Crop J 8:769–780
Zhao M, Zhao M, Gu S, Sun J, Ma Z, Wang L, Zheng W, Xu Z (2019) DEP1 is involved in regulating the carbon-nitrogen metabolic balance to affect grain yield and quality in rice (Oriza sativa L.). PloS One. 14: e0213504
Funding
The study was supported by the National Natural Science Foundation of China (31901526), Doctoral Research Foundation of Liaoning Province (2020-BS-299), and China Postdoctoral Science Foundation Grant (2019M651139).
Author information
Authors and Affiliations
Contributions
WZ and JL initiated the research. MZ designed and conducted all the experiments. YW, NH and XP analyzed genotype of RILs. LW, ZT, LZ, CW, ZM and HG, and LF conducted the field experiments and measured the phenotype. MZ prepared the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This study complied with the ethical standards of China, where this research.
work was carried out.
Consent for Publication
All authors are consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table S1 Correlation of grain length in different environments. Table S2 Grain length of rice recombinant inbred lines in different environments. Table S3 The Kompetitive Allele Specific PCR markers for fine mapping of qGL6.1. Table S4 The Kompetitive Allele Specific PCR markers for seven QTLs in this study. Table S5 The gene-specific primers for qPCR. Table S6 The gene-specific primers for DNA sequencing.
Additional file 2
. Fig. S1 The genotypic data at qGL11, qGL5.3, qGL5.1, qGL5.2, qGL6.2, qGL7.2 and qGL10 locus of some progenies derived from the heterozygous recombinants R5.
Additional file 3
. Fig. S2 Variations in the upstream, intron and downstream region of GW6a (A), SDT (B), qGL-6 (C), TGW6 (D) and GL6 (E) between Gangyuan8 (GY8) and Liaoxing1 (LX1).
Additional file 4
. Fig. S3 The expression level of GW6a, SDT, qGL-6, TGW6 and GL6 by qPCR analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, M., Wang, Y., He, N. et al. QTL Detection for Rice Grain Length and Fine Mapping of a Novel Locus qGL6.1. Rice 15, 60 (2022). https://doi.org/10.1186/s12284-022-00606-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-022-00606-z