Abstract
Necrotizing enterocolitis (NEC) is a devastating gastrointestinal disease that affects newborns, particularly preterm infants, and is associated with high morbidity and mortality. No effective therapeutic strategies to decrease the incidence and severity of NEC have been developed to date. Stem cell therapy has been explored and even applied in various diseases, including gastrointestinal disorders. Animal studies on stem cell therapy have made great progress, and the anti-inflammatory, anti-apoptotic, and intestinal barrier enhancing effects of stem cells may be protective against NEC clinically. In this review, we discuss the therapeutic mechanisms through which stem cells may function in the treatment of NEC.
Similar content being viewed by others
Introduction
Despite constant efforts on improving the diagnosis and treatment technique of premature infants, the morbidity and mortality rates associated with necrotizing enterocolitis (NEC), a devastating gastrointestinal inflammatory and necrotizing disease that affects newborns, particularly preterm infants, are rising. As a main cause of death in the neonatal intensive care unit, NEC has an incidence of approximately 8.9% (890/9956) in premature infants born at the gestational age of 22–28 weeks, and the mortality rate associated with NEC can be as high as 20–30%. Infants requiring surgery exhibit higher mortality rates (Bell et al. 2022; Meister et al. 2020). Survivors may suffer from lifelong gastrointestinal problems, including strictures, adhesions, cholestasis, short bowel syndrome with or without intestinal failure, and neurological sequelae (Bazacliu and Neu 2019).
NEC develops in response to hypoxic-ischemic injury of the intestinal mucosa, caused by exaggerated pro-inflammatory signals and compromised anti-inflammatory signals (Cho et al. 2020). As the main mediator regulating the balance between mucosal injury and repair in the intestines of premature infants, Toll-like receptor 4 is upregulated in infants with NEC, and inactivation of this protein has protective effects in stem cells (Hackam and Sodhi 2018; Liu et al. 2019; Niu et al. 2018). Because NEC is a multifactorial disorder, few radical treatments have been developed, so nonspecific supportive care based on diagnosis and surgery is the primary therapeutic approach. However, stem cell therapy has recently been evaluated in the treatment of NEC owing to the self-renewal potential, multidirectional differentiation capacity, good availability of stem cells, as well as their effects on protecting the intestinal barrier, inhibiting apoptosis, and reducing inflammation (Sajeesh et al. 2020; Pisano and Besner 2019).
In this review, we discuss current progress of stem cell therapy in NEC, with the goal of further elucidating the therapeutic mechanisms of stem cells in NEC and promoting breakthroughs in clinical trials. Accordingly, our review provides potential insight for the progress of new therapeutic method for NEC.
Stem cells
Stem cells are a class of unspecialized or undifferentiated cells that can self-renew and produce highly differentiated mature daughter cells. Stem cells can be divided into totipotent, pluripotent, and unipotent stem cells according to their differentiation potential. Totipotent cells, such as zygotes, have the potential for multidirectional differentiation; pluripotent stem cells, including embryonic stem cells (ESCs), can differentiate into multiple tissues, but cannot develop into complete individuals; and unipotent stem cells, including neural stem cells (NSCs), refer to cells that can only differentiate into one type of cell. Additionally, stem cells are classified into ESCs, adult stem cells (ASCs) and induced pluripotent stem cells (iPSCs), a novel type of stem cell identified in recent years according to developmental origins (Bozdağ et al. 2018).
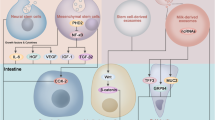
ASCs are pluripotent stem cells that are commonly used in the clinical setting. These cells can proliferate and differentiate directionally into certain unipotent stem cells, such as hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and intestinal stem cells (ISCs) (Suman et al. 2019). Such stem cells have beneficial functions in the intestine, including promoting intestinal epithelium growth, regulating inflammatory cytokines, reducing cell apoptosis, decreasing oxidant stress, repairing the intestinal barrier, and so on (Kandasamy et al. 2014; Jung et al. 2020; Hou et al. 2017; Burns and Thapar 2014) (Fig. 1).
Therapeutic effects of stem cells in NEC. Schematic illustrating the therapeutic effects of stem cells in the injured intestines of NEC model rats. With the injection of stem cells, the experimental NEC intestine has been shown to decrease inflammation, apoptosis, necrosis, and oxidant stress, balance bacteria, enhance barrier, inactivate TLR4, maintain ISC niche, improve motility, and promote IECs proliferation. ROS reactive oxygen species; LPS lipopolysaccharide; TLR4 toll-like receptor 4
Stem cell therapy in NEC
Considering the indispensable roles of stem cells in intestinal protection, stem cell therapy has attracted much interest in studies of NEC. A meta-analysis including nine animal experiments suggested that stem cells and stem cell-derived exosomes decreased the morbidity of NEC, particularly stage 2 NEC by enhancing intestinal motility and reducing intestinal permeability (Villamor-Martinez et al. 2020; Walsh et al. 1988) (Table 1). Current research indicates that ISCs, MSCs, and NSCs, which are derived from various tissues, are most commonly used for NEC treatment (Li et al. 2022; Zhou et al. 2013; Zeng et al. 2021; Drucker et al. 2018a). In the subsequent sections, we discuss how stem cells treat NEC as well as their advantages and disadvantages in the clinical setting (Table 2).
ISCs
Disruption of the viability and integrity of the intestinal epithelium and injury to the intestinal mucosa are commonly observed in infants with NEC (Yu et al. 2020). As a rapidly renewing tissue in mammals, the intestinal epithelium is mainly differentiated from ISCs located at the base of crypts. Thus, ISCs may be involved in the development of NEC (Venkatraman et al. 2021; Neal et al. 2012).
Two types of ISCs are present in intestinal crypts: active ISCs (actively proliferating) and reserve or quiescent ISCs (quiescent cycling). Active ISCs, which can be identified by the marker leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), are responsible for promoting homeostatic renewal and differentiation to intestinal epithelial cells (IECs) (Stewart et al. 2021). The regeneration of small intestinal crypts and villi is mainly attributed to the colonization of reserve ISCs. Reserve ISCs transform into active ISCs by silencing homeodomain-only protein X and active ISCs, then migrate to the damaged segment of the intestine and play important roles in injury-induced intestinal regeneration (Stewart et al. 2021; Gonzalez et al. 2019).
Goblet cells (Zhao et al. 2021), Paneth cells (Barreto et al. 2022), and enteroendocrine cells (Landeghem et al. 2012) derived from ISCs are crucial in modulating the proliferation and differentiation of IECs and promoting intestinal development via the paracrine signaling pathways, including the hedgehog, BMP, Wnt/β-catenin, and Notch signaling pathways; other pathways, such as endocrine signaling pathways and transcription factor pathways, also play important roles (Venkatraman et al. 2021). Owing to their roles in regulating the intestinal microbiota, mucosal immune responses, inflammatory cytokines, and cell apoptosis, paracrine pathways may protect infants from NEC.
MSCs
MSCs are ASCs that originate from the mesoderm and can differentiate into various mesenchymal cells, depending on the tissue in which they are located, e.g., the bone marrow, amniotic fluid, umbilical cord, placental tissues, dental pulp, and adipose tissue (Zhan et al. 2019). MSCs are the first type of stem cell studied in detail and are characterized by proliferation in vitro, multipotency, homing/migration, trophic effects, and immunosuppression; thus, these cells have been shown to have therapeutic potential in multiple autoimmune, inflammatory, and degenerative diseases (Naji et al. 2019). Moreover, MSCs have been shown to reduce the incidence and severity of experimental NEC in rats, although the mechanisms are still unclear (McCulloh et al. 2017a, b).
Notably, MSCs can home to injured intestinal segments; however, the number of MSCs observed in the intestine is not sufficient to exert protective effects, indicating that this protective action may be mainly related to another mechanism (Bahr et al. 2012). Additionally, the efficacy of MSCs may be predominantly mediated by paracrine chemokines and/or growth factors, e.g., interleukin (IL)-6 (Gu et al. 2022), IL-10 (Tu et al. 2022), vascular endothelial growth factor (Chou et al. 2016), and transforming growth factor-β (TGF-β) (Barati et al. 2022). IL-6 inhibits apoptosis, IL-10 exerts anti-inflammatory effects, vascular endothelial growth factor plays important roles in angiogenesis, and TGF-β blocks the expression of pro-inflammatory factors (Gu et al. 2022; Tu et al. 2022; Chou et al. 2016; Barati et al. 2022). These growth factors migrate to the ischemic intestine tissue and contribute to the treatment of NEC. It is worth noting that the paracrine effect of MSCs seems to be mediated through a “hit and run” mechanism. This short-acting and transient engraftment in the injured intestine may limit the adverse effects of MSCs therapy (Bahr et al. 2012).
The anti-inflammatory, antioxidant, anti-apoptotic, and local LGR5+ ISCs proliferative effects of MSCs, coupled with their effects on enhancement of gut microbial diversity in a colitis rat model could synergistically facilitate intestinal recovery (Jung et al. 2020; Weil et al. 2009; Soontararak et al. 2018). Moreover, regardless of the origin of tissue, transplantation of MSCs after intestinal ischemia/reperfusion prominently increases survival rates, reverses mesenteric perfusion, and blocks intestinal injury and inflammation (Jensen et al. 2016).
Bone marrow-derived MSCs (BM-MSCs)
As the name suggests, BM-MSCs are MSCs that originated from the bone marrow. Experiments evaluating the therapeutic effects of exogenous human BM-MSCs in a neonatal rat NEC model demonstrated that after intraperitoneal (IP) injection of transplanted BM-MSCs, the concentration of exogenous human BM-MSCs was increased in the area of the injured intestinal segment, with amelioration of intestinal pathological damage (Tayman et al. 2011).
The functions of BM-MSCs are mainly mediated by paracrine factors. Importantly, inhibition of the upstream transcription factor prolyl hydroxylase 2 enhances the paracrine efficacy of BM-MSCs and protects against NEC. The reason for this phenomenon is that prolyl hydroxylase 2 silencing promotes nuclear factor-κB activation to increase the release of the protective factors—insulin-like growth factor-1 and TGF-β2. Moreover, deficiency in prolyl hydroxylase 2 increases survival in NEC by modulating epithelial regeneration and inflammatory responses (Chen et al. 2020).
Amniotic fluid-derived MSCs (AF-MSCs)
AF-MSCs are a subset of MSCs extracted from amniotic fluid. These cells exhibit rapid proliferation and multidirectional differentiation, similar to pluripotent stem cells (Kaviani et al. 2001). AF-MSCs are easier to harvest and expand in vitro than ASCs and differentiate into cell lineages of all three embryonic germ layers (Dasgupta and Jain 2017; Rosner and Hengstschläger 2021). Additionally, AF-MSCs transiently stimulate healthy IECs proliferation and preserve LGR5+ ISCs, regardless of intestinal injury; thus, these cells may represent novel therapeutic agents in NEC (Li et al. 2022). Moreover, AF-MSCs have recently been shown to reduce the incidence and severity of NEC (Stenson 2014; Zani et al. 2014a; Li et al. 2020).
Wnt signaling is crucial for maintaining ISCs and IECs homeostasis, whose impairment has been observed in experimental NEC model rats (Li et al. 2019). Moreover, AF-MSCs have been shown to activate Wnt signaling, leading to the rescue of injured ISCs, decreased apoptosis and mucosal inflammation, proliferation of IECs, and restoration of intestinal construction (Li et al. 2020).
In addition, AF-MSCs rely on endoplasmic reticulum (ER) stress to mediate NEC. AF-MSCs activate the ER stress response to process the unfolded tight junction proteins, such as claudin-7, which can influence the function of the intestinal barrier by decreasing intestinal permeability (Li et al. 2021a). Meanwhile, AF-MSCs antagonize the apoptotic effects of ER stress by activating the binding immunoglobulin protein (ER stress central regulatory protein) and upregulating C/EBP homologous protein (modulator of apoptosis gene expression). These substances can further inhibit the expression of the pro-apoptotic marker, Bax, and stimulate the expression of the anti-apoptotic marker, Bcl-2, thereby inhibiting the necrosis and apoptosis of IECs (Li et al. 2021a; Lau et al. 2021).
IP injection of AF-MSCs was shown to reduce morbidity and mortality rates in NEC and prolong survival rates by increasing the expression of cyclooxygenase 2 (COX-2) in the lamina propria (Zani et al. 2014a). COX-2 is an important enzyme, whose expression is inversely proportional to the severity of NEC at 24 h (Lu and Zhu 2014). AF-MSCs secrete growth factors, which activate COX-2 either directly or indirectly by promoting the activation of epidermal growth factor receptors, thereby suppressing gut oxidation, facilitating villus cells proliferation, and reducing apoptosis (Zani et al. 2014a). Moreover, AF-MSC-mediated activation of COX-2 results in the secretion of tumor necrosis factor-induced protein 6, which can migrate to injured ileum tissue and attenuate intestinal ischemia/reperfusion injury, thereby blocking the onset of NEC (Koike et al. 2020; Klinke et al. 2020).
Other types of MSCs
The efficacy of stem cells may be negatively correlated with donor age; therefore, identification of original progenitor cell sources, such as umbilical cord blood or placental tissue, is essential for the collection of alternatives to traditional BM-MSCs (Alves et al. 2012). Umbilical cord-derived stem cells (UC-MSCs) have the same pluripotency as BM-MSCs and greater anti-inflammatory and immunomodulatory potential than BM-MSCs (Wegmeyer et al. 2013). Additionally, these cells can be easily isolated using noninvasive methods. Placental-derived MSCs (P-MSCs), initially regarded as medical waste after delivery, have also been shown to be a good source of abundant stem cells with low immunogenicity and strong anti-inflammatory effects (Damianos et al. 2022).
As prenatal stem cells, the effects of UC-MSCs and P-MSCs on amelioration of intestinal damage are primarily mediated by paracrine signaling. Indeed, the administration of UC-MSCs enhances mesenteric perfusion, maintains the intestinal barrier, increases the expression of anti-inflammatory cytokines, and decreases the expression of pro-inflammatory cytokines owing to activation of endothelial nitric oxide synthase (Jensen et al. 2018). The effects of UC-MSCs on inhibition of intestinal damage by secreting hydrogen sulfide suggest potential applications in NEC therapy (Drucker et al. 2019, 2018b). Additionally, significant restoration of the ISC niche with increased Wnt/β-catenin signaling is crucial for the efficacy of human P-MSCs therapy in NEC (Weis et al. 2021). The paracrine effects of P-MSCs can also attenuate inflammation, promote mucosal recovery, and inhibit oxidative stress (Duan et al. 2020). Collectively, human P-MSCs can halt the progression of NEC-related damage to the intestine by improving epithelial morphology and inhibiting intestinal destruction.
NSCs
The enteric neural system (ENS) refers to the multipotent cell population developing from enteric neural crest cells, which proliferate and differentiate into enteric neurons and glia cells in the developing intestine (Nagy and Goldstein 2017). ENS directly regulates gastrointestinal motility and secretes neurotransmitters to maintain intestinal mucosal epithelial barrier function independent from the central nervous system. The loss of enteric neurons and glia cells exacerbates the inflammatory cascades leading to intestinal ischemia (Nezami and Srinivasan 2010).
Both the immature ENS in premature infants and pathologically absent neurons and glial cells in the ENS leave infants susceptible to inflammatory injury observed in NEC (Chandramowlishwaran et al. 2022). Meanwhile, NEC epithelial injury also can cause ENS damage, and thus a vicious loop forms (Bellodas Sanchez and Kadrofske 2019). NSCs are responsible for repairing and replenishing neurons in the ENS, and their engraftment leads to enhancement of intestinal motility and prolongation of survival in experimental NEC (Burns and Thapar 2014; Zhou et al. 2013). Moreover, enteric NSCs (E-NSCs) significantly activate the neuronal nitric oxide synthase and increase nitric oxide, thereby preventing the ENS from damage and preserving intestinal integrity (Zhou et al. 2017).
However, it is difficult to extract NSCs from the gut, and researchers have instead focused on identifying convenient sources, such as the amniotic fluid. NSCs isolated from the amniotic fluid (AF-NSCs) also reduce the incidence and severity of NEC, and their protective effects have been shown to be equivalent to those of E-NSCs (Pisano and Besner 2019). Moreover, AF-NSCs can be collected at delivery or through amniocentesis and are more easily expanded in culture than E-NSCs (Coppi et al. 2007).
Accordingly, stem cells can exert intestinal protective functions through diverse mechanisms (Fig. 2); however, much more work is necessary to fully elucidate these mechanisms. In addition to the therapeutic mechanisms of stem cells described above, previously published studies have also shown that several interventions can affect NEC by enhancing or hampering the therapeutic effects of stem cells (Table 3).
The different types of stem cells used to treat NEC and their signaling pathways. Stem cells exert NEC therapeutic effects via various signaling pathways. Mainly, MSCs exert therapeutic effects through paracrine signaling. In addition, BM-MSCs inhibit prolyl hydroxylase 2 to promote nuclear factor-κB activation and increase the release of the intestinal protective factors. UC-MSCs activate endothelial nitric oxide synthase and secrete hydrogen sulfide in NEC therapy. NSCs can repair and replenish neurons in the ENS, activate the neuronal nitric oxide synthase to prevent the ENS from being damaged, and preserve intestinal integrity. AF-MSCs activate ER stress response to process the unfolded tight junction proteins and promote the expression of Bcl-2/Bax gene. Moreover, AF-MSCs increase the expression of COX-2 in the lamina propria. AF-MSCs and P-MSCs restore the ISC niche to promote IECs proliferation with increased Wnt/β-catenin signaling. ISCs are deservedly responsible for the differentiation to IECs via various signaling pathways. BM-MSCs bone marrow-derived mesenchymal stem cells; UC-MSCs umbilical cord-derived stem cells; NSCs neural stem cells; ENS enteric neural system; COX-2 cyclooxygenase 2; Bcl-2 B-cell lymphoma 2; Bax Bcl-2-associated X protein; AF-MSCs amniotic fluid-derived mesenchymal stem cells; ISCs intestinal stem cells; P-MSCs placental-derived mesenchymal stem cells
Applications of stem cells in NEC
Many advances have been made in the use of stem cell therapy in regeneration medicine in recent decades. Although stem cell-related research in NEC is limited, the feasibility of this approach is high. Further acceleration of progression in NEC treatment will require the removal of obstacles to stem cell transplantation.
Origins of stem cells
MSCs that are derived from bone marrow, amniotic fluid, umbilical cord, and placenta have the great properties of low immunogenicity and immunosuppression, so these stem cells can be transplanted into NEC infants not only by autologous but by allogenic donor transplantation (Naji et al. 2019). AF-MSCs, AF-NSCs, UC-MSCs, and P-MSCs with low expression of human leukocyte antigen (HLA) lower the risk of rejection in allogeneic transplantation and can be easily collected at delivery (Gorodetsky and Aicher 2021). ISCs and E-NSCs are extracted from healthy regions of the patients for autologous transplantation, which avoids the issue of HLA matching in HSCs engraftment (Fig. 3).
Stem cells from various sources are transplanted into NEC models. First, stem cells are isolated and extracted from various tissues including the intestine, bone marrow, amniotic fluid, umbilical cord, and placenta. Among, ISC and E-NSC are autologous, BM-MSC, AF-MSC, UC-MSC, P-MSC, and AF-NSC can be administered into NEC animal models by the donor or autologous transplantation. Then, extracted stem cells are propagated in an incubator. Finally, these cultured stem cells will be transported into NEC animal models via IP or IV injection. BM-MSC bone marrow-derived mesenchymal stem cell; UC-MSC umbilical cord-derived stem cell; AF-MSC amniotic fluid-derived mesenchymal stem cell; AF-NSC neural stem cell isolated from the amniotic fluid; E-NSC enteric neural stem cell; ISC Intestinal stem cell; P-MSC placental-derived mesenchymal stem cell; IP intraperitoneal; IV intravenous
Approach of transplantation
Classical stem cell transplantation involves either IP or intravenous (IV) injection of cells (Ramalho et al. 2018). No major differences in morbidity, pathological damage, or survival rate were observed when comparing IP and IV administration of MSCs, although IV administration is more convenient in preclinical and clinical trials and is regarded as a more efficient delivery route than IP injection (Yang et al. 2012a, b). Nevertheless, IP injection prevents retention of transplanted cells in the pulmonary capillary, which is observed after IV injection, and avoids embolism when administered intra-arterially. IP injection also results in diffuse implantation throughout the gastrointestinal tract, particularly when a broad area of the intestine is treated (Nikiforou et al. 2016; Furlani et al. 2009). Researchers have also shown that systemic delivery of MSCs by umbilical vein infusion, which is safe, noninvasive, and effective, has a high success rate and is related to a low death rate (Yang et al. 2012b, 2014). Each type of injection has specific advantages, and the appropriate approach should be chosen according to a patient’s individual characteristics.
Time of transplantation
It is still ambiguous whether the effects of stem cells on NEC are preventive or protective, which may affect the delivery time of stem cells (Eaton et al. 2013). Although the early use of stem cells is effective, prophylactic administration of stem cells in infants may raise ethical issues, and the treatment time window cannot be identified for therapeutic administration. Hence, it may be necessary to further optimize effective detection methods for NEC to facilitate early intervention with stem cell therapy.
Clinical therapy
Because of the roles of stem cells in experimental NEC models, stem cell therapy was successfully applied in a clinical case in 2019. A 26-day-old full-term infant suffering from NEC was provided with IV injection of UC-MSCs after surgery, and mesenteric blood supply was significantly improved, revealing the potential of stem cells in NEC therapy and preventing short bowel syndrome in this infant (Akduman et al. 2021). However, this is the only published case of the clinical application of stem cells in NEC, and few clinical trials are currently being performed; indeed, on ClinicalTrials.gov, there is only one registered clinical trial for stem cell therapy in NEC to date (trial no. NCT05138276).
Complications
Clinical transformation of stem cells is associated with multiple challenges, including ethical considerations, technical limitations, and adverse effects. Following transplantation, stem cells can exhibit abnormal differentiation after ectopic engraftment (Fennema et al. 2018), low survival rate (Reekmans et al. 2012), and can be physically trapped in the pulmonary capillary bed owing to the large diameter of the cells (Watanabe et al. 2019). Research has shown that undifferentiated stem cells can lead to teratoma formation in vivo (Li et al. 2021b). Therefore, stem cells with rapid growth and high differentiation capacity, such as UC-MSCs, may avoid tumorigenesis, immune rejection, and ethical problems, and are more suitable for current cell therapy approaches (Wang et al. 2022).
Other stem cells derivatives
The conditioned medium is a mixture of all organic and inorganic products secreted by stem cells and can exert functions similar to those of stem cells. Extracellular vesicles (EVs) are vesicles coated with lipids, proteins, and RNA secreted from parental stem cells. The paracrine substances secreted by MSCs can exert function in other organs and even other individuals through the fusion with EVs, which is promising and sustainable for future NEC therapy (Joo et al. 2020). The transition from cell therapy to cell-free therapy may broaden the availability and safety of stem cell treatment.
Given that paracrine mediators are collected in conditioned medium from MSC (MSC-CM), MSC-CM could contribute to mucosal recovery, reduce inflammation, and restore ISCs activity (Lykov et al. 2018; O'Connell et al. 2021). Thus, MSC-CM may have applications in NEC. Owing to problems with low grafting efficiency caused by deficiencies in trophic paracrine factors in MSC-CM, it is also necessary to evaluate how to enhance the secretion of trophic paracrine factors and elevate the therapeutic efficacy of MSC-CM. Stimulation with several factors, including hypoxia, cytokines, growth factors, hormones, and drugs can yield an MSC-CM with protective effects against NEC in the intestinal tract of newborn rats, regulate the balance of pro-inflammatory factors and anti-inflammatory factors, and thereby reduce intestinal injury (Ferreira et al. 2018).
As a subpopulation of EVs, exosomes do not induce immunoreactions between HLA and stem cells owing to intercellular fusion. Moreover, these vesicles exhibit lower immunogenicity than stem cells (Manchon et al. 2021). Additionally, exosomes can cross the blood–brain barrier, enabling applications in brain injury caused by neurological sequelae related to NEC. Exosomes can also be used as delivery vehicles, e.g., facilitating the transportation of intestinal protective growth factors to the damaged intestine (Ghosh et al. 2020; McCulloh et al. 2018). Although exosomes can overcome many drawbacks of stem cell therapy, standardized production of large amounts of exosomes has not yet been achieved, and the legal and ethical considerations have not yet been discussed (Watanabe et al. 2021).
iPSCs have the potential for multi-dermal differentiation, the excellent ability of living and infinite progeny MSCs generation (Hynes et al. 2018). Importantly, they are derived from human’s cells and the differentiation ability can be induced in vitro from those adult cells after birth (Suman et al. 2019; Zakrzewski et al. 2019). There is no immune rejection, and the tumorigenicity evaluation of implanting differentiated MSCs in non-human primates didn’t show any evidence of tumor formation (Hong et al. 2014). Mouse iPSCs-derived MSCs have been found to reduce inflammatory infiltration in local or systemic tissues, but the inflammation can’t be reduced as much as the BM-MSCs do when IP injection (Kagia et al. 2019).
Conclusion and perspectives
Stem cell therapy is a novel approach for treating NEC. Whereas most research is still limited to animal experiments, studies on its long-term outcomes are lacking. Various challenges have hindered the translation of preclinical studies to clinical applications, including the safety of stem cell transplantation in infants. Hence, the exact mechanisms through which stem cells exert beneficial effects in NEC and the pathogenesis of NEC need to be studied in greater detail to facilitate successful clinical trials. Stem cell derivatives or conjunctive treatments with other activators may also have applications in the treatment of NEC in the future.
Availability of data and materials
Not applicable.
Abbreviations
- NEC:
-
Necrotizing enterocolitis
- ESCs:
-
Embryonic stem cells
- NSCs:
-
Neural stem cells
- ASCs:
-
Adult stem cells
- iPSCs:
-
Induced pluripotent stem cells
- HSCs:
-
Hematopoietic stem cells
- MSCs:
-
Mesenchymal stem cells
- ISCs:
-
Intestinal stem cells
- LGR5:
-
Leucine-rich repeat-containing G protein-coupled receptor 5
- IECs:
-
Intestinal epithelial cells
- IL:
-
Interleukin
- TGF-β:
-
Transforming growth factor-β
- BM-MSCs:
-
Bone marrow-derived mesenchymal stem cells
- IP:
-
Intraperitoneal
- AF-MSCs:
-
Amniotic fluid-derived mesenchymal stem cells
- COX-2:
-
Cyclooxygenase 2
- UC-MSCs:
-
Umbilical cord-derived stem cells
- P-MSCs:
-
Placental-derived mesenchymal stem cells
- ENS:
-
Enteric neural system
- E-NSCs:
-
Enteric NSCs
- AF-NSCs:
-
NSCs isolated from the amniotic fluid
- IV:
-
Intravenous
- EVs:
-
Extracellular vesicles
- MSC-CM:
-
Conditioned medium from mesenchymal stem cell
- HLA:
-
Human leukocyte antigen
References
Akduman H, et al. Successful mesenchymal stem cell application in supraventricular tachycardia-related necrotizing enterocolitis: a case report. Fetal Pediatr Pathol. 2021;40:250–5.
Alves H, et al. A mesenchymal stromal cell gene signature for donor age. PLoS ONE. 2012;7: e42908.
Barati S, Kashani IR, Tahmasebi F. The effects of mesenchymal stem cells transplantation on A1 neurotoxic reactive astrocyte and demyelination in the cuprizone model. J Mol Histol. 2022;53:333–46.
Barreto EBL, Rattes IC, da Costa AV, Gama P. Paneth cells and their multiple functions. Cell Biol Int. 2022;46:701–10.
Bazacliu C, Neu J. Necrotizing enterocolitis: long term complications. Curr Pediatr Rev. 2019;15:115–24.
Bell EF, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013–2018. JAMA. 2022;327:248–63.
Bellodas Sanchez J, Kadrofske M. Necrotizing enterocolitis. Neurogastroenterol Motility. 2019;31: e13569.
Bozdağ SC, Yüksel MK, Demirer T. Adult stem cells and medicine. Adv Exp Med Biol. 2018;1079:17–36.
Burns AJ, Thapar N. Neural stem cell therapies for enteric nervous system disorders. Nat Rev Gastroenterol Hepatol. 2014;11:317–28.
Chandramowlishwaran P, Raja S, Maheshwari A, Srinivasan S. Enteric nervous system in neonatal necrotizing enterocolitis. Curr Pediatr Rev. 2022;18:9–24.
Chen CL, et al. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest. 2012;92:331–44.
Chen H, et al. Prolyl hydroxylase 2 silencing enhances the paracrine effects of mesenchymal stem cells on necrotizing enterocolitis in an NF-kappaB-dependent mechanism. Cell Death Dis. 2020;11:188.
Cho SX, et al. Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities. Nat Commun. 2020;11:5794.
Chou HC, Li YT, Chen CM. Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Am J Transl Res. 2016;8:342–53.
Damianos A, Xu K, Kalin GT, Kalinichenko VV. Placental tissue stem cells and their role in neonatal diseases. Semin Fetal Neonatal Med. 2022;27: 101322.
Dasgupta S, Jain SK. Protective effects of amniotic fluid in the setting of necrotizing enterocolitis. Pediatr Res. 2017;82:584–95.
De Coppi P, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–6.
Dong P, et al. Protective effects of human milk-derived exosomes on intestinal stem cells damaged by oxidative stress. Cell Transpl. 2020;29:963689720912690.
Drucker NA, et al. Stem cell therapy in necrotizing enterocolitis: current state and future directions. Semin Pediatr Surg. 2018a;27:57–64.
Drucker NA, Jensen AR, Ferkowicz M, Markel TA. Hydrogen sulfide provides intestinal protection during a murine model of experimental necrotizing enterocolitis. J Pediatr Surg. 2018b;53:1692–8.
Drucker NA, Te Winkel JP, Shelley WC, Olson KR, Markel TA. Inhibiting hydrogen sulfide production in umbilical stem cells reduces their protective effects during experimental necrotizing enterocolitis. J Pediatr Surg. 2019;54:1168–73.
Duan L, et al. Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int J Mol Med. 2020;46:1551–61.
Eaton S, Zani A, Pierro A, De Coppi P. Stem cells as a potential therapy for necrotizing enterocolitis. Expert Opin Biol Ther. 2013;13:1683–9.
Fennema EM, et al. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: a comparative study. J Tissue Eng Regen Med. 2018;12:e150–8.
Ferreira JR, et al. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9:2837.
Furlani D, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–6.
Ghosh S, Garg S, Ghosh S. Cell-derived exosome therapy: a novel approach to treat post-traumatic brain injury mediated neural injury. ACS Chem Neurosci. 2020;11:2045–7.
Gonzalez LM, et al. Preservation of reserve intestinal epithelial stem cells following severe ischemic injury. Am J Physiol Gastrointest Liver Physiol. 2019;316:G482-g494.
Gorodetsky R, Aicher WK. Allogenic use of human placenta-derived stromal cells as a highly active subtype of mesenchymal stromal cells for cell-based therapies. Int J Mol Sci. 2021;22:5302.
Gu C, et al. Human umbilical cord-derived mesenchymal stem cells affect urea synthesis and the cell apoptosis of human induced hepatocytes by secreting IL-6 in a serum-free co-culture system. Biotechnol J. 2022;17: e2100096.
Hackam DJ, Sodhi CP. Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol Hepatol. 2018;6:229-238.e221.
Hong SG, et al. Path to the clinic: assessment of iPSC-based cell therapies in vivo in a nonhuman primate model. Cell Rep. 2014;7:1298–309.
Hou Q, Ye L, Huang L, Yu Q. The research progress on intestinal stem cells and its relationship with intestinal microbiota. Front Immunol. 2017;8:599.
Hynes K, et al. Potential of iPSC-derived mesenchymal stromal cells for treating periodontal disease. Stem Cells Int. 2018;2018:2601945.
Jensen AR, Manning MM, Khaneki S, Drucker NA, Markel TA. Harvest tissue source does not alter the protective power of stromal cell therapy after intestinal ischemia and reperfusion injury. J Surg Res. 2016;204:361–70.
Jensen AR, Drucker NA, Ferkowicz MJ, Markel TA. Umbilical mesenchymal stromal cells provide intestinal protection through nitric oxide dependent pathways. J Surg Res. 2018;224:148–55.
Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21:727.
Jung KJ, et al. Mesenchymal stem cells decrease oxidative stress in the bowels of interleukin-10 knockout mice. Gut Liver. 2020;14:100–7.
Kagia A, et al. Therapeutic effects of mesenchymal stem cells derived from bone marrow, umbilical cord blood, and pluripotent stem cells in a mouse model of chemically induced inflammatory bowel disease. Inflammation. 2019;42:1730–40.
Kandasamy J, Huda S, Ambalavanan N, Jilling T. Inflammatory signals that regulate intestinal epithelial renewal, differentiation, migration and cell death: Implications for necrotizing enterocolitis. Pathophysiology. 2014;21:67–80.
Kaviani A, et al. The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg. 2001;36:1662–5.
Khalifeh Soltani S, et al. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy. 2019;21:54–63.
Klinke M, et al. Cardiac and inflammatory necrotizing enterocolitis in newborns are not the same entity. Front Pediatr. 2020;8: 593926.
Koike Y, et al. The intestinal injury caused by ischemia-reperfusion is attenuated by amniotic fluid stem cells via the release of tumor necrosis factor-stimulated gene 6 protein. FASEB J. 2020;34:6824–36.
Lau E, Lee C, Li B, Pierro A. Endoplasmic reticulum stress in the acute intestinal epithelial injury of necrotizing enterocolitis. Pediatr Surg Int. 2021;37:1151–60.
Lee C, et al. Influence of stress factors on intestinal epithelial injury and regeneration. Pediatr Surg Int. 2018;34:155–60.
Li B, et al. Inhibition of corticotropin-releasing hormone receptor 1 and activation of receptor 2 protect against colonic injury and promote epithelium repair. Sci Rep. 2017;7:46616.
Li J, et al. Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Mol Med Rep. 2018;18:4969–77.
Li B, et al. Impaired Wnt/β-catenin pathway leads to dysfunction of intestinal regeneration during necrotizing enterocolitis. Cell Death Dis. 2019;10:743.
Li B, et al. Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. 2020;11:750.
Li B, et al. Intestinal epithelial tight junctions and permeability can be rescued through the regulation of endoplasmic reticulum stress by amniotic fluid stem cells during necrotizing enterocolitis. FASEB J. 2021a;35: e21265.
Li C, Zhao H, Wang B. Mesenchymal stem/stromal cells: developmental origin, tumorigenesis and translational cancer therapeutics. Transl Oncol. 2021b;14: 100948.
Li B, et al. Amniotic fluid stem cell administration can prevent epithelial injury from necrotizing enterocolitis. Pediatr Res. 2022;91:101–6.
Liu J, Chen T, Lei P, Tang X, Huang P. Exosomes released by bone marrow mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF-κB pathway. Int J Med Sci. 2019;16:1238–44.
Lu H, Zhu B. Role of cyclooxygenase-2 in intestinal injury in neonatal rats. Biomed Rep. 2014;2:875–8.
Lykov AP, et al. Therapeutic potential of biomedical cell product in DSS-induced inflammation in the small intestine of C57Bl/6J mice. Bull Exp Biol Med. 2018;165:576–80.
Manchon E, Hirt N, Bouaziz JD, Jabrane-Ferrat N, Al-Daccak R. Stem cells-derived extracellular vesicles: potential therapeutics for wound healing in chronic inflammatory skin diseases. Int J Mol Sci. 2021;22:3130.
McCulloh CJ, Olson JK, Zhou Y, Wang Y, Besner GE. Stem cells and necrotizing enterocolitis: a direct comparison of the efficacy of multiple types of stem cells. J Pediatr Surg. 2017a;52:999–1005.
McCulloh CJ, et al. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J Surg Res. 2017b;214:278–85.
McCulloh CJ, et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. 2018;53:1215–20.
Meister AL, Doheny KK, Travagli RA. Necrotizing enterocolitis: it’s not all in the gut. Exp Biol Med (maywood, NJ). 2020;245:85–95.
Nagy N, Goldstein AM. Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin Cell Dev Biol. 2017;66:94–106.
Naji A, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76:3323–48.
Neal MD, et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem. 2012;287:37296–308.
Nezami BG, Srinivasan S. Enteric nervous system in the small intestine: pathophysiology and clinical implications. Curr Gastroenterol Rep. 2010;12:358–65.
Nikiforou M, et al. Global hypoxia-ischemia induced inflammation and structural changes in the preterm ovine gut which were not ameliorated by mesenchymal stem cell treatment. Mol Med. 2016;22:244–57.
Nino DF, et al. Retinoic acid improves incidence and severity of necrotizing enterocolitis by lymphocyte balance restitution and repopulation of LGR5+ intestinal stem cells. Shock. 2017;47:22–32.
Nitkin CR, et al. Stem cell therapy for preventing neonatal diseases in the 21st century: current understanding and challenges. Pediatr Res. 2020;87:265–76.
Niu GC, et al. Mesenchymal stem cell transplantation improves chronic colitis-associated complications through inhibiting the activity of toll-like receptor-4 in mice. BMC Gastroenterol. 2018;18:127.
O’Connell JS, et al. Treatment of necrotizing enterocolitis by conditioned medium derived from human amniotic fluid stem cells. PLoS ONE. 2021;16: e0260522.
Pisano C, Besner GE. Potential role of stem cells in disease prevention based on a murine model of experimental necrotizing enterocolitis. J Pediatr Surg. 2019;54:413–6.
Prather WR, Toren A, Meiron M. Placental-derived and expanded mesenchymal stromal cells (PLX-I) to enhance the engraftment of hematopoietic stem cells derived from umbilical cord blood. Expert Opin Biol Ther. 2008;8:1241–50.
Ramalho BDS, Almeida FM, Sales CM, de Lima S, Martinez AMB. Injection of bone marrow mesenchymal stem cells by intravenous or intraperitoneal routes is a viable alternative to spinal cord injury treatment in mice. Neural Regen Res. 2018;13:1046–53.
Reekmans K, et al. Current challenges for the advancement of neural stem cell biology and transplantation research. Stem Cell Rev Rep. 2012;8:262–78.
Rosner M, Hengstschläger M. Amniotic fluid stem cells: what they are and what they can become. Current Stem Cell Res Therapy. 2021. https://doi.org/10.2174/1574888X16666211210143640.
Sajeesh S, Broekelman T, Mecham RP, Ramamurthi A. Stem cell derived extracellular vesicles for vascular elastic matrix regenerative repair. Acta Biomater. 2020;113:267–78.
Shelby RD, Cromeens B, Rager TM, Besner GE. Influence of growth factors on the development of necrotizing enterocolitis. Clin Perinatol. 2019;46:51–64.
Soontararak S, et al. Mesenchymal stem cells (MSC) derived from induced pluripotent stem cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl Med. 2018;7:456–67.
Stenson WF. Preventing necrotising enterocolitis with amniotic fluid stem cells. Gut. 2014;63:218–9.
Stewart AS, et al. HOPX(+) injury-resistant intestinal stem cells drive epithelial recovery after severe intestinal ischemia. Am J Physiol Gastrointest Liver Physiol. 2021;321:G588-g602.
Suman S, Domingues A, Ratajczak J, Ratajczak MZ. Potential clinical applications of stem cells in regenerative medicine. Adv Exp Med Biol. 2019;1201:1–22.
Tayman C, et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res. 2011;70:489–94.
Tu C, et al. Human umbilical cord mesenchymal stem cells promote macrophage PD-L1 expression and attenuate acute lung injury in mice. Current Stem Cell Res Therapy. 2022;17:564–75.
Van Landeghem L, et al. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1111-1132.
Venkatraman A, Yu W, Nitkin C, Sampath V. Intestinal stem cell development in the neonatal gut: pathways regulating development and relevance to necrotizing enterocolitis. Cells. 2021;10:312.
Villamor-Martinez E, Hundscheid T, Kramer BW, Hooijmans CR, Villamor E. Stem cells as therapy for necrotizing enterocolitis: a systematic review and meta-analysis of preclinical studies. Front Pediatr. 2020;8: 578984.
von Bahr L, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells (dayton, Ohio). 2012;30:1575–8.
Walsh MC, Kliegman RM, Fanaroff AA. Necrotizing enterocolitis: a practitioner’s perspective. Pediatr Rev. 1988;9:219–26.
Wang G, et al. Pre-clinical study of human umbilical cord mesenchymal stem cell transplantation for the treatment of traumatic brain injury: safety evaluation from immunogenic and oncogenic perspectives. Neural Regen Res. 2022;17:354–61.
Watanabe Y, et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl Med. 2019;8:271–84.
Watanabe Y, Tsuchiya A, Terai S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin Mol Hepatol. 2021;27:70–80.
Watkins DJ, Zhou Y, Chen CL, Darbyshire A, Besner GE. Heparin-binding epidermal growth factor-like growth factor protects mesenchymal stem cells. J Surg Res. 2012;177:359–64.
Wegmeyer H, et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013;22:2606–18.
Wei J, Zhou Y, Besner GE. Heparin-binding EGF-like growth factor and enteric neural stem cell transplantation in the prevention of experimental necrotizing enterocolitis in mice. Pediatr Res. 2015;78:29–37.
Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Meldrum DR. Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery. 2009;146:190–7.
Weis VG, et al. Human placental-derived stem cell therapy ameliorates experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2021;320:G658–74.
Yang J, et al. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg. 2012a;215:534–45.
Yang J, et al. A technique for systemic mesenchymal stem cell transplantation in newborn rat pups. J Investig Surg. 2012b;25:405–14.
Yang J, Su Y, Besner GE. Stem cell therapy for necrotizing enterocolitis: innovative techniques and procedures for pediatric translational research. Methods Mol Biol. 2014;1213:121–37.
Yu Y, et al. Maternal administration of probiotics promotes gut development in mouse offsprings. PLoS ONE. 2020;15: e0237182.
Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68.
Zani A, et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut. 2014a;63:300–9.
Zani A, et al. Amniotic fluid stem cells prevent development of ascites in a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg. 2014b;24:57–60.
Zeng R, et al. Stem cells and exosomes: promising candidates for necrotizing enterocolitis therapy. Stem Cell Res Ther. 2021;12:323.
Zhan XS, et al. A comparative study of biological characteristics and transcriptome profiles of mesenchymal stem cells from different canine tissues. Int J Mol Sci. 2019;20:1485.
Zhao A, et al. Chemical conversion of human epidermal stem cells into intestinal goblet cells for modeling mucus-microbe interaction and therapy. Sci Adv. 2021;7:16.
Zhou Y, et al. Enteric nervous system abnormalities are present in human necrotizing enterocolitis: potential neurotransplantation therapy. Stem Cell Res Ther. 2013;4:157.
Zhou Y, Wang Y, Olson J, Yang J, Besner GE. Heparin-binding EGF-like growth factor promotes neuronal nitric oxide synthase expression and protects the enteric nervous system after necrotizing enterocolitis. Pediatr Res. 2017;82:490–500.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 82171709 and 81801500), the 345 Talent Project of Shengjing Hospital (Grant No. M0275 and M0279), Key R&D Guidance Plan Projects in Liaoning Province (Grant No. 2020JH1/10300001).
Author information
Authors and Affiliations
Contributions
Y-YS and S-JD designed the overall study; S-JD drafted the manuscript of the article. S-JD and S-YW performed a systematic literature search. S-JD, S-YW, T-JL and Y-YS revised the manuscript critically and approved final version of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di, SJ., Wu, SY., Liu, TJ. et al. Stem cell therapy as a promising strategy in necrotizing enterocolitis. Mol Med 28, 107 (2022). https://doi.org/10.1186/s10020-022-00536-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10020-022-00536-y