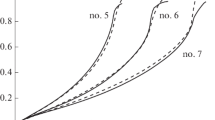
A study has been made of the influence of temperature, NaON, and concentrations of NaBH4 on the rate of catalytic hydrolysis under isothermal and adiabatic conditions. Finely divided Co/TiO2 powder was used as the model catalyst. The catalyst preserves its initial activity during 20 cycles, ensuring an NaBH4 conversion of 94–98%. Activation energies equal to 65.6 and 55 kJ/mole have been determined in the aqueous and aqueous-alkaline solution of NaBH4 respectively. It has been shown that the standard method of determining activation energy without account of sorption/ desorption processes leads to its dependence on the composition of the solution.
Similar content being viewed by others
References
T. Vilarinho-Franco, R. Tenu, J. Delmas, et al., Lifetime analysis of a hydrogen generator by hydrolysis of sodium borohydride, Energy Procedia, 36, 1192–1201 (2013).
H. I. Schlesinger, H. C. Brown, A. E. Finholt, et al., Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen, J. Am. Chem. Soc., 75, 215–219 (1953).
L. Wang, Z. Li, P. Zhang, et al., Hydrogen generation from alkaline NaBH4 solution using Co–Ni–Mo–P/γ–Al2O3 catalysts, Int. J. Hydrogen Energy, 41, Issue 3, 1468–1476 (2015).
L. Wang, Z. Li, Y. Zhang, et al., Hydrogen generation from alkaline NaBH4 solution using electroless-deposited Co–Ni–W–P/γ–Al2O3 as catalysts, J. Alloys Compd., 702, 649–658 (2017).
Z. Li, H. Li, L. Wang, et al., Hydrogen generation from catalytic hydrolysis of sodium borohydride solution using supported amorphous alloy catalysts (Ni–Co–P/γ–Al2O3), Int. J. Hydrogen Energy, 39, Issue 27, 14935–14941 (2014).
W. Ye, H. Zhang, D. Xua, et al., Hydrogen generation utilizing alkaline sodium borohydride solution and supported cobalt catalyst, J. Power Sources, 164, Issue 2, 544–548 (2007).
D. Xu, P. Dai, X. Liu, et al., Carbon-supported cobalt catalyst for hydrogen generation from alkaline sodium borohydride solution, J. Power Sources, 182, Issue 2, 616–620 (2008).
A. Didehban, M. Zabihi, and J. R. Shahrouzi, Experimental studies on the catalytic behavior of alloy and core-shell supported Co–Ni bimetallic nano-catalysts for hydrogen generation by hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 43, Issue 45, 20645–20660 (2018).
E. Ozdemir, Enhanced catalytic activity of Co–B/glassy carbon and Co–B/graphite catalysts for hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 40, Issue 40, 14045–14051 (2015).
X. Shen, Q. Wang, Q. Wu, et al., CoB supported on Ag-activated TiO2 as a highly active catalyst for hydrolysis of alkaline NaBH4 solution, Energy, 90, Issue 1, 464–474 (2015).
Y.-C. Lu, M.-S. Chen, and Y.-W. Chen, Hydrogen generation by sodium borohydride hydrolysis on nanosized CoB catalysts supported on TiO2, Al2O3, and CeO2, Int. J. Hydrogen Energy, 37, Issue 5, 4254–4258 (2012).
O. Sahin, M. S. Izgi, E. Onat, et al., Influence of the using of methanol instead of water in the preparation of Co–B–TiO2 catalyst for hydrogen production by NaBH4 hydrolysis and plasma treatment effect on the Co–B–TiO2 catalyst, Int. J. Hydrogen Energy, 41, Issue 4, 2539–2546 (2016).
S. I. Shabunya, V. G. Minkina, V. I. Kalinin, et al., Kinetics of the catalytic hydrolysis of concentrated aqueous solutions of NaBH4 on Co/TiO2 powder, Kinetics Catalysis, 62, Issue 3, 350–359 (2021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Inzhenerno-Fizicheskii Zhurnal, Vol. 96, No. 7, pp. 1853–1860, November–December, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Minkina, V.G., Shabunya, S.I. & Kalinin, V.I. Catalytic Hydrolysis of Sodium Borohydride. J Eng Phys Thermophy 96, 1820–1827 (2023). https://doi.org/10.1007/s10891-023-02851-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10891-023-02851-5