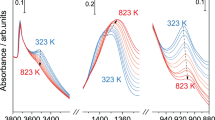
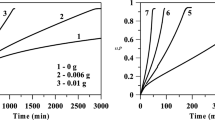
Abstract
In this study, Langmuir–Hinshelwood and Michaelis–Menten kinetic models are applied to describe the kinetic behaviour of the Co–B catalyst in the hydrolysis of 0.12 M aqueous solutions of boron hydrides at temperatures from 22 to 60°C. Boron hydrides are selected as sodium borohydride (NaBH4, 10 wt % NaOH) and ammonia borane (NH3BH3). Based on the Langmuir–Hinshelwood kinetic approach, it is found that under the same reaction conditions the NaBH4–Co–B catalyst interaction is more effective than that of the NH3BH3–Co–B. According to the Langmuir–Hinshelwood model, apparent activation energies (Ea) for the hydrolysis of NaBH4 and NH3BH3 over Co–B catalysts are calculated to be 45.38 and 57.37 kJ/mol, respectively.



Similar content being viewed by others
REFERENCES
Schlesinger, H.I., Brown, H.C., Finholt, A.E., Gilbreath, J.R., Hoekstra, H.R., and Hyde, E.K., J. Amer. Chem. Soc., 1953, vol. 75, no. 1, p. 215.
Retnamma, R., Novais, A.Q., and Rangel, C.M., Int. J. Hydrogen Energy, 2011, vol. 36, no. 16, p. 9772.
Fernandes, R., Patel, N., Miotello, A., Jaiswal, R., and Kothari, D.C., Int. J. Hydrogen Energy, 2012, vol. 37, no. 3, p. 2397.
Abo-Hamed, E.K., Pennycook, T., Vaynzof, Y., Toprakcioglu, C., Koutsioubas, A., and Scherman, O.A., Small, 2014, vol. 10, no. 15, p. 3145.
Sun, D., Mazumder, V., Metin, O., and Sun, S., ACS Nano, 2011, vol. 5, no. 8, p. 6458.
Xu, Q. and Chandra, M., J. Power Sources, 2006, vol. 163, no. 1, p. 364.
Metin, O., Mazumder, V., Ozkar, S., and Sun, S., J. Amer. Chem. Soc., 2010, vol. 132, no. 5, p. 1468.
Yamada, Y., Yano, K., Xu, Q., and Fukuzumi, S., J. Phys. Chem. C, 2010, vol. 114, no. 39, p. 16456.
Wu, C., Wu, F., Bai, Y., Yi, B., and Zhang, H., Mater. Lett., 2005, vol. 59, no. 14, p. 1748.
Ozerova, A.M., Simagina, V.I., Komova, O.V., Netskina, O.V., Odegova, G.V., Bulavchenko, O.A., and Rudina, N.A., J. Alloy Compd., 2012, vol. 513, p. 266.
Cho, K.W. and Kwon, H.S., Catal. Today, 2007, vol. 120, no. 3, p. 298.
Sahiner, N., Ozay, O., Inger, E., and Aktas, N., Appl. Catal., B, 2011, vol. 102, no. 1, p. 201.
Qiu, F.Y., Wang, Y.J., Wang, Y.P., Li, L., Liu, G., Yan, C., and Yuan, H.T., Catal. Today, 2011, vol. 170, no. 1, p. 64.
Groven, L.J., Pfeil, T.L., and Pourpoint, T.L., Int. J. Hydrogen Energy, 2013, vol. 38, no. 15, p. 6377.
Simagina, V.I., Komova, O.V., Ozerova, A.M., Netskina, O.V., Odegova, G.V., Kellerman, D.G., and Ishchenko, A.V., Appl. Catal., A, 2011, vol. 394, no. 1, p. 86.
Kaya, M., Zahmakiran, M., Özkar, S., and Volkan, M., ACS Appl. Mater. Interfaces, 2012, vol. 4, no. 8, p. 3866.
Liu, B.H. and Li, Z.P., J. Power Sources, 2009, vol. 187, no. 2, p. 527.
Zhang, J.S., Delgass, W.N., Fisher, T.S., and Gore, J.P., J. Power Sources, 2007, vol. 164, no. 2, p. 772.
Hung, A.J., Tsai, S.F., Hsu, Y.Y., Ku, J.R., Chen, Y.H., and Yu, C.C., Int. J. Hydrogen Energy, 2008, vol. 33, no. 21, p. 6205.
Demirci, U.B. and Miele, P., C. R. Chim., 2014, vol. 17, no. 7, p. 707.
Metin, Ö., Dinç, M., Eren, Z.S., and Özkar, S., Int. J. Hydrogen Energy, 2011, vol. 36, no. 18, p. 11528.
Luo, Y.C., Liu, Y.H., Hung, Y., Liu, X.Y., and Mou, C.Y., Int. J. Hydrogen Energy, 2013, vol. 38, no. 18, p. 7280.
Ye, W., Zhang, H., Xu, D., Ma, L., and Yi, B., J. Power Sources, 2007, vol. 164, no. 2, p. 544.
Xu, Q. and Chandra, M., J. Alloy Compd., 2007, vol. 446, p. 729.
Coşkuner, B., Figen, A.K., and Pişkin, S., React. Kinet. Mech. Catal., 2013, vol. 109, no. 2, p. 375.
Andrieux, J., Demirci, U.B., and Miele, P., Catal. Today, 2011, vol. 170, p. 13.
Levenspiel, O., Chemical Reaction Engineering, John Wiley & Sons, 1999.
Figen, A.K. and Coşkuner, B., Int. J. Hydrogen Energy, 2013, vol. 38, no. 6, p. 2824.
Kantürk Figen, A., Coşkuner, B., Pişkin, M.B., Dere Özdemir, Ö. J Int Sci Publications: Mater, Methods & Techologies, 2013, vol. 7, no. 1, p. 43.
Zhang, Q., Wu, Y., Sun, X., and Ortega, J., Ind. Eng. Chem. Res., 2007, vol. 46, no. 4, p. 1120.
ACKNOWLEDGMENTS
The authors would like to thank the Yildiz Technical University Research Foundation (Project no. 2012-07-01-GEP01) for its financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
B. Coşkuner Filiz, A. Kantürk Figen The Molecular-Kinetic Approach to Hydrolysis of Boron Hydrides for Hydrogen Production. Kinet Catal 60, 37–43 (2019). https://doi.org/10.1134/S0023158419010075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158419010075