Abstract
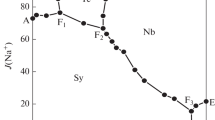
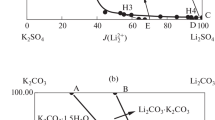
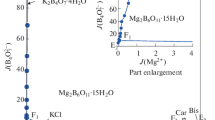
Solid-liquid phase equilibria of the quaternary system (LiBO2 + NaBO2 + KBO2 + H2O) and its subsystem are very important for the separation of lithium and boron salts from the salt lake brines. In this paper, the solubilities, densities and refractive indices of the quaternary system (LiBO2 + NaBO2 + KBO2 + H2O) and its subsystem (NaBO2 + KBO2 + H2O) at 288.15 K and 0.1 MPa were determined by the isothermal solution equilibrium method. The experimental results show that the (NaBO2 + KBO2 + H2O) system contains one invariant point and two single salt crystallization zones corresponding to NaBO2·4H2O and KBO2·1.33H2O. The crystallization zone of NaBO2·4H2O was much larger than that of KBO2·1.33H2O. The quaternary system (LiBO2 + NaBO2 + KBO2 + H2O) contains two invariant points, five univariate solubility curves, and four single salt crystallization regions corresponding to LiBO2·8H2O, LiBO2·2H2O, NaBO2·4H2O and KBO2·1.33H2O. LiBO2·8H2O is easily dehydrated and converted to LiBO2·2H2O in the high concentration of KBO2·1.33H2O.







Similar content being viewed by others
REFERENCES
Y. Liu, X. Liu, S. Liu, et al., Angew. Chem. Int. Edit. 59, 7793 (2020). https://doi.org/10.1002/anie.202001042
P. M. Wang, J. J. Kosinski, M. M. Lencka, et al., Pure Appl. Chem. 85, 2117 (2013). https://doi.org/10.1351/PAC-CON-12-07-09
H. W. Ge, M. Wang, Y. Yao, et al., J. Chem. Eng. Data 65, 26 (2019). https://doi.org/10.1021/acs.jced.9b00663
J. Li, B. Li, and S. Y. Gao, J. Chem. Thermodyn. 30, 681 (1998). https://doi.org/10.1006/jcht.1997.0324
N. S. Kistanova, A. R. Mukminova, I. N. Koneva, et al., Russ. J. Inorg. Chem. 66, 1736 (2021). https://doi.org/10.1134/S0036023621110127
X. F. Guo, S. H. Sang, H. Z. Zhang, et al., Russ. J. Inorg. Chem. 66, 916 (2021). https://doi.org/10.1134/S0036023621060097
O. S. Kudryashova, A. M. Elokhov, E. E. Garbuz, et al., Russ. J. Inorg. Chem. 65, 1905 (2020). https://doi.org/10.1134/S0036023620120104
D. L. Gao, Y. F. Guo, X. P. Yu, et al., J. Chem. Eng. Data 60, 2594 (2015). https://doi.org/10.1021/acs.jced.5b00121
S. Q. Wang, Y. F. Guo, W. J. Liu, et al., J. Solution Chem. 44, 1545 (2015). https://doi.org/10.1007/s10953-015-0359-4
S. Q. Chen, M. X. Wang, J. Y. Hu, et al., J. Chem. Eng. Data 63, 4662 (2018). https://doi.org/10.1021/acs.jced8b00715
T. Vilarinho-Franco, A. Teyssier, R., Tenu, et al., Fluid Phase Equilib. 360, 212 (2013). https://doi.org/10.1016/j.fluid.2013.09.035
A. V. Churikov, K. V. Zapsis, V. V. Khramkov, et al., J. Chem. Eng. Data 56, 383 (2011). https://doi.org/10.1021/je1007422
S. Q. Wang, J. Yang, C. C. Shi, et al., J. Chem. Eng. Data 64, 3122 (2019). https://doi.org/10.1021/acs.jced.9b00218
Y. F. Guo, L. Li, L. N. Cao, et al., J. Chem. Eng. Data 62, 508 (2017). https://doi.org/10.1021/acs.jced.6b00777
L. N. Cao, L. Li, N. Zhang, et al., Ciesc. J. 67, 1117 (2016).
W. J. Cui, H. F. Hou, J. Y. Hu, et al., J. Chem. 2019, 1983051 (2019). https://doi.org/10.1155/2019/1983051
L. Yang, D. Li, T. Zhang, et al., J. Chem. Thermodyn. 142, 106021 (2020). https://doi.org/10.1016/j.jct.2019.106021
L. X. Zhu, S. Y. Gao, B. Wang, et al., Chin. J. Inorg. Chem. 19, 333 (2003).
F. Yuan, J. Jiang, S. Q. Wang, et al., J. Mol. Liq. 337, 116334 (2021). https://doi.org/10.1016/j.molliq.2021.116334
S. Q. Wang, Y. Song, X. M. Du, et al., Rus. J. Inorg. Chem. 63, 116 (2018). https://doi.org/10.1134/S0036023618010175
L. Zhang, J. T. Wu, S. Q. Wang, et al., Rus. J. Inorg. Chem. 66, 924 (2021). https://doi.org/10.1134/S0036023621060231
P. S. Song, Chin. J. Salt Lake Res. 1, 15 (1991).
S. Q. Wang, C. C. Shi, J. Yang, et al., J. Solution Chem. 49, 353 (2020). https://doi.org/10.1007/s10953-020-00962-8
Q. Yang, J. Li, and H. X. Li, Chin. J. Salt Lake Res. 9, 24 (2001).
N. P. Nies and R. W. Hulbert, J. Chem. Eng. Data 12, 303 (1967).
O. Krol, J. Andrieux, J. J. Counioux, et al., in Proceedings of the 35th Conference Phase Equilibria XXV-JEEP, Annecy, France, 2009, 00023. https://doi.org/10.1051/jeep/200900023
Funding
The work was supported by the Program of the National Natural Science Foundation of China (22078247, U1707602, and U1507109), Tianjin Key Laboratory of Brine Chemical Engineering Foundation (BCERE201905), and the Yangtze Scholars and Innovative Research Team in University of Ministry of Education of China (IRT-17R81).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hai-juan Qin, Zhang, L., Han, Sj. et al. Solubilities, Densities, and Refractive Indices of the Quaternary System (LiBO2 + NaBO2 + KBO2 + H2O) and Subsystem (NaBO2 + KBO2 + H2O) at 288.15 K and 0.1 MPa. Russ. J. Inorg. Chem. 67, 691–698 (2022). https://doi.org/10.1134/S0036023622050138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622050138