Abstract
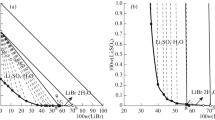
The solubilities and physicochemical properties, including density and pH, in the ternary system (LiBO2 + CaB2O4 + H2O) at (288.15 and 298.15) K were investigated using the isothermal dissolution equilibrium method. According to the experimental results of the ternary system at (288.15 and 298.15) K, the stable phase diagrams and the diagrams of physicochemical properties were plotted versus composition. In the phase diagrams of the ternary system at (288.15 and 298.15) K, there is one invariant point (LiBO2·8H2O + CaB2O4·6H2O), two univariant isotherm dissolution curves, and two crystallization regions corresponding to lithium metaborate octahydrate (LiBO2·8H2O) and calcium metaborate hexahydrate (CaB2O4·6H2O), respectively. This system at both temperatures belongs to hydrate type I classification and neither double salt nor solid solution were found. A comparison of the phase diagrams for the ternary system at (288.15 and 298.15) K shows that the numbers of solid phase and the minerals present are the same; the crystallized area of CaB2O4·6H2O increased at the higher temperature, while that of LiBO2·8H2O decreased. The physicochemical properties (density and pH values) of the ternary system at the two temperatures change regularly with increasing lithium metaborate concentration. The calculated values of density using empirical equations of the ternary system are in good agreement with the experimental values.



Similar content being viewed by others
References
Zheng, X.Y., Zhang, M.G., Xu, Y.: Salt Lakes in China. Chinese Science Press, Beijing (2002)
Fu, J.L., Yu, S.S., Li, S.J., Ren, H.Y.: Availability of tertiary oilfield water resources in Western Qaidam Basin. J. Salt Lake Res. 13, 17–21 (2005) (in Chinese)
Song, P.S., Sun, B., Zeng, D.W.: Solubility phenomena studies concerning brines in China. Pure Appl. Chem. 85, 2097–2116 (2013)
Song, P.S., Li, W., Sun, B., Nie, Z., Bu, L.Z., Wang, Y.S.: Recent development on comprehensive utilization of salt lake resources. Chin. J. Inorg. Chem. 27, 801–815 (2011)
Deng, T.L., Li, D.C., Wang, S.Q.: Metastable phase equilibrium in the aqueous ternary system (KCl–CaCl2–H2O) at (288.15 and 308.15) K. J. Chem. Eng. Data 53, 1007–1011 (2008)
Li, D.C., Deng, T.L.: Liquid–solid metastable phase equilibria for the quaternary system (NaCl–KCl–CaCl2–H2O) at 308.15 K. J. Therm. Anal. Calorim. 95, 361–367 (2009)
Deng, T.L., Yu, X., Li, D.C.: Metastable phase equilibrium in the aqueous ternary system K2SO4 + MgSO4 + H2O at (288.15 and 308.15) K. J. Solution Chem. 38, 27–34 (2009)
Guo, Y.F., Gao, D.L., Han, H.J., Yu, X.P., Wang, S.Q., Deng, T.L.: Metastable phase equilibria in the aqueous ternary system (Na2SO4 + Li2SO4 + H2O) at 308.15 and 348.15 K. Fluid Phase Equilib. 358, 56–59 (2013)
Yang, J.M., Yao, Y., Zhang, R.Z., Sun, A.D., Li, B.H., Lu, H.Z., Xia, Q.Y.: Osmotic coefficients of the Li2B4O7 + LiCl + H2O system at T = 273.15 K. J. Solution Chem. 38, 429–439 (2009)
Deng, T.L., Li, D.C.: Solid–liquid metastable equilibria in the quaternary system (NaCl–KCl–CaCl2–H2O) at 288.15 K. Fluid Phase Equilib. 269, 98–103 (2008)
Yin, S.T., Yao, Y., Li, B., Tian, H.B., Song, P.S.: Isopiestic studies of aqueous MgB4O7 and MgSO4 + MgB4O7 at 298.15 K and representation with Pitzer’s ion-interaction model. J. Solution Chem. 36, 1745–1761 (2007)
Deng, T.L., Li, D.: Solid–liquid metastable equilibria in the quaternary system (NaCl–LiCl–CaCl2–H2O) at 288.15 K. J. Chem. Eng. Data 53, 2488–2492 (2007)
Hu, M.C., Zhang, W.J., Li, S.N., Zhai, Q.G., Jiang, Y.C., Li, Y., Wang, J., Chen, N.: Thermodynamic study of the mixed system (CsCl + CaCl2 + H2O) by EMF Measurements at T = 298.15 K. J. Chem. Thermodyn. 41, 1016–1019 (2009)
Wang, S.Q., Guo, Y.F., Li, D.C., Tang, P., Deng, T.L.: Experimental determination and modeling of the solubility phase diagram of the ternary system (Li2SO4 + K2SO4 + H2O) at 288.15 K. Thermochim. Acta 601, 75–81 (2015)
Li, D., Li, D.C., Sun, B., Deng, T.L.: Titration method for Ca2+ measurement in brine systems with large amount of Li+. Chin. Chem. World 7, 397–401 (2007) (in Chinese)
Song, P.S., Du, X.H., Xu, H.C.: Studies on the phase equilibrium and physico-chemical properties of the ternary system Li2B4O7–Li2SO4–H2O at 25 °C. Chin. Sci. Bull. 28, 106–110 (1983) (in Chinese)
Speight, J.M.: Lange’s Handbook of Chemistry, 16th edn. McGeaw-Hill, New York (2005)
Acknowledgments
These research financially supported by the Program of the National Natural Science Foundation of China (Nos. 21106103, 21276194, and 21306136), the Natural Science Foundation of Tianjin (No. 12JCQNJC03400), the Specialized Research Fund for the Doctoral Program of Chinese Higher Education (No. 20111208120003), the Training Program for Changjiang Scholars and Innovative Research Team in University ([2013]373), and the Innovative Research Team of Tianjin Municipal Education Commission (TD12-5004) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Guo, Y., Liu, W. et al. Phase Equilibria in the Aqueous Ternary System (LiBO2 + CaB2O4 + H2O) at 288.15 and 298.15 K. J Solution Chem 44, 1545–1554 (2015). https://doi.org/10.1007/s10953-015-0359-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0359-4