Abstract
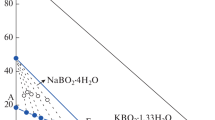
Solid-liquid phase equilibria of the quaternary system (NaCl + NaBO2 + KCl + KBO2 + H2O) is very important for the separation of potassium and boron salts from the salt lake brines. In this paper, the solubilities, densities and refractive indices of the quaternary system (NaCl + NaBO2 + KCl + KBO2 + H2O) at 288.15 K were determined by the isothermal solution equilibrium method. The experimental results show that the (NaCl + NaBO2 + KCl + KBO2 + H2O) system contains three invariant points, seven univariate solubility curves, and five salt crystallization regions corresponding to halite (NaCl), sodium metaborate tetrahydrate (NaBO2·4H2O), hydrated potassium metaborate (KBO2·4/3H2O), sylvite (KCl), and the double salt teepleite (NaCl·NaBO2·2H2O, Te) existed in this system. KCl occupies the greatest part of phase region, while KBO2·4/3H2O covers the smallest. These results show that KCl could be easily crystallized and separated from this system. The physicochemical properties (refractive index and density) presents a regular variation with the increasing of Jänecke index values of \(J({\text{BO}}_{2}^{ - })\) in the solution.





Similar content being viewed by others
REFERENCES
D. Ciceri, D. A. C. Manning, and A. Allanore, Sci. Total Environ. 502, 590 (2015). https://doi.org/10.1016/j.scitotenv.2014.09.013
R. Liu, X. X. Xue, X. Liu, et al., Bull. Chin. Ceram. Soc. 25, 107 (2006). https://doi.org/10.3969/j.issn.1001-1625.2006.06.025
L. R. Hu and S. J. Wang, Technol. Chem. Ind. Miner. 35, 1 (2006).
H. W. Ge, M. Wang, Y. Yao, et al., J. Chem. Eng. Data 65, 26 (2019). https://doi.org/10.1021/acs.jced.9b00663
V. Dolnik, Electrophoresis 41, 1073 (2020). https://doi.org/10.1002/elps.201900470
H. I. Schlesinger, H. C. Brown, A. E. Finholt, et al., J. Am. Chem. Soc. 75, 215 (1953). https://doi.org/10.1021/ja01097a057
J. Goubeau, H. Kallfass, Z. Anorg. Allg. Chem. 299, 160 (1959). https://doi.org/10.1002/zaac.19592990308
Y. Kojima, Y. Kawai, and H. Nakanishi, J. Power Sources 135, 36 (2004). https://doi.org/10.1016/j.jpowsour.2004.03.079
R. Biniwale, S. Rayalu, S. Devotta, et al., Int. J. Hydrogen Energy 33, 360 (2008). https://doi.org/10.1016/j.ijhydene.2007.07.028
E. Y. Marrero-Alfonso, A. M. Beaird, T. A. Davis, et al., Ind. Eng. Chem. Res. 48, 3703 (2009). https://doi.org/10.1021/ie8016225
L. Z. Ouyang, H. Zhong, and Z. M. Li, J. Power Sources 269, 768 (2014). https://doi.org/10.1016/j.jphotochem.2006.12.028
Z. Y. Li, X. Huang, S. Xu, et al., J. Photoch, and Photobio. A: Chem. 188, 311 (2014). https://doi.org/10.1016/j.jpowsour.2014.07.074
B. H. Liu, Z. P. Li, N. Morigasaki, et al., Int. J. Hydrogen Energy 33, 1323 (2008). https://doi.org/10.1016/j.ijhydene.2007.12.033
X. C. Liu, Y. Y. Zhou, J. C. Zhang, et al., ACS Appl. Mater. Interface 9, 20255 (2017). https://doi.org/10.1021/acsami.7b02563
S. A. Mazunin, M. N. Noskov, and A. V. Elsukov, Russ. J. Inorg. Chem. 62, 539 (2017). https://doi.org/10.1134/S0036023617050163
S. Q. Wang, D. Zhao, Y. Song, et al., Russ. J. Inorg. Chem. 64, 661 (2019). https://doi.org/10.1134/S003602361905019X
C. C. Shi, J. Yang, S. Q. Wang, et al., Chin. J. Salt Lake Res. 27, 78 (2019).
S. Q. Wang, X. M. Du, Y. Jing, et al., J. Chem. Eng. Data 62, 253 (2017). https://doi.org/10.1021/acs.jced.6b00626
H. W. Ge, H. Yang, and M. Wang, J. Chem. Eng. Data 65, 628 (2020). https://doi.org/10.1021/acs.jced.9b00852
X. P. Zhao, X. P. Zhang, and S. H. Sang, Russ. J. Phys. Chem. A 91, 1932 (2017). https://doi.org/10.1134/s0036024417100417
D. C. Li, J. S. Yuan, and S. Q. Wang, Russ. J. Phys. Chem. A 88, 42 (2014). https://doi.org/10.1134/S0036024414010300
T. L. Deng, S. Q. Wang, and B. Sun, J. Chem. Eng. Data 53, 411(2008). https://doi.org/10.1021/je700472p
L. Yang, X. F. He, Y. Y. Gao, et al., J. Chem. Eng. Data 63, 1206 (2018). https://doi.org/10.1021/acs.jced.7b00800
S. Q. Wang, X. M. Du, Y. Jing, et al., Russ. J. Phys. Chem. A 91, 2503 (2017). https://doi.org/10.1021/acs.jced.6b00626
S. Q. Wang, Y. Song, X. M. Du, et al., Russ. J. Inorg. Chem. 63, 116 (2018). https://doi.org/10.1134/S0036023618010175
X. P. Zhao, X. P. Zhang, Y. Y. Yang, et al., J. Chem. Eng. Data 62, 1377 (2017). https://doi.org/10.1021/acs.jced.6b00926
S. H. Song and J. Peng, Chin. J. Chem. 28, 755 (2010). https://doi.org/10.1002/cjoc.201090142
S. Tursunbadalov, Russ. J. Inorg. Chem. 65, 412 (2020). https://doi.org/10.1134/s0036023620030195
X. D. Yu, Y. Zeng, S. S. Guo, et al., J. Chem. Eng. Data 61, 1246 (2016). https://doi.org/10.1021/acs.jced.5b00888
L. X. Zhu, S. Y. Gao, B. Wang, et al., Chin. J. Inorg. Chem. 19, 491 (2003). https://doi.org/10.3321/j.issn:1001-4861.2003.03.023
F. Yuan, J. Jiang, S. Q. Wang, et al., J. Mol. Liq. 337, 116334 (2021). https://doi.org/10.1016/j.molliq.2021.116334
S. Q. Wang, Y. Song, X. M. Du, et al., Russ. J. Inorg. Chem. 63, 116 (2018). https://doi.org/10.1134/S0036023618010175
Funding
The work was supported by the Program of the National Natural Science Foundation of China (22078247, U1707602, and U1507109), the Natural Science Foundation of Hebei Province (B2021202058), and the Yangtze Scholars and Innovative Research Team in University of Ministry of Education of China (IRT-17R81).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jia-ying He, Wang, Dy., Wang, Mm. et al. Solubilities, Densities, and Refractive Indices of the Quaternary System (NaCl + NaBO2 + KCl + KBO2 + H2O) at 288.15 K. Russ. J. Inorg. Chem. 67, 2239–2246 (2022). https://doi.org/10.1134/S0036023622700127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622700127