Abstract
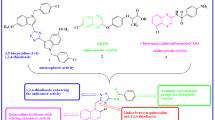
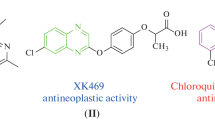
In the current study, some new quinoxaline linked 1,3,4-oxadiazole sulfonamide hybrids have been designed, synthesized and characterized by IR, 1H and 13C NMR, and mass spectra. The products have been evaluated for their in vitro anti-proliferative activity against four human cancer cell lines, namely HeLa (cervical), A549 (lung), MCF-7 (breast), HEK-293 (embryonic kidney). Three compounds have demonstrated high anticancer activity when compared with Etoposide used as a reference drug.


Similar content being viewed by others
REFERENCES
Shepard, D.R. and Dreicer, R., Expert Opin. Investig. Drugs., 2010, vol. 19, p. 899. https://doi.org/10.1517/13543784.2010.491822
Carta, A., Paglietti, G., Nikookar, M.E.R., Sanna, P., Sechi, L., and Zanetti, S., Eur. J. Med. Chem., 2002, vol. 37, p. 355. https://doi.org/10.1016/S0223-5234(02)01346-6
Stokes, S.S., Albert, R., Buurman, E.T., Andrews, B., Shapiro, A.B., Green, O.M., McKenzie, A.R., and Otterbein, L.R., Bioorg. Med. Chem. Lett., 2012, vol. 22, p. 7019. https://doi.org/10.1016/j.bmcl.2012.10.003
Lal, J., Gupta, S.K., Thavaselvam, D., and Agarwal, D.D., Eur. J. Med. Chem., 2013, vol. 64, p. 579. https://doi.org/10.1016/j.ejmech.2013.03.012
Ning, X., Guo, Y., Ma, X., Zhu, R., Tian, C., Zhang, Z., Wang, X., Ma, Z., and Liu, J., Bioorg. Med. Chem., 2013, vol. 21, p. 5589.
Abbas, S.A., Murtaza, S., Tahir, M.N., Shamim, S., Sirajuddin, M., Rana, U.A., Naseem, K., and Rafique, H., J. Mol. Struct., 2016, vol. 1117, p. 269. https://doi.org/10.1016/j.molstruc.2016.03.066
Chandna, N., Kumar, S., Kaushik, P., Kaushik, D., Roy, S.K., Gupta, G.K., Jachak, S.M., Kapoor, J.K., and Sharma, P.K., Bioorg. Med. Chem., 2013, vol. 21, p. 4581. https://doi.org/10.1016/j.bmc.2013.05.029
Lu, X.Y., Wang, Z.C., Ren, S.Z., Shen, F.Q., Man, R.J., and Zhu, H.L., Bioorg. Med. Chem. Lett., 2016, vol. 26, p. 3491. https://doi.org/10.1016/j.bmcl.2016.06.037
Bano, S., Javed, K., Ahmad, S., Rathish, I.G., Singh, S., and Alam, M.S., Eur. J. Med. Chem., 2011, vol. 46, p. 5763. https://doi.org/10.1016/j.ejmech.2011.08.015
Navarrete-V.G., Azquez, M.G., Morales-Vilchis, S., Estrada-Soto, J.J., Ramírez-Espinosa, S., Hidalgo-Figueroa, C., Nava-Zuazo, H., Tlahuext, I., Leon-Rivera,J.L., Medina-Franco, F., Lopez-Vallejo, S.P., Webster, M., Binnie, R., Ortiz-Andrade, H., and Moreno-Diaz., Eur. J. Med. Chem., 2014, vol. 74, p. 179. https://doi.org/10.1016/j.ejmech.2013.12.042
Solomon, V.R., Hu, C., and Lee, H., Bioorg. Med. Chem., 2009, vol. 17, p. 7585. https://doi.org/10.1016/j.bmc.2009.08.068
Patpi, S.R., Pulipati, L., Yogeeswari, P., Sriram, D., Jain, N., Sridhar, B., Murthy, R., Anjana Devi, T., Kalivendi, S.V., and Kantevari, S., J. Med. Chem., 2012, vol. 55, p. 3911. https://doi.org/10.1021/jm300125e
Ono, Y., Ninomiya, M., Kaneko, D., Sonawane, A.D., Udagawa, T., Tanaka, K., Nishina, A., and Koketsu, M., Bioorg. Chem., 2020, vol. 104, p. 104245. https://doi.org/10.1016/j.bioorg.2020.104245
ACKNOWLEDGMENTS
The authors are thankful to the Department of Chemistry, Chaitanya Deemed to be University for providing Laboratory facilities and Department of Biotechnology, Chaitanya Deemed to be University for their support in anticancer activity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies involving animals or human participants performed by any of the authors.
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Ravula, S., Nukala, S.K., Thirukovela, N.S. et al. Design, Synthesis, and Anti-Proliferative Activity of Some New Quinoxaline-1,3,4-oxadiazole Sulfonamide Hybrids. Russ J Gen Chem 92, 702–708 (2022). https://doi.org/10.1134/S1070363222040119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222040119