Abstract
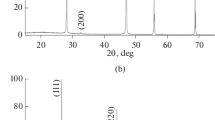
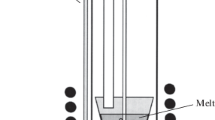
The ionic compositions of lithium metaniobate and metatantalate nanopowders are modified in calcium-containing chloride melts. The reaction products are studied by X-ray diffraction, Raman spectroscopy, scanning electron microscopy, energy dispersive X-ray spectroscopy, and elemental chemical analysis. The results of the studies show that the surface layer of the modified small-size particles of the lithium niobate or lithium tantalate powders is enriched in calcium metaniobate or metatantalate (CaNb2O6 or CaTa2O6) formed due to the isomorphic heterovalent substitution of calcium ions for lithium ions in molten LiCl–CaCl2 and KCl–CaCl2 mixtures at 700 and 750°C.







Similar content being viewed by others
REFERENCES
Yu. S. Kuz’minov, Lithium Niobate and Tantalate as Materials for Nonlinear Optics (Nauka, Moscow, 1975).
M. N. Palatnikov, N. V. Sidorov, and V. T. Kalinnikov, Segnetoelectric Solid Solutions Based on Niobium and Tantalum Oxides (Nauka, St. Petersburg, 2002).
T. Kimura, “Molten salt synthesis of ceramic powders,” in Advances in Ceramics—Synthesis and Characterization, Processing and Specific Application, Ed. by C. Sikalidis (In Tech., 2011), pp. 75–100.
L. Li, J. Deng, J. Cheng, and X. Xing, Chem. Sci. 7, 855–865 (2016). https://doi.org/10.1039/c5sc03521j
Y. Nakayama, P. J. Pauzauskie, A. Radenovic, R. M. Onorato, R. J. Saykally, J. Liphardt, and P. Yang, Nature 447, 1098–1101 (2007). https://doi.org/10.1038/nature05921
Ch.-Y. Xu, L. Zhen, R. Yang, and Z. L. Wang, J. Am. Chem. Soc. 129, 15444–15445 (2007). https://doi.org/10.1021/ja077251t
F. Subhan, S. Azam, G. Khan, M. Irfan, S. Muhammad, A. G. Al-Sehemi, S. H. Naqib, R. Khenata, S. Khan, I. V. Kityki, and B. Amin, J. Alloys Compd. 785, 232–239 (2019). https://doi.org/10.1016/j.jallcom.2019.01.140
V. A. Fedorov, V. A. Ganshin, Yu. N. Korkishko, and T. V. Morozova, Ferroelectrics 138, 23–36 (1993). https://doi.org/10.1080/00150199308017712
W. T. Hsu, Z. B. Chen, C. C. Wu, R. K. Choubey, and C. W. Lan, “Optical properties of Mg, Fe, Co-doped near-stoichiometric LiTaO3 single crystals,” Materials 5, 227–238 (2012).
V. Khokhlov, D. Modenov, V. Dokutovich, V. Kochedykov, I. Zakir’yanova, E. Vovkotrub, and I. Beketov, “Lithium oxide solution in chloride melts as a medium to prepare LiCoO2 nanoparticles,” MRS Commun. 4, 15–18 (2014).
D. V. Modenov, V. N. Dokutovich, V. A. Khokhlov, B. D. Antonov, V. A. Kochedykov, and I. D. Zakir’yanova, Russ. Metall. (Metally), No. 2, 86–89 (2013). https://doi.org/10.1134/S0036029513020092
V. A. Khokhlov, V. N. Dokutovich, N. A. Viugin, and K. O. Bobrova, Russ. Metall. (Metally), No. 2, 90–96 (2019). https://doi.org/10.1134/S0036029519020125
Y. Zhu, S. Lin, Y. Liu, D. Ma, and B. Wang, Mater. Manufact. Proc. 30, 1342–1347 (2015). https://doi.org/10.1080/10426914.2015.1037915
T. Yang, Y. Liu, L. Zhang, M. Hu, Q. Yang, Z. Huang, and M. Fang, Adv. Powder Technol. 25, 933–936 (2014). https://doi.org/10.1016/j.apt.2014.01.01i
RRUFF Database. http://rruff.info/Rynersonite/ R080064.
ACKNOWLEDGMENTS
The authors are grateful to A.A. Pankratov and N.I. Moskalenko (Center for Collective Use “Composition of Matter” at the Institute of High-Temperature Electrochemistry, Ural Branch, Russian Academy of Sciences) for the analysis of the composition and morphology of the modified oxide products.
Funding
This work was supported in part by the Russian Foundation for Basic Research, project no. 18-03-00475a.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Khokhlov, V.A., V’yugin, N.A., Dokutovich, V.N. et al. Modification of the Cationic Composition of LiNbO3 and LiTaO3 Nanopowders in Calcium-Containing Chloride Melts. Russ. Metall. 2020, 144–149 (2020). https://doi.org/10.1134/S0036029520020081
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029520020081