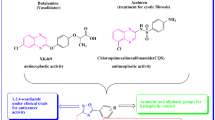
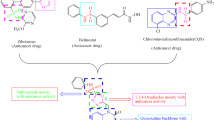
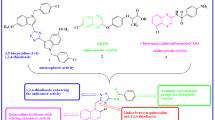
Abstract
Herein, we synthesized some new quinoxaline-1,3,4-oxadiazole hybrids (Va–n) and evaluated for their in vitro anticancer potency towards A549 (lung), MCF-7 (breast), HeLa (cervical) and HEK-293 (embryonic kidney) and etoposide acts as a standard drug. Compounds 1-((5-(3,5-dimethoxyphenyl)-1,3,4-oxadiazol-2-yl) methyl) quinoxaline-2(1H)-one, 1-((5-(4-methoxy phenyl)-1,3,4-oxadiazol-2-yl) methyl) quinoxalin-2(1H)-one and 1-((5-(2,4-dimethyl phenyl)-1,3,4-oxadiazol-2-yl) methyl) quinoxalin-2(1H)-one showed promising anticancer activities against four cancer cell lines. Predominantly, the compound 1-((5-(3,5-dimethoxy phenyl)-1,3,4-oxadiazol-2-yl) methyl) quinoxaline-2(1H)-one has shown greater potency against all cell lines than the standard Etoposide with IC50 ranging from 0.93 ± 0.03, 1.95 ± 0.04, 1.87 ± 0.02 and 2.13 ± 0.05 μM. Furthermore, the compounds1-((5-(3,5-dimethoxyphenyl)-1,3,4-oxadiazol -2-yl) methyl) quinoxaline-2(1H)-one and 1-((5-(4-methoxyphenyl)-1,3,4-oxadiazol -2-yl) methyl) quinoxalin-2(1H)-one displayed promising inhibitory activity over tyrosine kinase EGFR when compared with the standard erlotinib.


Similar content being viewed by others
REFERENCES
Global Status Report on Noncommunicable Diseases 2010. www.who.int/nmh/publications/ncd. report fullen.pdf. Accessed March 1, 2016.
Hanahan, D. and Weinberg, R.A., Cell., 2000, vol. 100, pp. 57–70. https://doi.org/10.1016/s0092-8674(00)81683-9
Thanikachalam, P.V., Maurya, R.K., Garg, V., Monga, V., Kakkar, S., Narasimhan, B., Mandewale, M.C., Patil, U.C., Shedge, S.V., Dappadwad, U.R., Yamgar, R.S., Xu, Z., Zhao, S.J., and Liu, Y., Eur. J. Med. Chem., 2019, vol. 180, pp. 562–612. https://doi.org/10.1016/j.ejmech.2019.07.019
Narasimhan, B., BMC Chem., 2019, vol. 13, pp. 113–117. https://doi.org/113.10.1186/ s13 065-019-0629-0
Mandewale, M.C., Patil, U.C., Shedge, S.V., Dappadwad, U.R., and Yamgar, R.S., BeniSuef Univ. J. Basic Appl. Sci., 2017, vol. 6, pp. 354–361. https://doi.org/10.1016/j.bjbas.2017.07.005
Xu, Z., Zhao, S.J., and Liu, Y., Eur. J. Med. Chem., 2019, vol. 183, p. 111700. https://doi.org/10.1016/j.ejmech.2019.111700
Nayak, S. and Gaonkar, S.L., Mini-Rev. Med. Chem., 2019, vol. 19, pp. 215–238. https://doi.org/10.2174/1389557518666180816112151
Luczynski, M. and Kudelko, A., Appl. Sci., 2022, vol. 12, p. 3756. https://doi.org/10.3390/app12083756
Bostro, J., Hogner, A., Llina, A., Wellner, E., and Plowright, A.T., J. Med. Chem., 2012, vol. 55, pp. 1817–1830. https://doi.org/10.1021/jm2013248
Nayak, S., Gaonkar, S.L., Musad, E.A., and Al Dawsar, A.M., J. Saudi Chem. Soc., 2021, vol. 25, p. 101284. https://doi.org/10.1016/j.jscs.2021.101284
Montana, M., Montero, V., Khoumeri, O., and Vanelle, P., Molecules, 2020, vol. 25, pp. 2784. https://doi.org/10.3390/molecules25122784
Naylor, M.A., Stephen, M.A., Nolan, J., Sutton, B., Tocher, J.H., Fielden, E.M., Adams, J.E., and Strafford, I., Anticancer Drug Des., 1993, vol. 8, pp. 439–461. PMID: 8286012
Hui, X., Desrivot, J., Bories, C., Loiseau, C.P.M., Franck, X., Hocquemiller, R., abd Figadere, B., Bioorg. Med. Chem. Lett., 2006, vol. 16, pp. 815–820. https://doi.org/10.1016/j.bmcl.2005.11.025
Gonzalez, M. and Cerecetto, H., Expert Opin. Ther. Pat., 2012, vol. 22, pp. 1289–1302. https://doi.org/10.1517/13543776.2012.724677
Corbett, T.H., Lorusso, P.M., Demchick, L., Simpson, C., Pugh, S., White, K., Kushner, J., Polin, L., Meyer, J., Czarnecki, J., Heilbrun, L., Horwitz, J.P., Gross, J.L., Behrens, C.H., Harrison, B.A., McRipley, R.J., and Trainor, G., Invest. New Drugs, 1998, vol. 16, pp. 129–139. https://doi.org/10.1023/A:1006174622061
Lorusso, P.M., Parchment, R., Demchik, L., Knight, J., Polin, L., Dzubow, J., and Behrens, C., Invest. New Drugs, 1998, vol. 16, pp. 287–296. https://doi.org/10.1007/BF00180810
Rigas, J.R., Miller, V.A., Tong, W.P., Roistacher, N., Kris, M.G., Orazem, J.P., Young, C.W., and Warrell, R.P., Cancer Chemother. Pharmacol., 1995, vol. 35, pp. 483–488. https://doi.org/10.1007/BF00686832
Yogeeswari, P., Ragavendran, J.V., Sriram, D., Nageswari, Y., Kavya, R., Sreevatsan, N., Vanitha, K., and Stables, J., J. Med. Chem., 2007, vol. 50, pp. 2459–2467. https://doi.org/10.1021/jm061431g
Solomon, V.R., Pundir, S., and Lee, H., Sci. Rep., 2019, vol. 9, p. 6315. https://doi.org/10.1038/s41598-019-42816-4
Solomon, V.R., Hu, C., and Lee, H., Bioorg. Med. Chem., 2010, vol. 18, pp. 1563–1572. https://doi.org/10.1016/j.bmc.2010.01.001
Tron, G.C., Pirali, T., Billington, R.A., Canonico, P.L., and Sorba, G., Med. Res. Rev., 2008, vol. 28, pp. 278. https://doi.org/10.1002/med.20107
ACKNOWLEDGMENTS
The authors are thankful to the Department of Chemistry, Chaitanya Deemed to be University for providing Laboratory facilities and Department of Biotechnology, Chaitanya Deemed to be University for their support in carrying out anticancer activity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Srinivas Bandari and Bhaskar Juluru are main authors of this paper. Material preparation, data collection and analysis in biology were performed by Srinivas Bandari. Material preparation, data collection and analysis in chemistry were performed by Bhaskar Juluru. The manuscript was written by Bhaskar Juluru.
Supplementary Information
Rights and permissions
About this article
Cite this article
Kandukuri, P., Dasari, G., Nukala, S.K. et al. Design and Synthesis of Some New Quinoxaline Containing 1,3,4-Oxadiazole Hybrids and Evaluation of Their Anti-Cancer Activity. Russ J Bioorg Chem 49, 139–146 (2023). https://doi.org/10.1134/S1068162023010132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162023010132