Abstract
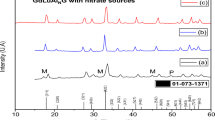
Calcium fluoride and strontium fluoride powders were prepared from respective alkaline-earth metal sulfates by a chemical reaction carried out in molten salt flux made up of lithium and sodium sulfates at 700°С. The fluorinating agent used was sodium fluoride. The irregular shapes of powder particles are likely to arise from the topotaxic phenomena occurring on the precursor calcium sulfate and strontium sulfate particles.



Similar content being viewed by others
REFERENCES
H. Guggenheim, J. Phys. Chem. 64, 938 (1960).
C. E. Bamberger, Advances in Molt Salt Chemistry, Ed. by J. Braunstein, G. Mamantov, and G. P. N. Y. Smith (Plenum, New York, 1975), p. 177.
P. H. Bergmann, Handbuch der anorganischen Chemie. Syst. Nummer 39: Seltenerdelemente, Teil C.3: Sc, Y, La und Lanthanide. Fluoride, Oxifluoride und zugehogige Alkalidoppelverbindungen (Springer, Berlin, 1976).
B. M. Wanklyn, F. R. Wondre, B. J. Garrard, et al., J. Mater. Sci. 16, 2303 (1981).
L. N. Demianets, Prog. Cryst. Growth Charact. 21, 299 (1990).
B. P. Sobolev, The Rare Earth Trifluorides, Pt. 2: Introduction to Materials Science of Multiconoinent Metal Fluoride Crystals (Barcelona, 2001).
C. Zhang, J. Chen, Y. Zhou, and D. Li, J. Phys. Chem. C 112, 10083 (2008). https://doi.org/10.1021/jp802083q
P. P. Fedorov, A. A. Luginina, S. V. Kuznetsov, and V. V. Osiko, J. Fluor. Chem. 132, 1012 (2011). https://doi.org/10.1016/j.jfluchem.2011.06.025
P. P. Fedorov, S. V. Kuznetsov, M. N. Mayakova, et al., Russ. J. Inorg. Chem. 56, 1525 (2011). https://doi.org/10.1134/S003602361110007X
V. Bartůněk, V. Jakeš, V. Král, and J. Rak, J. Fluorine Chem. 135, 358 (2012). https://doi.org/10.1016/j.jfluchem.2011.09.003
B. E. G. Lucier, K. E. Johnston, D. C. Arnold, et al., J. Phys. Chem. 118, 1213 (2014). https://doi.org/10.1021/jp408148b
R. Naccache, Q. Yu, and J. A. Capabianco, Adv. Opt. Mater. 3, 482 (2015). https://doi.org/10.1002/adom20140062.8
M. Wilkening, A. Duvel, F. Preishuber-Pflugl, et al., Z. Kristallogr. 232, 107 (2016). https://doi.org/10.1515/zkri-2016-1963
S. V. Kuznetsov, A. N. Kozlova, V. V. Voronov, et al., Russ. J. Inorg. Chem. 64, 293 (2018). https://doi.org/10.1134/S0036023618030130
P. P. Fedorov and A. A. Alexandrov, J. Fluorine Chem. 227, 109374 (2019). https://doi.org/10.1016/j.jfluchem.2019.109374
L. R. Batsanova, A. K. Kupriyanova, and V. I. Doroshenko, Neorg. Mater. 7, 1876 (1971).
L. R. Batsanova, Usp. Khim. 40, 945 (1971).
M. Ding, C. Lu, L. Cao, et al., CrystEngComm 15, 6015 (2013). https://doi.org/10.1039/c3ce40477c
X. Huang, G. Hu, Q. Xu, et al., J. Alloys Compd. 616, 652 (2014). https://doi.org/10.1016/j.jallcom.2014.07.067
L. Hu, J. Chen, L. Fan, et al., J. Am. Ceram. Soc. 97, 1009 (2014). https://doi.org/10.1111/jace.12855
N. Niu, F. He, L. Wang, et al., J. Nanosci. Nanotechnol. 14, 3509 (2014). https://doi.org/10.1166/jnn.2014.7976
M. Ding, J. Xi, S. Yin, and Z. Ji, Superlattices Microstruct. 83, 390 (2015). https://doi.org/10.1016/j.spmi.2015.03.026
X. Huang, L. Jiang, Q. Xu, et al., RSC Adv. 7, 41190 (2017). https://doi.org/10.1039/c7ra05479c
X. Huang, L. Jiang, X. Li, and A. He, J. Alloys Compd. 721, 374 (2017). https://doi.org/10.1016/j.jallcom.2017.05.320
T. Pornpatdetaudom and K. Serivalsatit, Key Eng. Mater. 766, 34 (2018). https://doi.org/10.4028/www.scientific.net/KEM.766.34
J.-W. Ha, E.-H. Sohn, I. J. Park, et al., Mater. Lett. 209, 357 (2017). https://doi.org/10.1016/j.matlet.2017.08.029
P. P. Fedorov, M. N. Mayakova, A. A. Alexandrov, et al., Inorganics 6, 38 (2018). https://doi.org/10.3390/inorganics6020038
P. P. Fedorov, M. N. Mayakova, V. V. Voronov, et al., J. Fluorine Chem. 218, 69 (2019). https://doi.org/10.1016/j.jfluchem.2018.11.018
T. Kimura, in Advances in Ceramics Synthesis and Characterization, Processing and Specific Applications, Ed. by C. Sikalidis (London, 2011), p. 750. https://doi.org/10.5772/20472
V. I. Posypaiko, E. A. Alekseeva, N. A. Vasina, et al., Melting Diagrams of Salt Systems (Metallurgiya. Moscow, 1977) [in Russian].
P. P. Fedorov, V. Yu. Proydakova, S. V. Kuznetsov, et al., J. Am. Ceram. Soc., 103, 3390 (2020). https://doi.org/10.1111/jace.16996
https://chemiday.com/ru/thermodynamic_inorganic.
P. P. Fedorov, V. Y. Proidakova, S. V. Kuznetsov, and V. V. Voronov, Russ. J. Inorg. Chem. 62, 1508 (2017).
J. C. Warf, W. D. Cline, and R. D. Tevebaugh, Anal. Chem. 26, 342 (1954). https://doi.org/10.1021/ac60086a019
D. R. Messier, J. Electrochem. Soc. 115, 397 (1968).
P. P. Fedorov, M. N. Mayakova, S. V. Kuznetsov, et al., Russ. J. Inorg. Chem. 61, 14728 (2016).
P. P. Fedorov and V. V. Osiko, Dokl. Phys. 64, 353 (2019). https://doi.org/10.1134/S1028335819090076
N. N. Oleinikov, Ros. Khim. Zh. 39 (2), 85 (1995).
ACKNOWLEDGMENTS
Equipment of the Shared Facilities Centers of the Prokhorov General Physics Institute and the Kurnakov Institute of General and Inorganic Chemistry was used in the study. The authors appreciate the help of A.E. Baranchikov in SEM experiments.
Funding
This work was fulfilled in the frame of the R&D plan of the Prokhorov General Physics Institute and was partially supported by the Russian Foundation for Basic Research (project no. 2018-18-29-12050-MK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Proydakova, V.Y., Alexandrov, A.A., Voronov, V.V. et al. Synthesis of Calcium and Strontium Fluorides Using Li2SO4–Na2SO4 Eutectic Melts. Russ. J. Inorg. Chem. 65, 834–838 (2020). https://doi.org/10.1134/S0036023620060169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620060169