Abstract
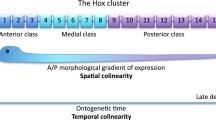
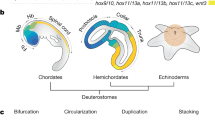
Paleontological data on the appearance and development of metazoan axial symmetry are compared with molecular genetic evidence to determine the axial body plan and growth vectors of bilaterian organisms by the coordinated functioning cascades of signaling pathways Wnt, BMP, Nodal, Hedgehog, and regulatory Hox and ParaHox gene clusters. The axial body symmetry in most bilaterians is associated with the uniaxial growth vector. Posterior body growth ensured by the progenitor stem cell pool of the growth zone localized at the preterminal end of the body became a cardinal evolutionary innovation in Bilateria. The conservatism of ancestral molecular mechanisms of bilaterian body plan specification is combined with their flexibility, which provided the possibility of evolutionary transformations of the axial pattern of the organism. Radical evolutionary changes with the formation of additional growth zones are reported in the representatives of Deuterostomia (Vertebrata and Echinodermata). In modern vertebrates, while the main ancestral axis of body growth is preserved, two pairs of additional growth zones (limb buds) emerged. They are considered paramorphic homologs (replicates) of the posterior part of the main body axis. The pentamerous pattern of the body plan and growth of recent echinoderms can be explained as a result of the multiplication of the ancestral organizing center of axial architectonics and its radialization during larval metamorphosis (or in early embryogenesis during lecithotrophic development) with a corresponding multiplication of growth zones localized at the ends of rays (ambulacra). It is consistent with paleontological data and the concept of echinoderm rays as paramorphic homologs of the main body axis of the bilaterian ancestors of echinoderms.










Similar content being viewed by others
REFERENCES
Adachi, S., Niimi, I., Sakai, Y., et al., Anteroposterior molecular registries in ectoderm of the echinus rudiment, Devel. Dynamics, 2018, vol. 247, pp. 1297–1307.
Anderson, C. and Stern, C.D., Organizers in development, Curr. Top. Dev. Biol., 2016, vol. 117, pp. 435–454.
Angerer, L.M., Yaguchi, S., Angerer, R.C., and Burke, R.D., The evolution of nervous system patterning: Insights from sea urchin development, Development, 2011, vol. 138, pp. 3613–3623.
Annunziata, R., Martinez, P., and Arnone, M.I., Intact cluster and chordate-like expression of ParaHox genes in a sea star, BMC Biol., 2013, vol. 11. http://www.biomedcentral.com/1741-7007/11/68.
d’Arcy, W., On Growth and Form, Cambridge: Univ. Press: Cambridge, 2nd ed., 1942.
Arenas-Mena, C., Martinez, P., Cameron, R.A., and Davidson, E.H., Expression of the Hox gene complex in the indirect development of a sea urchin, Proc. Natl. Acad. Sci. USA, 1998, vol. 95, pp. 13062–13067.
Arias, A.M. and Steventon, B., On the nature and function of organizers, Development, 2018, vol. 145, no. 5, dev159525. https://doi.org/10.1242/dev.159525
Arnone, M.I., Rizzo, F., Annunciata, R., et al., Genetic organization and embryonic expression of the ParaHox genes in the sea urchin S. purpuratus: Insights into the relationship between clustering and colinearity, Dev. Biol., 2006, vol. 300, pp. 63–73.
Arnone, M.I., Byrne, M., and Martinez, P., Echinodermata, in Evolutionary Developmental Biology of Invertebrates. Vol. 6: Deuterostomia, Wanninger, A., Ed., Wien: Springer-Verlag, 2015, pp. 1–58.
Bajard, L., Morelli, L.G., Ares, S., et al., Wnt-regulated dynamics of positional information in zebrafish somitogenesis, Development, 2014, vol. 141, pp. 1381–1391.
Baughman, K.W., McDougall, C., Cummins, S.F., et al., Genomic organization of Hox and ParaHox clusters in the echinoderm, Acanthaster planci, Genesis, 2014, vol. 52, pp. 952–958.
Bayramov, A.V., Ermakova, G.V., Kucheryavyy, A.V., and Zaraysky, A.G., Genome duplications as the basis of vertebrates’ evolutionary success, Russ. J. Dev. Biol., 2021, vol. 52, no. 3, pp. 141–163.
Benton, M.J. and Harper, D.A.T., Introduction to Paleobiology and the Fossil Records, Oxford: Wiley-Blackwell, 2009.
Bertrand, S., Aldea, D., Oulion, S., et al., Evolution of the role of RA and FGF signals in the control of somitogenesis in chordates, PLoS ONE, 2015, vol. 10, no. 9, e0136587. https://doi.org/10.1371/journal.pone.0136587
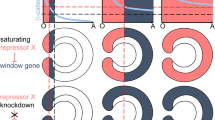
Bier, E. and De Robertis, E.M., BMP gradients: A paradigm for morphogen-mediated developmental patterning, Science, 2015, vol. 348, no. 6242, aaa5838. https://doi.org/10.1126/science.aaa5838
Borkhvardt, V.G., The origin of paired fins: the state of the problem, Russ. Ornitol. Zh., 2016, vol. 25, Express Iss. 1253, pp. 657–668.
Budd, G.E. and Jackson, I.S.C., Ecological innovations in the Cambrian and the origins of the crown group phyla, Philos. Trans. R. Soc., B, 2016, vol. 371, no. 1685, 20150287. https://doi.org/10.1098/rstb.2015.0287
Byrne, M. and Selvakumaraswamy, P., Evolutionary modification of gastrulation in Parvulastra exigua, an asterinid seastar with holobenthic lecithotrophic development, Evol. Devel., 2021, vol. 23, pp. 63–71.
Byrne, M., Cisternas, P., Elia, L., and Relf, B., Engrailed is expressed in larval development and in the radial nervous system of the Patiriella sea stars, Dev. Genes Evol., 2006, vol. 215, pp. 608–617.
Byrne, M., Koop, D., and Cisternas, P., Transcriptomic analysis of Nodal- and BMP-associated genes during juvenile development of the sea urchin Heliocidaris erythrogramma, Mar. Genom., 2015, vol. 24 (Pt. 1), pp. 41–45. https://doi.org/10.1016/j.margen.2015.05.019
Byrne, M., Martinez, P., and Morris, V., Evolution of a pentameral body plan was not linked to translocation of anterior Hox genes: The echinoderm HOX cluster revisited, Evol. Devel., 2016, pp. 1–7. https://doi.org/10.1111/ede.12172
Byrne, M., Koop, D., Morris, V.B., et al., Expression of genes and proteins of the Pax-Six-Eya-Dach network in the metamorphic sea urchin: Insights into development of the enigmatic echinoderm body plan and sensory structures, Dev. Dyn., 2018, vol. 247, pp. 239–249.
Byrne, M., Koop, D., Strbenac, D., et al., Transcriptomic analysis of sea star development through metamorphosis to the highly derived pentameral body plan with a focus on neural transcription factors, DNA Res., 2020, pp. 1–10. https://doi.org/10.1093/dnares/dsaa007
Byrne, M., Koop, D., Strbenac, D., et al., Transcriptomic analysis of Nodal- and BMP- associated genes during development to the juvenile seastar in Parvulastra exigua (Asterinidae), Mar. Genom., 2021, vol. 59, no. 12, 100857. https://doi.org/10.1016/j.margen.2021.100857
Byrum, C.A. and Wikramanayake, A.H., Nuclearization of β-catenin in ectodermal precursors confers organizer-like ability to induce endomesoderm and pattern a pluteus larva, EvoDevo, 2013, vol. 4, no. 1. http://www.evodevojournal.com/content/4/1/31.
Cameron, C.B., Garey, J.R., and Swalla, B.J., Evolution of the chordate body plan: New insights from phylogenetic analyses of deuterostome phyla, PNAS, 2000, vol. 97, pp. 4469–4474.
Cameron, R.A., Rowen, L., Nesbitt, R., et al., Unusual gene order and organisation of the sea urchin Hox cluster, J. Exp. Zool. (Mol. Dev. Evol.), 2006, vol. M306B, pp. 45–58.
Cavalieri, V. and Spinelli, G., Symmetry breaking and establishment of Dorsal/Ventral polarity in the early sea urchin embryo, Symmetry, 2015, vol. 7, pp. 1721–1733.
Cavodeassi, F.,·Creuzet, S., and Etchevers, H.C., The hedgehog pathway and ocular developmental anomalies, Human Genet., 2019, vol. 138, pp. 917–936.
Chipman, A.D., Hexapoda: Comparative aspects of early development, in Evolutionary Developmental Biology of Invertebrates, Vol. 5. Ecdysozoa III: Hexapoda, Wanninger, A., Ed., Wien: Springer-Verlag, 2015, pp. 92–109.
Cisternas, P. and Byrne, M., Expression of Hox4 during development of the pentamerous juvenile sea star, Parvulastra exigua, Dev. Genes Evol., 2009, vol. 219, pp. 613–618.
Constantinou, S.J., Duan, N., Nagy, L.M., Chipman, A.D., and Williams, T.A., Elongation during segmentation shows axial variability, low mitotic rates, and synchronized cell cycle domains in the crustacean, Thamnocephalus platyurus, EvoDevo, 2020, vol. 11, no. 1. https://doi.org/10.1186/s13227-020-0147-0
Crick, F.H.C., Diffusion in embryogenesis, Nature, 1970, vol. 225, pp. 420–421.
Cui, M., Siriwon, N., Li, E., Davidson, E.H., and Peter, I.S., Specific functions of the Wnt signaling system in gene regulatory networks throughout the early sea urchin embryo, PNAS, 2014, vol. 111, no. 47, E5029-38. https://doi.org/10.1073/pnas.1419141111
Davidson, E.H., The Regulatory Genome: Gene Regulatory Networks in Development and Evolution, San Diego: Acad. Press. 2006.
Davidson E.H., Erwin D.H. An integrated view of Precambrian eumetazoan evolution, Cold Spring Harb. Symp. Quant. Biol., 2009, vol. 74, pp. 1–16.
Deschamps, J. and Duboule, D., Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock, Genes Devel., 2017, vol. 31, pp. 1406–1416.
Ding, Y., Colozza, G., Sosa, E.A., Moriyama, Y., Rundle, S., Salwinski, L., and De Robertis, E.M., Bighead is a Wnt antagonist secreted by the Xenopus Spemann organizer that promotes Lrp6 endocytosis, Proc. Nat. Acad. Sci. USA, 2018, vol. 115, pp. E9135–E9144.
Driesch, H., Analytische Theorie der Organischen Entwicklung, Leipzig: Engelman, 1894.
DuBuc, T.Q., Stephenson, T.B., Rock, A.Q., and Martindale, M.Q., Hox and Wnt pattern the primary body axis of an anthozoan cnidarian before gastrulation, Nature Comm., 2018, vol. 9, no. 1, 2007. https://doi.org/10.1038/s41467-018-04184-x
Dunn, F.S., Liu, A.G., and Donoghue, P.C.J., Ediacaran developmental biology, Biol. Rev. Cambridge Philos. Soc., 2017, vol. 93, no. 4. https://doi.org/10.1111/brv.12379
Durston, A.J., Some questions and answers about the role of Hox temporal collinearity in vertebrate axial patterning, Front. Cell Dev. Biol., 2019, vol. 29. https://doi.org/10.3389/fcell.2019.00257
Edgar, A., Byrne, M., McClay, D.R., and Wray, G.A., Evolution of abbreviated development in Heliocidaris erythrogramma dramatically re-wired the highly conserved sea urchin developmental gene regulatory network to decouple signaling center function from ultimate fate, BioRxiv, 2019. https://doi.org/10.1101/712216
Erwin, D.H., The origin of animal body plans: a view from fossil evidence and the regulatory genome, Development, 2020, vol. 147, no. 4, dev182899. https://doi.org/10.1242/dev.182899
Erwin, D.H. and Valentine, J.W., The Cambrian Explosion: The Construction of Animal Biodiversity, Greenwood: Roberts & Co., 2013.
Evans, S.D., Droser, M.L., and Gehling, J.G., Highly regulated growth and development of the Ediacara macrofossil Dickinsonia costata, PloS ONE, 2017, vol. 12, no. 5, e0176874. https://doi.org/10.1371/journal.pone.0176874
Evans, S.D., Hughes, I.V., Gehling, J.G., and Droser, M.L., Discovery of the oldest bilaterian from the Ediacaran of South Australia, Proc. Natl. Acad. Sci. USA, 2020, vol. 117, pp. 7845–7850.
Fedonkin, M.A., Precambrian metazoans: the problems of preservation, systematics and evolution, Phil. Trans. Roy. Soc., B, 1985, vol. 311, pp. 27–45.
Fedonkin, M.A., Metameric features in the Vendian metazoans, Italian J. Zool., 1998, vol. 65, pp. 11–27.
Fedonkin, M.A., The origin of the Metazoa in the light of the Proterozoic fossil records, Palentol. Res., 2003, vol. 7, pp. 9–41.
Fedonkin, M.A., Simonetta, A., and Ivantsov, A.Y., New data on Kimberella, the Vendian mollusc-like organism (White Sea region, Russia): palaeoecological and evolutionary implications, in The Rise and Fall of the Ediacaran Biota, Vickers-Rich, P. and Komarover, P., Eds., Spec. Publ.—Geol. Soc. London, 2007, vol. 286, pp. 1–13.
Ferrier, D.E.K., Space and time in Hox/ParaHox gene cluster evolution, in Perspectives on Evolutionary and Developmental Biology. Essays for Alessandro Minelli, Fusco, G., Ed., Padova: Padova Univ. Press, 2019, pp. 245–258.
Fields, C. and Levin, M., Does regeneration recapitulate phylogeny? Planaria as a model of body-axis specification in ancestral eumetazoa, Comm. Integrat. Biol., 2020, vol. 13, pp. 27–38.
Garcia-Fernandez, J. and Benito-Gutierrez, E., It’s a long way from amphioxus: Descendants of the earliest chordate, BioEssays, 2009, vol. 31, pp. 665–675.
Genikhovich, G. and Technau, U., On the evolution of bilaterality, Development, 2017, vol. 144, pp. 3392–3404.
Giribet, G. and Edgecombe, G.D., “Perspectives in Animal Phylogeny and Evolution”: A decade later, in Perspectives on Evolutionary and Developmental Biology, Fusco, G., Ed., Padova Univ. Press, 2019, pp. 167–178.
Gould, S.J., Wonderful Life. The Burgess and the Nature of History, New York, London: Norton & Co, 1989.
Guensburg, T.E. and Sprinkle, J., Solving the mystery of crinoid ancestry: New fossil evidence of arm origin and development, J. Paleontol., 2009, vol. 83, pp. 350–364.
Guensburg, T.E., Sprinkle, J., Mooi, R., Lefebvre, B., David, B., Roux, M., and Derstler, K., Athenacrinus n. gen. and other early echinoderm taxa inform crinoid origin and arm evolution, J. Paleontol., 2009, vol. 94, pp. 311–333. https://doi.org/10.1017/jpa.2019.87
Gurwitsch, A.G., Über den Begriff des embryonalen Feldes, Wilhelm Roux’ Arch. Entwicklungsmech. Org., 1922, vol. 52, pp. 383–415.
Hara, Y., Yamaguchi, M., Akasaka, K., Nakano, H., Nonaka, M., and Amemiya, S., Expression patterns of Hox genes in larvae of the sea lily Metacrinus rotundus, Dev. Genes Evol., 2006, vol. 216, pp. 797–809.
Hinman, V.F. and Burke, R.D., Embryonic neurogenesis in echinoderms, WIREs Dev Biol., 2018, vol. 7, no. 27, e316. https://doi.org/10.1002/wdev.316
Hogvall, M., Vellutini, B.C., Martín-Durán, J.M., Hejnol, A., Budd, G.E., and Janssen, R., Embryonic expression of priapulid Wnt genes, Devel. Genes. Evol., 2019, vol. 229, pp. 125–135.
Holland, L.Z., Tunicates, Curr. Biol., 2016, vol. 26, pp. R141–R156.
Holland, N.D., Holland, L.Z., and Holland, P.W.H., Scenarios for the making of vertebrates, Nature, 2015, vol. 520, pp. 450–455.
Holland, P.W.H., Did homeobox gene duplications contribute to the Cambrian explosion? Zool. Lett., 2015, vol. 1, no. 1. https://doi.org/10.1186/s40851-014-0004-x
Holló, G. and Novák, M., The manoeuvrability hypothesis to explain the maintenance of bilateral symmetry in animal evolution, Biol. Direct., 2012, vol. 7, no. 1. https://doi.org/10.1186/1745-6150-7-22
Holstein, T.W., The evolution of the Wnt pathway, Cold Spring Harb. Perspect. Biol., 2012, vol. 4, a007922. https://doi.org/10.1101/cshperspect.a007922
Hotchkiss, F.H.C., Growth zones and extraxial-axial skeletal homologies in Asteroidea (Echinodermata), Proc. Biol. Soc. Washington, 2012, vol.125, pp. 106–121.
Hyman, L., The Invertebrates. Vol. 5. Phylum Echinodermata, New York: Mc-Graw-Hill, 1955.
Iglesias, M., Gomez-Skarmeta, J.L., Saló, E., and Adell, T., Silencing of Smed-betacatenin1 generates radial-like hypercephalized planarians, Development, 2008, vol. 135, pp. 1215–1221.
Isaeva, V.V., Cells in Morphogenesis, Moscow: Nauka, 1994 [in Russian].
Isaeva, V.V. and Kasyanov, N.V., Symmetry transformations in metazoan evolution and development, Symmetry, 2021a, vol. 13, no. 2. https://doi.org/10.3390/sym13020160
Isaeva, V.V. and Rozhnov, S.V., Evolutionary transformations of the metazoan body plan: Genomic-morphogenetic correlations, Paleontol. J., 2021, vol. 55, no. 7, pp. 97–110.
Ivantsov, A.Yu., The most probable Eumetazoa among late Precambrian macrofossils, Invertebr. Zool., 2017, vol. 14, pp. 127–133.
Ivantsov, A.Y.;. and Leonov, M.V., The Imprints of Vendian Animals—Unique Paleontological Objects of the Arkhangelsk Region, Arkhangelsk, 2008 [in Russian].
Ivantsov, Yu.A., Zakrevskaya, M.A., and Nagovitsyn, A.L., Morphology of integuments of the Precambrian animals, Proarticulata, Invertebr. Zool., 2019, vol. 16, pp. 19–26.
Jacobs, D.K., Hughes, N.C., Fitz-Gibbon, S.T., and Winchell, C.J., Terminal addition, the Cambrian radiation and the Phanerozoic evolution of bilaterian form, Evol. Devel., 2005, vol. 7, pp. 498–514.
Jefferies, R. P. S., The subphylum Calcichordata (Jefferies 1967) primitive fossil chordates with echinoderm affinities, Bull. British Museum (Nat. Hist.) Geol., 1968, vol. 16, pp. 243–339.
Kauffman, J.S. and Raff, R.A., Patterning mechanisms in the evolution of derived developmental life histories: The role of Wnt signaling in axis formation of the direct-developing sea urchin Heliocidaris erythrogramma, Dev. Genes Evol., 2003, vol. 213, pp. 612–624.
Koop, D., Cisternas, P., Morris, V.B., et al., Nodal and BMP expression during the transition to pentamery in the sea urchin Heliocidaris erythrogramma: insights into patterning the enigmatic echinoderm body plan, BMC Dev. Biol., 2017, vol. 17, no. 1. https://bmcdevbiol.biomedcentral.com/articles/10.1186/s12861-017-0145-1.
Kraus, Y., Fritzenwanker, J.H., Genikhovich, G., and Technau, U., The blastoporal organiser of a sea anemone, Curr. Biol., 2007, vol. 17, pp. R874–R876.
Kraus, Y., Aman, A., Technau, U., and Genikhovich, G., Pre-bilaterian origin of the blastoporal axial organizer, Nat. Commun., 2016, vol. 7, no. 11694, pp 1–9.
Lallier, R., Recherches sur les modifications expérimentales de la différenciation de la larve d’Oursin par les enzymes protéolytiques, C. R. Soc. Biol., 1969, vol. 163, pp. 2028–2032.
Lapraz, F., Haillot, E., and Lepage, T., A deuterostome origin of Spemann organiser suggested by Nodal and ADMPs functions in Echinoderms, Nat. Commun., 2015, vol. 6, 8434. https://doi.org/10.1038/ncomms9434
Lefebvre, B., Guensburg, T., Martin, E., et al., Exceptionally preserved soft parts in fossils from the Lower Ordovician of Morocco clarify stylophoran affinities within basal deuterostomes, Geobios, 2019, vol. 52, pp. 27–36.
Li, Y., Omori, A., Flores, R.L., et al., Genomic insights of body plan transitions from bilateral to pentameral symmetry in Echinoderms, Comm. Biol., 2020, vol. 3. https://doi.org/10.1038/s42003-020-1091-1
Loh, K.M., van Amerongen, R., and Nusse, R., Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals, Dev. Cell., 2016, vol. 38, pp. 643–655.
Lowe, C.J., Clarke, D.N., Medeiros, D.M., Rokhsar, D.S., and Gerhart, J., The deuterostome context of chordate origins, Nature, 2015, vol. 520, pp. 456–465.
Manuel, M., Early evolution of symmetry and polarity in metazoan body plans, C.R. Biol., 2009, vol. 332, pp. 184–209.
Maroto, M., Bone, R.A., and Dale, J.K., Somitogenesis, Development, 2012, vol. 139, pp. 2453–2456.
Martin, B.L. and Kimelman, D., Wnt signaling and the evolution of embryonic posterior development, Curr. Biol., 2009, vol. 19, pp. R215–R219.
McClay, D.R., Evolutionary crossroads in developmental biology: Sea urchins, Development, 2011, vol. 138, pp. 2639–2648.
Meinhardt, H., The primary body axes of vertebrates: the generation of a near-Cartesian coordinate system and the role of Spemann-type organizer, Dev. Dyn., 2006, vol. 235, pp. 2907–2919.
Minelli, A., Limbs and tail as evolutionarily diverging duplicates of the main body axis, Evol. Dev., 2000, vol. 2, pp. 157–165.
Minelli A. The Development of Animal Form. Ontogeny, Morphology, and Evolution, Cambridge: Cambridge Univ. Press, 2003.
Minelli, A., EvoDevo and its significance for animal evolution and phylogeny, in Evolutionary Developmental Biology of Invertebrates. Vol. 1, Wanninger, A., Ed., Wien: Springer, 2015, pp. 1–24.
Minsuk, S.B., Turner, F.R., Andrews, M.E., and Raff, R.A., Axial patterning of the pentaradial adult echinoderm body plan, Dev. Genes Evol., 2009, vol. 219, pp. 89–101.
Molina, M.D., de Croze, N., Haillot, E., and Lepage, T., Nodal: master and commander of the dorsal-ventral and left-right axes in the sea urchin embryo, Curr. Opin. Genet. Dev., 2013, vol. 23, pp. 445–453.
Mooi, R. and David, B., Radial symmetry, the anterior/posterior axis, and echinoderm Hox genes, Ann. Rev. Ecol. Evol. Syst., 2008, vol. 39, pp. 43–62.
Mooi, R., David, B., and Wray, G.A., Arrays in rays: Terminal addition in echinoderms and its correlation with gene expression, Evol. Dev., 2005, vol. 7, pp. 542–555.
Morris, V.B., Early development of coelomic structures in an echinoderm larva and a similarity with coelomic structures in a chordate embryo, Dev. Genes Evol., 2012, vol. 222, pp. 313–323.
Morris, V.B., Analysis of coelom development in the sea urchin Holopneustes purpurescens yielding a deuterostome body plan, Biol. Open, 2016, vol. 5, pp. 348–358.
Morris, V.B. and Byrne, M., Involvement of two Hox genes and Otx in echinoderm body-plan morphogenesis in the sea urchin Holopneustes purpurescens, J. Exp. Zool. (Mol. Dev. Evol.), 2005, vol. 304B, pp. 456–467.
Morris, V.B. and Byrne, M., Oral–aboral identity displayed in the expression of HpHox3 and HpHox11 /13 in the adult rudiment of the sea urchin Holopneustes purpurescens, Dev. Genes Evol., 2014, vol. 224, pp. 1–11.
Muscente, A.D., Boag, T.H., Bykova, N., and Schiffbauer, J.D., Environmental disturbance, resource availability, and biologic turnover at the dawn of animal life, Earth-Sci. Rev., 2018, vol. 177, pp. 248–264.
Neijts, R., Simmini, S., Giuliani, F., van Rooijen, C., and Deschamps, J., Region-specific regulation of posterior axial elongation during vertebrate embryogenesis, Dev. Dyn., 2014, vol. 243, pp. 88–98.
Neijts, R., Amin, S., van Rooijen, C., Tan, S., Creyghton, M.P., de Laat, W., and Deschamps, J., Polarized regulatory landscape and Wnt responsiveness underlie Hox activation in embryos, Genes Devel., 2016, vol. 30, pp. 1937–1942.
Neijts, R., Amin, S., van Rooijen, C., and Deschamps, J., Cdx is crucial for the timing mechanism driving colinear Hox activation and defines a trunk segment in the Hox cluster topology, Dev. Biol., 2017, vol. 422, pp 146–154.
Niehrs, C., On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes, Development, 2010, vol. 137, pp. 845–857.
Omori, A., Kikuchi, M., and Kondo, M., Larval and adult body axes in echinoderms, in Reproductive and Developmental Strategies: The Continuity of Life, Kobayashi, K., Kitano T., Iwao, Y., and Kondo, M., Eds., Tokyo: Springer Japan KK, 2018, pp. 760–789.
Owlarn, S. and Bartscherer, K., Go ahead, grow a head! A planarian’s guide to anterior regeneration, Regeneration, 2016, vol. 3, pp. 139–155.
Paul, C.R.C. and Hotchkiss, F., Origin and significance of Lovén's Law in echinoderms, J. Paleontol., 2020, vol. 94, no. 6, pp. 1–14. https://doi.org/10.1017/jpa.2020.31
Peter, I.S. and Davidson, E.H., Evolution of gene regulatory networks controlling body plan development, Cell, 2011, vol. 144, pp. 970–985.
Petersen, C.P. and Reddien, P.W., Wnt signaling and the polarity of the primary body axis, Cell, 2009, vol. 139, pp. 1056–1068.
Peterson, K.J. and Eernisse, D.J., The phylogeny, evolutionary developmental biology, and paleobiology of the Deuterostomia: 25 years of new techniques, new discoveries, and new idea, Org. Divers. Evol., 2016, vol. 16, pp. 401–418.
Peterson, K.J. and Davidson, E.H., Regulatory evolution and the origin of the bilaterians, Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, pp. 4430–4433.
Raff, R. and Popodi, E.M., Evolutionary approaches to analyzing development, in Molecular Zoology: Advances, Strategies and Protocols, Ferraris, J.D. and Palumbi, S.R., Eds., New York: Wiley-Liss, 1996, pp. 245–265.
Rahman, I.A. and Zamora, S., The oldest cinctan carpoid (stem-group Echinodermata), and the evolution of the water vascular system, Zool. J. Linn. Soc., 2009, vol. 157, no. 2, pp. 420–432. https://doi.org/10.1111/j.1096-3642.2008.00517.x
Range, R.C., Angerer, R.C., and Angerer, L.M., Integration of canonical and noncanonical Wnt signaling pathways patterns the neuroectoderm along the anterior-posterior axis of sea urchin embryos, PLoS Biol., vol. 11, no. 1, e1001467. https://doi.org/10.1371/journal.pbio.1001467
De Robertis, E.M., Evo-devo: Variations on ancestral themes, Cell, 2008, vol. 132, pp. 185–195.
De Robertis, E.M., Spemann’s organizer and the self-regulation of embryonic fields, Mech. Devel., 2009, vol. 126, pp. 925–941.
Rozhnov, S. V., New representatives of the class Stylophora (echinoderms), Paleontol. Zh., 1990, no. 4, pp. 37–48.
Rozhnov, S.V., Morphogenesis and evolution of crinoids and other pelmatozoan echinoderms in the Early Paleozoic, Paleontol. J., 2002, vol. 36, no. 6, pp. S525–S674.
Rozhnov, S.V., From Vendian to Cambrian: The beginning of morphological disparity of modern metazoan phyla, Russ. J. Dev. Biol., 2010, vol. 41, no. 6, pp. 357–368.
Rozhnov, S.V., The anteroposterior axis in echinoderms and displacement of the mouth in their phylogeny and ontogeny, Biol. Bull., 2012a, vol. 39, no. 2, pp. 162–171
Rozhnov, S.V., Development of symmetry and asymmetry in the early evolution of the echinoderms, Paleontol. J., 2012b, vol. 46, no. 8, pp. 780–792.
Rozhnov, S.V., Symmetry of echinoderms: From initial bilaterally-asymmetric metamerism to pentaradiality, Nat. Sci., 2016, vol. 6, no. 4, pp. 171–183. https://doi.org/10.4236/ns.2014.64021
Rozhnov, S.V., Aboral nervous system in two ordovician crinoids: reconstruction and comparison of Baltic Pentamerocrinus Jaekel and Grammocrinus Eichwald, Paleontol. J., 2016, vol. 50, no. 2, pp. 163–173. https://doi.org/10.1134/S0031030116020064
Rozhnov, S.V., The origin and homology of the jointed appendages of carpoid and pelmatozoan echinoderms, Invertebr. Zool., 2017, vol. 14, no. 2, pp. 174–181. https://doi.org/10.15298/invertzool.14.2.12
Rozhnov, S., Solutans: Between torsion and pentaradiality, in Abstr. 16th Int. Echinoderm Conf., Nagoya, Japan. May 28 to June 1, 2018, Nagoya, Jappan: Nagoya Univ., 2018.
Rozhnov, S. V. and Parsley, R. L., A new cornute (Homalozoa: Echinodermatahttps://doi.org/10.1134/S0031030117050100le Cambrian (Stage 3, Furongian) from Northern Iran: Its systematicsand functional morphology, Paleontol. J., 2017, vol. 51, no. 5, pp. 500–509. 10.1134/S0031030117050100
Seilacher, A., The nature of vendobionts, in The Rise and Fall of the Ediacaran Biota, Vickers-Rich P. and Komarower, P., Eds., Spec. Publ.—Geol. Soc. London, 2007, vol. 286, pp. 387–397.
Seilacher, A. and Gishlick, A., Morphodynamics, Boca Raton FL: CRC Press, Taylor & Francis Group, 2015, pp. 136–150.
Simakov, O. and Kawashima, T., Independent evolution of genomic characters during major metazoan transitions, Dev. Biol., 2017, vol. 427, pp. 179–192.
Smith, A.B., Deuterostomes in a twist: The origin of a radical new body plan, Evol. Dev., 2008, vol. 10, pp. 493–503.
Smith, A.B. and Zamora, S., Cambrian spiral-plated echinoderms from Gondwana reveal the earliest pentaradial body plan, Proc. R. Soc. B, 2013, vol. 280, 20131197. https://doi.org/10.1098/rspb.2013.1197
Soukup, V., Left-right asymmetry specification in amphioxus: review and prospects, Int. J. Dev. Biol., 2017, vol. 61, pp. 611–620.
Spemann, H. and Mangold, H., Induction of embryonic primordia by implantation of organizers from a different species, Roux’s Arch. Entwicklungsmech. Org., 1924, vol. 100, pp. 599–638.
Sperling, E.A. and Vinther, J.A., Placozoan affinity for Dickinsonia and the evolution of late Proterozoic metazoan feeding modes, Evol. Dev., 2010, vol. 12, no. 2, pp. 201–209.
Sprinkle, J., Morphology and Evolution of Blastozoan Echinoderms, Harvard Univ. Museum of Comparative Zoology, 1973.
Srivastava, M. A., Comparative genomics perspective on the origin of multicellularity and early animal evolution, in Evolutionary Transitions to Multicellular Life. Principles and Mechanisms, Ruiz-Trillo, I. and Nedelcu, A.M., Eds., Dordrecht: Springer Science+Business Media, 2015, pp. 269–300.
Stern, C.D., Charité, J., Deschamps, J., Duboule, D., Durston, A.J., Kmita, M., Nicolas, J.-F., Palmeirim, I., Smith, J.C., and Wolpert, L., Head-tail patterning of the vertebrate embryo: one, two or many unresolved problems? Int. J. Dev. Biol., 2006, vol. 50, pp. 3–15.
Swalla, B.J., Building divergent body plans with similar genetic pathways, Heredity, 2006, vol. 97, pp. 235–243.
Szabó, R. and Ferrier, D.E.K., Two more posterior Hox genes and Hox cluster dispersal in echinoderms, BMC Evol. Biol., 2018, vol. 18. no. 1, p. 203. https://doi.org/10.1186/s12862-018-1307-x
Thompson d’Arcy, W., On Growth and Form (2nd ed.), Cambridge: Cambridge Univ. Press, 1942.
Tsuchimoto, J., Expression Patterns of Hox Genes in the Direct-Type Developing Sand Dollar Peronella japonica: Insights into the Evolution of Echinoderms, Kanazawa University Graduate School of Natural Sciences Doctoral Dissertation, 2012. http://hdl.handle.net/2297/34907
Tsuchimoto, J. and Yamaguchi, M., Hox expression in the direct-type developing sand dollar Peronella japonica, Dev. Dyn., 2014, vol. 243, no. 8. https://doi.org/10.1002/dvdy.24135
Turing, A.M., The chemical basis of morphogenesis, Philos. Trans. R. Soc., B, 1952, vol. 237, pp. 37–72.
Ubaghs, G., Sur la nature de l’organe appelé tige ou pédoncule chez les carpoïdes Cornuta et Mitrata, C. R. Séances Acad. Sci., Paris, 1961, vol. 253, pp. 2738–2740.
Yasuoka, Y. and Taira M., The molecular basis of the gastrula organizer in amphibians and cnidarians, in Reproductive and Developmental Strategies. Diversity and Commonality in Animals, Kobayashi, K., Kitano, T., Iwao, Y., and Kondo, M., Eds., Tokyo: Springer Japan KK, 2018, pp. 667–708.
Zamora, S, Rahman, I.A., and Smith, A.B., Plated Cambrian bilaterians reveal the earliest stages of echinoderm evolution, PLoS One, 2012, vo. 7, e38296.
Zamora, S., Lefebvre, B., Alvaro, J.J., et al., Cambrian echinoderm diversity and palaeobiogeography, in Early Palaeozoic Biogeography and Palaeogeography, Harper, D.A.T. and Servais, T., Eds., Geol. Soc.London, Mem., Bath, 2013, pp. 157–171.
Zhang, X., Sun, L., Yaun, J., et al., The sea cucumber genome provides insights into morphological evolution and visceral regeneration, PLoS Biol., 2017, vol. 15, e2003790.
Zhong, Y., Herrera-Ubeda, C., Garcia-Fernàndez, J., Li, G., and Holland, P.W.H., Mutation of amphioxus Pdx and Cdx demonstrates conserved roles for ParaHox genes in gut, anus and tail patterning, BMC Biol., 2020, vol. 18, no. 1. https://doi.org/10.1186/s12915-020-00796-2
ACKNOWLEDGMENTS
We are grateful to N.D. Ozernyuk and an anonymous reviewer for their comments. Special thanks to G.A. Anekeeva for her assistance with preparing drawings for the manuscript.
Funding
This study was supported by the Russian Science Foundation (project no. 19-14-00346).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by D. Voroshchuk
Rights and permissions
About this article
Cite this article
Isaeva, V.V., Rozhnov, S.V. Transformation of the Ancestral Body Plan and Axial Growth in Echinoderms: Ontogenetic and Paleontological Data. Paleontol. J. 56, 863–886 (2022). https://doi.org/10.1134/S0031030122080032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031030122080032