Abstract
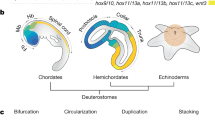
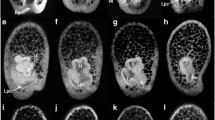
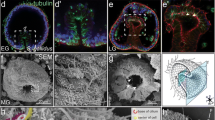
Adult echinoderms possess a highly diverged, pentaradial body plan. Developmental mechanisms underlying this body plan are completely unknown, but are critical in understanding how echinoderm pentamery evolved from bilateral ancestors. These mechanisms are difficult to study in indirect-developing species; in this study, we use the direct-developing sea urchin Heliocidaris erythrogramma, whose accelerated adult development can be perturbed by NiCl2. We introduce a new nomenclature for the adult echinoderm axes to facilitate discussion of the radially symmetric body plan and the events required to pattern it. In sea urchins, the adult oral–aboral axis is often conflated with the long axes of the five rays; we identify these as distinct body axes, the proximodistal (PD). In addition, we define a circular axis, the circumoral (CO), along which the division into five sectors occurs. In NiCl2-treated larvae, aspects of normal PD pattern were retained, but CO pattern was abolished. Milder treatments resulted in relatively normal juveniles ranging from biradial to decaradial. NiCl2 treatment had no effect either on mesodermal morphology or on the ectodermal gene expression response to an inductive mesodermal signal. This suggests that the mesoderm does not mediate the disruption of CO patterning by NiCl2. In contrast, mesodermal signaling may explain the presence of PD pattern in treated larvae. However, variations in appendage pattern suggest that ectodermal signals are also required. We conclude that CO patterning in both germ layers is dependent on ectodermal events and PD patterning is controlled by mutual ectoderm–mesoderm signaling.








Similar content being viewed by others
References
Aihara M, Amemiya S (2001) Left–right positioning of the adult rudiment in sea urchin larvae is directed by the right side. Development 128:4935–4948
Angerer LM, Angerer RC (1991) Localization of mRNAs by in situ hybridization. Methods Cell Biol 35:37–71
Bury H (1889) Studies in the embryology of the echinoderms. Q J Microsc Sci 29:409–447 (+plates 37–39)
David B, Mooi R (1998) Major events in the evolution of echinoderms viewed by the light of embryology. In: Mooi R, Telford M (eds) Echinoderms: San Francisco. Balkema, Rotterdam, pp 21–28
Duboc V, Röttinger E, Lapraz F, Besnardeau L, Lepage T (2005) Left–right asymmetry in the sea urchin embryo is regulated by Nodal signaling on the right side. Developmental Cell 9:147–158
Emlet RB (1995) Larval spicules, cilia, and symmetry as remnants of indirect development in the direct developing sea urchin Heliocidaris erythrogramma. Dev Biol 167:405–415
Ferkowicz MJ, Raff RA (2001) Wnt gene expression in sea urchin development: heterochronies associated with the evolution of developmental mode. Evol Dev 3:24–33
Haag ES, Raff RA (1998) Isolation and characterization of three mRNAs enriched in embryos of the direct-developing sea urchin Heliocidaris erythrogramma: evolution of larval ectoderm. Dev Genes Evol 208:188–204
Hadfield MG (1975) Hemichordata. In: Giese AC, Pearse JS (eds) Reproduction of marine invertebrates, vol 2. Entoprocts and lesser coelomates. Academic, New York, pp 185–240
Hardin J, Coffman JA, Black SD, McClay DR (1992) Commitment along the dorsoventral axis of the sea urchin embryo is altered in response to NiCl2. Development 116:671–685
Hendler G (1991) Echinodermata: ophiuroidea. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates, vol 6. Echinoderms and lophophorates. Boxwood, Pacific Grove, CA, pp 355–511
Hinegardner RT (1975) Morphology and genetics of sea urchin development. Am Zool 15:679–689
Hotchkiss FHC (1998) A “rays-as-appendages” model for the origin of pentamerism in echinoderms. Paleobiology 24:200–214
Hyman LH (1955) The invertebrates, vol. 4. Echinodermata. McGraw-Hill, New York
Hyman LH (1959) The Invertebrates. vol. 5. Smaller coelomate groups. McGraw-Hill, New York
Lowe CJ, Wray GA (1997) Radical alterations in the roles of homeobox genes during echinoderm evolution. Nature 389:718–721
Lowe CJ, Issel-Tarver L, Wray GA (2002) Gene expression and larval evolution: changing roles of distal-less and orthodenticle in echinoderm larvae. Evol Dev 4:111–123
MacBride EW (1903) The development of Echinus esculentus, together with some points in the development of E. miliaris and E. acutus. Phil Trans R Soc Lond B 195:285–327 (+plates 7–16)
Marsh AG, Watts SA, Chen CP, McClintock JB (1986) The effect of high salinity on development, mortality and ray number of Echinaster spinulosus (Echinodermata: Asteroidea) at different developmental stages. Comp Biochem Physiol 83A:229–231
McCain ER, McClay DR (1994) The establishment of bilateral asymmetry in sea urchin embryos. Development 120:395–404
Minsuk SB, Raff RA (2002) Pattern formation in a pentameral animal: induction of early adult rudiment development in sea urchins. Dev Biol 247:335–350
Minsuk SB, Raff RA (2005) Co-option of an oral-aboral patterning mechanism to control left–right differentiation: the direct-developing sea urchin Heliocidaris erythrogramma is sinistralized, not ventralized, by NiCl2. Evol Dev 7:289–300
Minsuk SB, Andrews ME, Raff RA (2005) From larval bodies to adult body plans: patterning the development of the presumptive adult ectoderm in the sea urchin larva. Dev Genes Evol 215:383–392
Morris VB (1999) Bilateral homologues in echinoderms and a predictive model of the bilateral echinoderm ancestor. Biol J Linn Soc 66:293–303
Morris VB, Byrne M (2005) Involvement of two Hox genes and Otx in echinoderm body-plan morphogenesis in the sea urchin Holopneustes purpurescens. J Exp Zoolog B Mol Dev Evol 304B:456–467
Nielsen MG, Popodi E, Minsuk S, Raff RA (2003) Evolutionary convergence in Otx expression in the pentameral adult rudiment in direct-developing sea urchins. Dev Genes Evol 213:73–82
Okazaki K (1975) Normal development to metamorphosis. In: Czihak G (ed) The sea urchin embryo. Springer, New York, pp 177–232
Paul CRC, Smith AB (1984) The early radiation and phylogeny of echinoderms. Biol Rev 59:443–481
Peterson KJ, Cameron RA, Tagawa K, Satoh N, Davidson EH (1999) A comparative molecular approach to mesodermal patterning in basal deuterostomes: the expression pattern of Brachyury in the enteropneust hemichordate Ptychodera flava. Development 126:85–95
Peterson KJ, Arenas-Mena C, Davidson EH (2000) The A/P axis in echinoderm ontogeny and evolution: evidence from fossils and molecules. Evol Dev 2:93–101
Raff RA (1996) The shape of life. The University of Chicago Press, Chicago
Raff RA, Popodi EM (1996) Evolutionary approaches to analyzing development. In: Ferraris JD, Palumbi SR (eds) Molecular zoology: advances, strategies, and protocols. Wiley, New York, pp 245–265
Sly BJ, Hazel JC, Popodi EM, Raff RA (2002) Patterns of gene expression in the developing adult sea urchin central nervous system reveal multiple domains and deep-seated neural pentamery. Evol Dev 4:189–204
Smiley S (1986) Metamorphosis of Stichopus californicus (Echinodermata: holothuroidea) and its phylogenetic implications. Biol Bull 171:611–631
Smiley S, McEuen FS, Chafee C, Krishnan S (1991) Echinodermata: holothuroidea. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates, vol 6. Echinoderms and lophophorates. Boxwood, Pacific Grove, CA, pp 663–750
Smith AB (2008) Deuterostomes in a twist: the origins of a radical new body plan. Evol Dev 10:493–503
Summers RG, Piston DW, Harris KM, Morrill JB (1996) The orientation of first cleavage in the sea urchin embryo, Lytechinus variegatus, does not specify the axes of bilateral symmetry. Dev Biol 175:177–183
Urata M, Yamaguchi M (2004) The development of the enteropneust hemichordate Balanoglossus misakiensis Kuwano. Zool Sci 21:533–540
Watts SA, Scheibling RE, Marsh AG, McClintock JB (1983) Induction of aberrant ray numbers in Echinaster sp. (Echinodermata: Asteroidea) by high salinity. Fla Sci 46:125–128
Williams DHC, Anderson DT (1975) The reproductive system, embryonic development, larval development and metamorphosis of the sea urchin Heliocidaris erythrogramma (Val.) (Echinoidea: Echinometridae). Aust J Zool 23:371–403
Wilson KA, Andrews ME, Raff RA (2005) Dissociation of expression patterns of homeodomain transcription factors in the evolution of developmental mode in the sea urchins Heliocidaris tuberculata and H. erythrogramma. Evol Dev 7:401–415
Wray GA (1998) Origin and diversification of echinoderm body architecture: insights from the expression of body-patterning genes. In: Mooi R, Telford M (eds) Echinoderms: San Francisco. Balkema, Rotterdam, p 90
Acknowledgments
We thank the Sydney Aquarium and the School of Biological Sciences, University of Sydney for providing resources and for making our work in Australia possible; Gerd Müller and Wolfgang Weninger for the use of equipment; Meg Snoke for help with specimen treatment and transport; and Ulrich Krohs and Ellen Popodi for helpful discussion. We would also like to thank the anonymous reviewers for comments on the manuscript and Javier Capdevila for comments on an earlier draft. New South Wales Fisheries provided collection permits. This work was funded by an NIH Postdoctoral Fellowship to SBM and an NSF research grant to RAR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Satoh
Rights and permissions
About this article
Cite this article
Minsuk, S.B., Turner, F.R., Andrews, M.E. et al. Axial patterning of the pentaradial adult echinoderm body plan. Dev Genes Evol 219, 89–101 (2009). https://doi.org/10.1007/s00427-009-0270-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-009-0270-3