Abstract
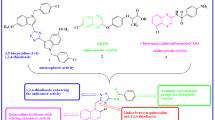
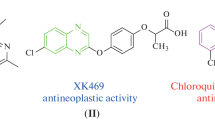
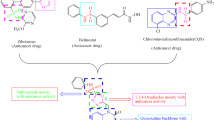
Herein, synthesis of some new quinoxaline-1,2,4-oxadiazole derivatives is presented, and their in vitro anti-cancer activity against four human cancer cell lines like MCF-7 (human breast), HeLa (human cervical), HCT116 (human colon carcinoma) HepG2 (liver hepato cellular carcinoma) is tested. The results reveal promising results for four products. One of the products displays higher activity against all tested cell lines than the standard drug etoposide. Molecular docking studies of the products on EGFR receptor suggest that the most potent compound strongly binds to protein EGFR (pdbid: 4HJO). Two compounds exhibit promising inhibitory activity of tyrosine kinase EGFR.



Similar content being viewed by others
REFERENCES
Vitale, R.M., Gatti, M., Carbone, M., Barbieri, F., Felicita, V., Gavagnin, M., Florio, T., and Amodeo, P.A., ACS Chem. Biol., 2013, vol. 8, p. 2762. https://doi.org/10.1021/cb400521b
Zhang, L., Jiang, C.S., Gao, L.X., Gong, J.X., Wang, Z.H., Li, J.Y., Li, J., Li, X.W., and Guo, Y.W., Bioorg. Med. Chem. Lett., 2016, vol. 26, p. 778. https://doi.org/10.1016/j.bmcl.2015.12.097
Zhang, H.Z., Kasibhatla, S., Kuemmerle, J., Kemnitzer, W., Ollis-Mason, K., Qiu, L., Crogan-Grundy, C., Tseng, B., Drewe, J., and Cai, S.X., J. Med. Chem., 2005, vol. 48, p. 5215. https://doi.org/10.1021/jm050292k
Pace, A., Buscemi, S., Piccionello, A.P., and Pibiri, I., Cambridge, MA, USA, 2015, vol. 116, p. 85.
Rasool, I., Ahmad, M., Khan, Z.A., Mansha, A., Maqbool, T., Zahoor, A.F., and Aslam, S., Trop. J. Pharm. Res., 2017, vol. 16, p. 723.
Maftei, C.V., Fodor, E., Jones, P.G., Daniliuc, C.G., Franz, M.H., Kelter, G., Fiebig, H.H., Tamm, M., and Neda, I., Tetrahedron, 2016, vol. 72, p. 1185. https://doi.org/10.1016/j.tet.2016.01.011
Zaimoku, H., Taniguchi, T., and Ishibashi, H., Org. Lett., 2012, vol. 14, p. 1656.
Galal, S.A., Abdelsamie, A.S., Soliman, S.M., Mortier, J., Wolber, G., Ali, M.M., Tokuda, H., Suzuki, N., Lida, A., Ramadan, R.A., and El Diwani, H.I., Eur. J. Med. Chem. 2013, vol. 69, p. 115. https://doi.org/10.1016/j.ejmech.2013.07.049
El-Enany, M.M., Kamel, M.M., Khalil, O.M., and El-Nassan, H.B., Eur. J. Med. Chem., 2010, vol. 45, p. 5286. https://doi.org/10.1016/j.ejmech.2010.08.048
Ahmed, H.E.A., Ihmaid, S.K., Omar, A.M., Shehata, A.M., Rateb, H.S., Zayed, M.F., Ahmed, S., and Elaasser, M.M., Bioorg. Chem., 2018, vol. 76, p. 332. https://doi.org/10.1016/j.bioorg.2016.02.007
Gediya, L.K. and Njar, V.C., Expert Opin. Drug Discov., 2009, vol. 4, p. 1099. https://doi.org/10.1517/17460440903341705
Huang, X. and Cheng-Chu, C., Synthesis, 1984, vol. 6, p. 851. https://doi.org/10.1021/acs.Orglett.7b01025
Tron, G.C., Pirali, T., Billington, R.A., Canonico, P.L., and Sorba, G., Med. Res. Rev., 2008, vol. 28, p. 278. https://doi.org/10.1002/med.20107
Mc Bryan, J., Howlin, J., Napoletano, S., and Martin, F., J. Mammary Gland Biol. Neoplasia, 2008, vol. 13, p. 159. https://doi.org/10.1007/s10911-008-9075-7
Roskoski, Jr.R., Pharmacol Res., 2014, vol. 79, p. 34. https://doi.org/10.1016/j.phrs.2019.104609
Sebastian, J., Richards, R.G., Walker, M.P., Wiesen, J.F., Werb, Z., Derynck, R., Hom, Y.K., Cunha, G.R., and Di Augustine, R.P., Cell Grow. Diff., 1998, vol. 9, p. 777.
Walker, F., Abramowitz, L., Benabderrahmane, D., Duval, X., Descatoire, V., Henin, D., Lehy, T., and Aparicio, T., Hum. Path., 2009, vol. 40, p. 1517. https://doi.org/10.1016/j.humpath.2009.05.010
Park, J.H. and Lemmon, M.A., Biochem. J., 2012, vol. 448, p. 417. https://doi.org/10.1042/BJ20121513
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Badithapuram, V., Nukala, S.K., Thirukovela, N.S. et al. Design, Synthesis, and Anti-Proliferative Activity of Quinoxaline Linked 1,2,4-Oxadiazole Hybrids. Russ J Gen Chem 92, 117–124 (2022). https://doi.org/10.1134/S1070363222010169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222010169