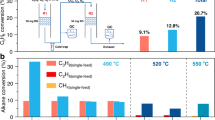
Abstract
Boron-based catalysts exhibit high alkene selectivity in oxidative dehydrogenation of propane (ODHP) but the mechanistic understanding remains incomplete. In this work, we show that the hydroxylation of framework boron species via steaming not only enhances the ODHP rate on both B-MFI and B-BEA, but also impacts the kinetics of the reaction. The altered activity, propane reaction order and the activation energy could be attributed to the hydrolysis of framework [B(OSi≡)3] unit to [B(OSi≡)3−x(OH···O(H)Si≡)x] (x = 1, 2, “···” represents hydrogen bonding). DFT calculations confirm that hydroxylated framework boron sites could stabilize radical species, e.g., hydroperoxyl radical, further facilitating the gas-phase radical mechanism. Variations in the contributions from gas-phase radical mechanisms in ODHP lead to the linear correlation between activation enthalpy and entropy on borosilicate zeolites. Insights gained in this work offer a general mechanistic framework to rationalize the kinetic behavior of the ODHP on boron-based catalysts.
Similar content being viewed by others
Introduction
Propylene (C3H6) is a fundamental building block of the chemical industry to produce bulk chemicals such as polypropylene, acrylonitrile, propylene oxide and acrylic acid1. Non-oxidative propane (C3H8) dehydrogenation (PDH) has been commercialized to meet the growing demand of C3H6, but the efficiency of PDH is limited by the unfavorable thermodynamics and coking-induced rapid catalyst deactivation2. Oxidative dehydrogenation of propane (ODHP), which introduces molecular oxygen (O2) to facilitate the C–H activation of propane, has the potential to overcome the challenges faced by PDH3,4. However, conventional ODHP catalysts, such as vanadium-based catalysts, exhibit poor alkene selectivity, which is caused by the deep oxidation of C3H6 to form carbon monoxide (CO) and carbon dioxide (CO2)5. The development of catalysts with high C3H6 yield and long-term stability is critical to make ODHP commercially viable.
ODHP activity of supported boron oxide (B2O3) catalysts has been recognized since the late 1980s, and the first application of hexagonal boron nitride (h-BN) after oxyfunctionalization was reported in 20166,7,8. Distinct from vanadium-based catalysts, boron-based catalysts are able to suppress further oxidation of alkenes in the presence of O28,9. A second-order dependence on the partial pressure of C3H8 (pC3H8) and the apparent activation energy (Eapp) higher than 150 kJ·mol−1 were observed on most boron-based catalysts (Table S1). The supra-linear reaction order of C3H8 cannot be rationalized by classical surface-mediated mechanisms in heterogeneous catalysis, which led to much research effort in understanding the catalytic mechanism. Hermans and co-workers found that the conversion rate of C3H8 was proportional to the volume of catalyst bed, rather than the catalyst loading, in the h-BN catalyzed ODHP, implying that a surface-mediated pathway is unlikely to be able to account for the majority of observed activity10. They proposed a combined surface and gas-phase radical reaction mechanism capable of rationalizing the product distribution of ODHP observed on h-BN11. Our recent kinetic study on oxidative co-dehydrogenation of ethane (C2H6) and C3H8 over h-BN found that the apparent second-order dependence on the partial pressure of alkane in ODHP could be rationalized by two roles played by the alkane, i.e., radical generator and reactant12. The high propylene selectivity of bulk boron-based catalysts in ODHP is likely due to the involvement of gas-phase radicals in activating propane, though the exact mechanism for suppressing the further conversion of propylene remains unclear13,14,15. The study using the magic angle spinning (MAS) 11B solid-state nuclear magnetic resonance (NMR) spectroscopy revealed that oxidized boron species, denoted as B(OH)xO3−x (where x = 0–3), were gradually formed on h-BN in ODHP16,17. Similar oxidized boron species could also be formed on other boron-containing materials under ODHP conditions, and are likely responsible for the catalytic activity18.
The identities of active structure of isolated boron sites and reaction mechanism are less clear. Hermans and coworkers synthesized boron-substituted MWW (B-MWW) zeolites with negligible ODHP activity19. 11B NMR results showed that most framework boron atoms were in the trigonal coordination environment, which led to the conclusion of isolated boron sites being inactive. Meanwhile, B-MWW prepared by Lu and coworkers showed quite similar overall and deconvoluted NMR features and exhibited high ODHP activity20. Xiao and coworkers synthesized boron-substituted MFI- and BEA-type (B-MFI and B-BEA) zeolites with most framework boron atoms located in the tetrahedral coordination environment both in as-synthesized and spent samples21. These B-MFI and B-BEA catalysts showed remarkable ODHP activities and durability. A first-order kinetics with respect to pC3H8 was determined on both borosilicate zeolites. Combined with computational results, a surface-mediated mechanism was proposed on trigonally coordinated framework boron atoms in ODHP. Qiu et al. assigned aggregated boron species in incompletely crystallized B-MFI as the most active structure22,23. These inconsistent literature claims highlight the need for further mechanistic studies.
Determination of kinetic parameters, i.e., the reaction order of C3H8 and the apparent activation energy (Eapp), proves to be informative in mechanistic studies of boron-catalyzed ODHP. The likely involvement of gas-phase radicals, as well as the high reaction temperature, in ODHP makes the detection of surface intermediates challenging and less relevant. In contrast, C3H8 reaction order could serve as a diagnostic variable to identify the dominant type of mechanism at play. A first-order kinetics for C3H8 indicates a surface-mediated mechanism24,25,26, which is typical on vanadium-based catalysts. Most bulk boron-based catalysts exhibit a second-order reaction for C3H8, indicating the involvement of a gas-phase radical mechanism11,12. Our recent work showed that spatial confinement of B2O3 nanoparticles in mesoporous support could lead to C3H8 reaction orders up to 327, which was attributed to the introduction of a branching radical pathway.
In this study, we investigated the correlation between the boron coordination environment and the ODHP kinetics. Fresh B-MFI with majority of boron species incorporated in the framework exhibits a first-order dependence on pC3H8 and Eapp ranging from 100 to 120 kJ·mol−1. Steaming treatment gradually hydrolyzes the B–O–Si bonds in B-MFI, which proceeds from [B(OSi≡)3] to hydroxyl group substituted [B(OSi≡)3−x(OH···O(H)Si≡)x] (x = 1, 2), and enhances the ODHP activity by up to a factor of 9 on B-MFI. Computational results show that the hydroxylation of the isolated boron sites could stabilize potential gas-phase radical species derived from O2, facilitating the initiation of the radical chain mechanism. Similar observations were made on B-BEA. Degree of hydroxylation on framework boron species is proposed to be the key in understanding the kinetic behaviors of boron-containing zeolites after different pretrement conditions and in different ranges of pC3H8.
Results
Boron coordination and ODHP performance of the fresh B-MFI Zeolite
B-MFI was synthesized via a solvent-free crystallization method reported by Zhou et al.21 as conventional hydrothermal method was prone to forming non-framework boron species28,29. The as-prepared sample is referred to as fresh B-MFI below. Fresh B-MFI consists of sphere-like particles with an average diameter of ~1 μm (Fig. S1). XRD patterns of fresh B-MFI, H-ZSM-5 and Silicalite-1 exhibit diffraction peaks characteristic of the MFI-type zeolites with no detectable phase impurities. (Fig. S2) Lattice parameters and unit cell volumes of the MFI-type zeolites were calculated based on the peak positions in the XRD patterns. (Table S2) Compared to H-ZSM-5 and Silicalite-1, the unit cell volume of B-MFI is slightly contracted, indicating that the boron atoms are successfully incorporated into the framework of zeolite30. Micropore volume of fresh B-MFI, H-ZSM-5 and Silicalite-1 are in line with typical MFI-type zeolites31. (Fig. S3 and Table S3)
To understand the coordination environment of the boron species, 11B and 29Si NMR spectra of fresh B-MFI were collected. (Figs. S4 and S5) 11B chemical shift of fresh B-MFI shows a strong peak at −3.2 ppm and a broad peak at 5.9 ppm. The former has been assigned to the tetra-coordinated boron species in zeolite frameworks (denoted as B[4]-Fr)32. The band at −3.2 ppm exhibits a Lorentzian lineshape, which is in line with B[4]-Fr33. Distinct from tetrahedrally coordinated boron species, the strong quadrupolar interaction of trigonally coordinated boron leads to a peak shift and anisotropic line broadening of tri-coordinated boron species34. Combing the chemical shift and the lineshape, the peak at 5.9 ppm could be attributed to tri-coordinated boron species in the zeolite framework (denoted as B[3]-Fr)35. Non-framework tri-coordinated boron species (denoted as B[3]-Nf), for example H3BO3 (δiso = 18.0–18.5 ppm)36,37 and B2O3 (δiso = 14.6 ppm)38, are absent in fresh B-MFI, indicating that most boron atoms are incorporated in the framework and isolated by neighboring –O–Si–O– units. 29Si NMR spectrum of fresh B-MFI exhibits a strong peak at −113 ppm and a shoulder peak at −103 ppm, corresponding to [Si(OSi≡)4] and [Si(OSi≡)3OH] in the zeolite framework, respectively39. (Fig. S5) According to the peak area in Fig. S4, B[4]-Fr is estimated to account for 92% of all boron atoms at room temperature in fresh B-MFI, which is consistent with a previous study21.
Although most framework boron atoms are in the form of B[4]-Fr at room temperature, in-situ IR spectroscopy shows that B[3]-Fr is the dominant species at reaction temperature. To study the boron coordination under the ODHP conditions, transmission IR spectra of dehydrated fresh B-MFI at different temperatures were collected. (Fig. S6) The peak around 3720 cm−1 is attributed to silanol groups (Si–OH) on zeolite and the peak at 3520 cm−1 is assigned to the Brønsted acid site in borosilicate zeolite39,40. (Fig. 1) The band at 910 cm−1 is attributed to the symmetric stretching of B[4]-Fr while the band located in the 1400 to 1360 cm−1 range corresponds to the B–O asymmetric stretching vibration of B[3]-Fr34,41. (Fig. 1) As temperature rises, the intensity of IR peaks at 3520 and 910 cm−1 decrease concomitantly while the peak at 1380 cm−1 strengthens, indicating that B[4]-Fr is transformed to B[3]-Fr at temperatures relevant in ODHP. Together with the absence of IR band for B–OH at 3680 cm−1 37,42, it could be inferred that [B(OSi≡)3] unit is the dominant boron specie of fresh B-MFI at the ODHP temperature21,29,41.
Fresh B-MFI exhibits distinct catalytic behavior from most bulk boron-based catalysts in ODHP (Fig. 2a, b). To verify the initial ODHP activity prior to activation is attributed to framework boron species, we conducted two control experiments (Table S4 and Fig. S7). Silicalite-1 showed no ODHP selectivity to C3H6 (Table S4), and C3H6 formation rate of fresh B-MFI in ODHP exhibited a positive correlation to boron content in B-MFI (Fig. S7). The control experiments confirmed that the framework boron species are likely to be the active sites responsible for the initial ODHP activity in fresh B-MFI. The formation rate of C3H6 (rC3H6) over fresh B-MFI exhibits a first-order dependence on pC3H8. (Fig. 2a) This result is different from the supra-linear order observed over most bulk boron-based catalysts, but in line with a recent report on borosilicates21. (Table S1) Product distribution in ODHP on fresh B-MFI as a function of the C3H8 conversion is shown in Fig. 2b. The propylene selectivity increases almost linearly with the propane conversion when the conversion is below 1%, and then gradually levels off at conversion above 1.5% at 85%. While ethylene (C2H4) is the main by-product in ODHP on most bulk boron-based catalysts43, CO is the main by-product on fresh B-MFI, and its selectivity increases with decreasing C3H8 conversion. The high CO selectivity in ODHP on boron-substituted zeolites was also observed by Lin and co-workers44. The distinct C3H8 reaction order and product distribution on fresh B-MFI indicate that ODHP on fresh B-MFI likely proceeds via a distinct pathway from that on catalysts with aggregated boron species, e.g., B2O3 and h-BN.
a The reaction order of rC3H6 with respect to pC3H8. Reaction conditions: 773 K, pO2 = 0.125 atm with balancing N2. Error bars show represent standard deviation of ln (rC3H6) in three replicate data. b Product selectivity as a function of C3H8 conversion. Reaction conditions: Reaction conditions: 773 K, pC3H8 = 0.25 atm, pO2 = 0.125 atm with balancing N2, space velocity from 1500 to 24000 LC3H8‧kgcat−1‧h−1.
The promotion of ODHP activity via steaming treatment
Steaming treatment markedly promotes the ODHP activity on fresh B-MFI. Steaming is an effective strategy to accelerate the hydrolysis of Si–O–X bonds (X represents a heteroatom) in zeolites, and thus tuning the coordination of framework heteroatoms such as boron45,46. The steaming of fresh B-MFI was carried out at high temperature (823 K) and low partial pressure of water (pH2O = 1.8 kPa). rC3H6 increases almost linearly with the duration of the steaming treatment within the first 3 h and levels off after 4 h regardless of pC3H8 (Fig. 3a–c). The increase in the ODHP activity after steaming is more pronounced at high pC3H8, and the apparent reaction order of C3H8 increases with the steaming duration. (Fig. S8) The specific C3H6 formation rate on fully steamed B-MFI (with steaming duration > 4 h) reaches 0.67 molC3H6·molB−1·h−1 at pC3H8 = 0.25 atm and 803 K, which is nearly one order of magnitude higher than that of fresh B-MFI. (Fig. 3d) The ODHP activity of fully steamed B-MFI could be further enhanced in ODHP, i.e., there is an induction period47. (Fig. S9) Steamed B-MFI after the induction period is referred to as activated B-MFI later in this work. The fact that activated B-MFI exhibits superior ODHP activity than fully steamed sample indicates that species involved in the reaction are able to induce structrual changes at boron site that are inaccessible via steaming. The steady-state C3H6 formation rates after the induction period on fresh and fully steamed B-MFI are comparable, implying that ODHP could activate fresh B-MFI without the steaming treatment. This is expected considering that ODHP produces water, so the catalyst is subject to conditions similar to the steaming treatment during reaction. The specific C3H6 formation rate on activated B-MFI sample is 0.88 molC3H6·molB−1·h−1 at pC3H8 = 0.25 atm, (Fig. 3d) which is comparable to the PDH activity on noble metals48. To avoid interference of the induction period in determining the kinetic parameters of ODHP, activated B-MFI was employed in the following investigations.
a–c Influence of steaming duration on rC3H6 at different pC3H8: a pC3H8 = 0.05 atm; b pC3H8 = 0.125 atm; c pC3H8 = 0.20 atm. d Specific C3H6 formation rate of different B-MFI. Error bars show represent standard deviation of specific rates in three replicate data. Reaction conditions for (a–d): 803 K, total gas flow = 40 mL·min−1, 50 mg catalyst, pO2 = 0.125 atm with balancing N2. e Correlation between Eapp and Aapp. The inset are corresponding Arrhenius plots. Reaction conditions for (e): 773 to 793 K, total gas flow = 40 mL·min−1, 50 mg catalyst, pO2 = 0.125 atm with balancing N2. f Reaction order of rC3H6 with respect to pC3H8 on activated B-MFI. Error bars show represent standard deviation of ln (rC3H6) in three replicate data. g Product selectivity (% of carbon atoms in C3H8 converted to products) as a function of C3H8 conversion on activated B-MFI. Reaction conditions for (f, g): 773 K, total gas flow = 40 mL·min−1, pO2 = 0.125 atm with balancing N2.
Activated B-MFI exhibits a higher Eapp than that on fresh B-MFI. (Fig. 3e and Table S5) At pC3H8 = 0.25 atm, Eapp of activated and fresh B-MFI were determined to be 163 and 125 kJ·mol−1, respectively. These measured Eapp values appear counterintuitive as the more active activated B-MFI exhibits a higher Eapp. The apparent pre-exponential factor (Aapp) of ODHP on activated B-MFI is higher than that of fresh B-MFI by a factor of ~400. Values of Eapp and Aapp also depend on pC3H8. When pC3H8 is decreased from 0.25 to 0.15 atm, both Eapp and Aapp of fresh and activated B-MFI are reduced, leading to a linear correlation between ln(Aapp) and Eapp (Fig. 3e and Table S5). Literature suggest that Eapp is typically higher with mechanisms involving gas-phase radicals (>150 kJ·mol−1) than that with surface-mediated mechanisms (< 120 kJ·mol−1)27. Thus, measured Eapp values suggest that ODHP on activated B-MFI likely occurs via a radical mechanism.
C3H8 reaction order and product distribution in ODHP on activated B-MFI differ fundamentally from those on fresh B-MFI. The apparent C3H8 order on activated B-MFI increases from 1.04 to 2.05 as pC3H8 rises beyond 0.15 atm (Fig. 3f), while no such transition is present on fresh B-MFI (Fig. 2a). A first-order kinetics for C3H8 indicates that the reaction proceeds via a surface-mediated mechanism, while a second-order suggests a gas-phase radical mechanism11,12,24,25,26,27. This claim is also supported by the experiments that using silicon carbide (SiC) as a diluent to differentiate surface-mediated reactions from gas-phase radical reactions10. The introduction of SiC does not influence ODHP activity of fresh B-MFI, but markedly increases C3H6 formation rate of activated B-MFI (Fig. S10a). Enhancements in catalytic activity induced by diluent is a characteristic of gas-phase reaction, which could be attributed to the extended residence time of gas-phase active species (Fig. S10b). Moreover, similar to bulk boron species but distinct from fresh B-MFI, C2H4, rather than CO, is the main by-product on activated B-MFI. Further, the trends of C3H6 and CO selectivity with decreasing C3H8 conversion are opposite on fresh and activated B-MFI (Figs. 2b, 3g), indictive of distinct reaction mechanisms. C3H8 reaction order (pC3H8 > 0.15 atm) and product distribution on activated B-MFI are similar to those on catalysts with aggregated boron species, suggesting that the nature of active boron species on fresh B-MFI has been altered during the steaming treatment and the induction period.
Evolution of active boron species in B-MFI
Characterization results show that elemental composition, morphology and crystalline structure of B-MFI remain largely unchanged during the steaming treatment and in the induction period. The melting point of B2O3 (723 K) is close to ODHP temperature and the volatilization of boron species was considered as a potential cause for catalyst deactivation43. B/Si molar ratios of both fresh and activated B-MFI determined by ICP-AES are consistent with that employed in the synthesis, indicating negligible boron loss. (Fig. S11) SEM images, XRD pattern and 29Si NMR spectra show that activated B-MFI retains the crystalline size, morphology, crystallographic parameters and silicon coordination environment after the steaming treatment and the induction period. (Figs. S12–14 and Table S6) Characterization results indicate that the enhanced ODHP activity on activated B-MFI is unlikely caused by changes in the compositional and structural change in the framework of B-MFI.
11B NMR results indicate that the boron coordination in fresh B-MFI is gradually hydroxylated during the steaming treatment, but most boron atoms remain in the zeolite framework in fully steamed and activated B-MFI. 11B NMR spectra of various B-MFI samples were fitted with three peaks attributed to B[4]-Fr (red), B[3]-Fr (green) and B[3]-Nf (blue) (Fig. 4a, S15 and Table S7)29,34,49. B[4]-Fr is the dominant boron species (88%) in fresh B-MFI, with the rest being B[3]-Fr. With increasing steaming duration (1–4 h) at 823 K, an increasing fraction of B[4]-Fr is hydrolyzed to B[3]-Fr. B[3]-Fr bonded with hydroxyl, i.e., [B(OSi)x(OH)3−x] (x = 1, 2), are stabilized by the formation of hydrogen bonding network, suppressing the conversion of B[3]-Fr with hydroxyl to B[4]-Fr via condensation34. After steaming for 2 h, B[3]-Nf, i.e., B2O3 and hydroxylated B2Ox(OH)3−x, appears in 11B NMR spectra due to the further hydrolysis of B–O–Si bond. The conversion from B[4]-Fr to B[3]-Fr, as well as the further hydroxylation of B[3]-Fr to B[3]-Nf, slows after 4 h of steaming. ODHP activity parallels the increase in the fraction of boron in B[3]-Fr within the first 4 h of steaming, and levels off afterwards (Fig. 3a–c). The fraction of boron species in the form of B[3]-Nf increases from 11% to 15% when B-MFI is steamed for additional 8 h after the initial 4 h, but with no appreciable change in the ODHP activity. The main difference in the distribution of various boron species between fully steamed and activated B-MFI is that B[3]-Fr accounts for a slightly higher fraction in activated B-MFI at the expense of B[4]-Fr (Fig. S15 and Table S7). The combined fraction of B[4]-Fr and B[3]-Fr in fully steamed and activated B-MFI is ≥ 85%, (Fig. S15 and Table S7) indicating that the majority of boron species are present as isolated in the zeolite framework. As discussed in Fig. 1, B[4]-Fr in 11B NMR spectra is converted into [B(OSi≡)3] at high temperature. It can be inferred that B[3]-Fr species, i.e., [B(OSi≡)3−x(OH)x] characterized by the 11B NMR spectroscopy, are largely responsible for the enhanced ODHP activity during steaming treatment. (Fig. 4b) The values of fitting lines on y-axis corresponds to the intrinsic activity of [B(OSi≡)3], which is the dominant boron specie on fresh B-MFI. As the duration of the steaming treatment increases, [B(OSi≡)3] is gradually hydroxylated to [B(OSi≡)3−x(OH)x], leading to a linear increase in the ODHP activity. (Fig. 4b) The slope of fitted lines in Fig. 4b, which is the growth rate of the ODHP activity with the content of [B(OSi≡)3−x(OH)x], is highly dependent on pC3H8. This is beacuse the supra-linear kinetics on steamed and activated B-MFI substantially enhances the ODHP rate at higher pC3H8. Due to the conversion of B[3]-Fr to B[4]-Fr induced by water uptake at room temperature21,34, the B[4]-Fr /B[3]-Fr ratios determined based on 11B NMR spectra collected at room temperature are likely overestimated compared to those at the reaction temperature, however, the increasing trend of the B[3]-Fr/B[4]-Fr ratio with the duration of the steaming treatment should hold regardless. We note that the observation that the steaming treatment itself is insufficient to fully activate B-MFI has been made on other boron-based catalysts in ODPH50. For example, the steaming treatment can only reduce the duration of, rather than replace, the induction period in ODHP (Fig. S9). Since the key difference between the steaming treatment and ODHP is the presence of reactive intermediates in the latter, we speculate that radical species generated in ODHP could help activate boron-based catalysts, though the exact mechanism remains unclear.
a 11B NMR spectra and peak fitting of boron species on fresh, steamed and activated B-MFI. b Correlation between the specific ODHP activity and the percentage of [B(OSi ≡)3−x(OH)x] in B-MFI at various pC3H8 values. c Transmission IR spectra of dehydrated fresh and activated B-MFI under vacuum at 823 K. d 2D 1H-1H DQ MAS NMR spectrum of activated B-MFI. e Calcualted binding energy of HOO• on different boron sites. Balls and sticks in the inset pictures represent atoms: hydrogen (white), boron (pink), oxygen (red), and silicon (yellow).
Hydroxylation of isolated boron sites during steaming is further supported by IR results. (Fig. 4c, S16 and S17) The intensity of the band attributable to intraporous B−OH groups with hydrogen bond interaction (~3680 cm−1)21,51,52 on activated B-MFI is stronger than that of fresh B-MFI at reaction temperature (823 K, Fig. 4c), which is strong evidence supporting the formation of B−OH group during the steaming treatment and the induction period. The peak located at 3710-3720 cm−1 is typically attributed to the silanol group on the intraporous and external surface of zeolite crystals21,51,52. Transmission IR spectra collected at different temperatures confirm that B[3]-Fr instead of B[4]-Fr is also the dominant boron species on activated B-MFI at elevated temperature, which is consistent with the discussion of fresh B-MFI. (Figs. S16 and S17) Combined with 11B NMR results, the appearance of B–OH group indicates that the isolated boron sites are derived from the hydroxylation of [B(OSi≡)3] to [B(OSi≡)3−x(OH)x]. (Fig. 4c and S18)
Results of 1H NMR experiments on activated B-MFI indicate the spatial proximity between boron hydroxyl in [B(OSi≡)3−x(OH)x] and silanol group. In the 1H MAS NMR spectrum of activated B-MFI (Fig. S19), 1H signals at 1.8, 2.1 and 3.1 ppm were observed, which could be assigned to terminal silanol groups (≡SiOH), silanol groups adjacent to oxygen (≡SiOH···O(H)X, X = Si≡ or B= , “···” represents hydrogen bonding), and boron hydroxyl group adjacent to oxygen (=BOH···O(H)X, X = Si≡ or B= )21. 1H-11B rotational-echo double resonance (REDOR) experiment was then conducted to identify which hydrogen species are adjacent to boron. (Fig. S20) Besides [≡SiOH···O(H)X] at 2.1 ppm and [=BOH···O(H)X] at 3.1 ppm, a 1H signal at 2.5 ppm was observed in 1H-11B REDOR experiment, which is assigned to silanol hydroxyl groups adjacent to boron [≡SiOH···B≡] according to previous studies21,29. Since most boron species are still in the zeolite framework (Fig. 4a), the presence of silanol adjacent to boron supports the existence of [B(OSi≡)3−x(OH)x] in activated B-MFI. 2D 1H-1H double-quantum (DQ) MAS NMR spectra are shown in Fig. 4d. The autocorrelation signal at (1.8, 3.6) ppm is derived from the interaction between terminal silanol hydroxyl groups29. The presence of the off-diagonal peak pair at (3.3, 5.4) and (2.1, 5.4) ppm indicates the spatial proximity of the silanol and boron hydroxyl groups [=BOH···O(H)Si≡] (δDQ(5.4 ppm) = 2.1 ppm + 3.3 ppm), supporting the hydroxylation of B–O–Si linkage during the activation of B-MFI. The peak pair at (3.7, 7.0) and (3.3, 7.0) ppm is likely derived from the spatial proximity of boron hydroxyl groups. Since the boron content is B-MFI is relatively low (the molar ratio of Si/B ≈ 60), it is unlikely for two framework boron atoms to be located close to one another. Thus, this NMR spectral feature could be better attributed to [B(OSi≡)(OH)2], where two hydroxyl groups are bonded to the same boron atom. However, the existence of [B(OSi≡)2(OH)] species cannot be excluded in activated B-MFI by these experiments. The strong signals at 2.1 and 2.5 ppm in the 1H-11B REDOR spectra of activated B-MFI suggest that most boron hydroxyl groups interact extensively with adjacent silanol groups (Fig. S20), and thus the [B(OSi≡)3−x(OH)x] species could be more accurately denoted by [B(OSi≡)3−x(OH···O(H)Si≡)x] to highlight to spatial proximity of boron and silanol groups.
DFT calculations suggest that the hydroxylation of isolated boron sites is beneficial to the stabilization of potential gas-phase radicals. (Fig. 4e) Hydroperoxyl radical (HOO•) was proposed as a potential H-abstractor of alkane in oxidative dehydrogenation11,12. Our previous study show that treating HOO• as the active intermediate could rationalize the observed second-order dependence on pC3H8 on boron-based catalysts12. The binding energy of HOO• on [B(OSi≡)3] was calculated to be 1.61 eV (more positive values indicate less favorable binding), which gradually decreased to 0.87 and 0.49 eV as one and two B−O−Si bonds were hydrolyzed, respectively (Fig. 4e). Stabilization of HOO• on hydroxylated framework boron species could be largely attributed to the hydrogen-bonding interaction between HOO• and the hydroxyl group(s).
ODHP on B-BEA
To probe the generality of findings on B-MFI, effect of steaming treatment on ODHP activity and kinetics on B-BEA was investigated. XRD and N2 physisorption results confirm the framework type and crystallinity of the synthesized B-BEA. (Figs. S21, S22 and Table S8) 11B NMR spectra indicate that most boron atoms are incorporated into the zeolite framework and remain unchanged during the steaming treatment. (Figs. S23, S24 and Table S9) After 12 h of steaming treatment, the ODHP activity of fresh B-BEA increases from 0.25 to 0.50 molC3H6·molB−1·h−1 at pC3H8 = 0.25 atm. (Fig. 5a) Similar to B-MFI, fully steamed B-BEA can be further activated in ODHP during the induction period (Fig. S25). The apparent C3H8 order of B-BEA also increases from 0.98 to 2.36 as pC3H8 increases beyond 0.15 atm. (Fig. 5b) The measured Eapp and ln (Aapp) also exhibits the linear correlation similar to that on B-MFI. (Fig. 5c and Table S10) Overall, the catalytic behaviors of B-BEA and B-MFI in ODHP are consistent.
a Specific C3H6 formation rate of different B-BEA samples. Reaction conditions: 803 K, total gas flow = 40 mL·min−1, 50 mg catalyst, pO2 = 0.125 atm with balancing N2. b The reaction order of rC3H6 with respect to pC3H8 on fresh and activated B-BEA samples. Reaction conditions: 773 K, total gas flow = 40 mL·min−1, pO2 = 0.125 atm with balancing N2. c The compensation effect between Eapp and Aapp. The inset are corresponding Arrhenius plots. Reaction conditions: 773 to 793 K, total gas flow = 40 mL·min−1, 50 mg catalyst, pO2 = 0.125 atm with balancing N2. d Transmission IR spectra of dehydrated fresh and activated B-BEA in the OH stretching vibration region under vacuum at 373 K.
Hydroxylation of boron sites also occurs during the steaming treatment on B-BEA. The conversion from B[4]-Fr to B[3]-Fr, which corresponds to the hydroxylation of isolated boron site, was also observed on activated B-BEA in 11B NMR spectra34. (Fig. S23 and Table S9) A shoulder peak at −103 ppm for [Si(OSi≡)3(OH)] in 29Si NMR spectra appears after steaming treatment, implying the hydrolysis of Si−O−X53. (Fig. S24) The increase of B−OH is further supported by IR spectra. (Fig. 5d) The IR peaks of isolated Si−OH (3742 cm−1) and isolated B−OH (3700 cm−1) were observed in OH stretching vibration region on B-BEA22,27,53. The intensity of IR peak corresponding to B−OH becomes much stronger on activated B-BEA than that on fresh B-BEA, indicating the hydrolysis of B−O−Si linkages. The similarities in the correlation between boron speciation and ODHP kinetics on B-MFI and B-BEA suggest that effect of the degree of framework boron hydroxylation on ODHP is general among isolated boron sites embedded in zeolites.
Discussion
Correlation among kinetic variables of ODHP on boron-based catalysts
Trends of C3H8 reaction order and Eapp provide insights into ODHP mechanisms on isolated boron sites in zeolite frameworks. There is a general linear correlation between C3H8 reaction order and Eapp on boron-containing catalysts in ODHP (Fig. 6a). Our recent work demonstrated that this correlation among aggregated boron species stemmed from the gradual shift from a surface-mediated mechanism to a gas-phase radical mechanism as the C3H8 reaction order increased beyond unity (grey symbols in Fig. 6a)27. Surface-mediated mechanisms typically lead to first-order kinetics with respect to C3H8, while gas-phase radical mechanisms could lead to C3H8 reaction orders of up to 327. It should be noted that kinetic studies only probe the most populated reaction pathway, so that a first-order kinetics does not rule out the presence of radicals in the reaction system. Instead, the radical-mediated pathway is insufficiently populated to contribute significantly to the overall reactivity, potentially due to the slow production rates or fast quenching of radicals. Since B-MFI and B-BEA fit in the same trend (blue and red symbols in Fig. 6a), we propose that ODHP on isolated boron sites also have contributions from both the surface-mediated and gas-phase radical mechanisms, but with a key difference. Aggregated boron species, e.g., B2O3 and h-BN, were known to mediate gas-phase radical pathway, so the first-order kinetics for C3H8 on B2O3 nanoparticles in mesopores of SBA-15 and MCM-41 at low pC3H8 (< 0.10 atm) was attributed to the rapid quenching of radicals by colliding with the pore walls27. On fresh B-MFI and B-BEA, first-order kinetics for C3H8 was observed within the entire pC3H8 range investigated (0.025 atm < pC3H8 < 0.25 atm), suggesting that [B(OSi≡)3] likely only facilitates the surface-mediated pathway in ODHP. The pore sizes of B-MFI (0.55 nm) and B-BEA (0.67 nm) are smaller than those in MCM-41 (3.2 nm) and SBA-15 (8.7 nm), so quenching of gas-phase radicals is expected to be more effective in zeolites. Meanwhile, the difference in pore sizes among these materials is minor in comparison to the mean-free-path of gas at ODHP temperature (~150 nm)27. In addition, second-order kinetics for C3H8 was observed on activated B-MFI and B-BEA, confirming that the size of zeolitic pores alone cannot suppress the gas-phase radical pathway at high pC3H8. The degree of its hydroxylation of framework boron species plays a decisive role, i.e., [B(OSi≡)3] for the surface-mediated pathway and [B(OSi≡)3−x(OH···O(H)Si≡)x] for the gas-phase radical pathway in ODHP. (Fig. 4e) The detection of hydroxyl groups on spent h-BN and B2O3 catalysts, on which the radical mechanism is dominant in ODHP, is also consistent this hypothesis16,17,18.
a Correlation between apparent C3H8 order and Eapp on different B-containing catalysts in ODHP. The data of supported B2O3 catalysts (grey symbols) comes from our previous study27. b The influence of the degree of hydroxylation on the compensation effect of activation enthalpies and entropies in ODHP on B-MFI. The color of dots represent the percentage of [B(OSi ≡)3−x(OH‧‧‧O(H)Si ≡)x] in borosilicate zeolites, quantified by the results of 11B NMR spectra. (Tables S7, S9).
The highly consistent linear correlation between activation enthalpies and entropies in ODHP on B-MFI at different stages of activation (Fig. 6b and S26) indicating that the ODHP mechanism is largely independent of zeolite framework type but sensitive to the degree of hydroxylation or the coordination environment of boron. As the extent of hydroxylation in B-MFI increases from 8 to 41% (Fig. 6b), both ΔH‡ and ΔS‡ increases, suggesting increasing contributions from the gas-phase radical mechanism to the overall reactivity. The activation enthalpy-entropy correlation on B-MFI could be rationalized as changes in the relative contributions from the surface-mediated and gas-phase radical mechanisms with varying [B(OSi≡)3]/[B(OSi≡)3−x(OH···O(H)Si≡)x] ratios. While framework [B(OSi≡)3] is unique in being able to facilitate ODHP only through a mostly surface-mediated pathway, hydroxylated boron species, regardless whether in isolated or aggregated form, are able to enable both mechanisms, with the gas-phase radical mechanism being dominant at high pC3H8. Direct comparison of ODHP between boron-containing zeolites and aggregated boron species, e.g., B2O3 and h-BN, is hampered by the difficulty in determining the density of surface boron sites. When normalized by the mass loading of boron, supported B2O3/SBA-15 catalyst with a low boron loading (0.5 wt%, B2O3 basis) exhibits comparable ODHP activity with borosilicate zeolites (Fig. S27). The specific ODHP activity of h-BN is much lower, likely due to the much lower dispersion of boron. With the concomitant evolution of the degree of hydroxylation of framework boron species and kinetic variables, including C3H8 reaction order, Eapp and Aapp on B-MFI, it could be inferred that [B(OSi≡)3] is likely responsible for the surface-mediated mechanism, while [B(OSi≡)3−x(OH···O(H)Si≡)x] stabilizes key radcial species (such as HOO•) to initiate the gas-phase radical pathway, leading to a second-order kinetics with respect to C3H8 (Fig. 7).
In summary, we demonstrated that the degree of hydroxylation of isolated boron sites in zeolites has a major impact on the rate and mechanism in ODHP. Steaming treatment markedly promotes the ODHP activity of B-MFI by hydrolyzing framework [B(OSi≡)3] to [B(OSi≡)3−x(OH···O(H)Si≡)x]. The hydroxylated boron sites are able to facilitate the gas-phase radical reaction by stabilizing radical species, which leads to more than an order of magnitude increase in the C3H6 formation rate on B-MFI. This mechanism is supported by the shift from first- to second-order kinetics with respect to C3H8 as [B(OSi≡)3] is converted to [B(OSi≡)3−x(OH···O(H)Si≡)x]. Similar observations were made on B-BEA, suggesting the generality of proposed mechanism. The linear correlation between the activation enthalpy and entropy on B-MFI with different degrees of hydroxylation shows that the relative contributions of the surface-mediated and the gas-phase radical-mediated mechanisms determine the measured kinetic variables.
Methods
Catalysts preparation
Materials
Boric acid (H3BO3), 25 wt% tetrapropylammonium hydroxide (TPAOH) and 25 wt% tetraethylammonium hydroxide (TEAOH) were purchased from Alfa Aesar. Amorphous silica (SiO2), sodium hydroxide (NaOH) and sodium metaborate tetrahydrate (NaBO2·4H2O) were purchased from Aladdin Industrial Inc. Silicalite-1 and H-ZSM-5 samples were purchased from Tianjin Nankai University Catalyst Co., Ltd. Home-made secondary distilled water with conductivity less than 1.5 μS·cm−1 was used in preparation.
Synthesis of B-MFI zeolite
B-MFI zeolites were prepared by a solvent-free method according to the previous procedure21. In a typical synthesis, 0.03 g of H3BO3 and 2 g of SiO2 were firstly mixed in an agate mortar. Then, 2.6 g of 25 wt% TPAOH solution was added and the mixture was ground for 15 min. The mixture was transferred into a 10 mL Teflon autoclave and heated at 453 K for 72 h. After the thermal treatment, the obtained white powders were dried at 373 K for 12 h and finally calcined at 873 K for 4 h in flowing air. The as-prepared sample is referred to as fresh B-MFI. The steaming treatment was carried out at 823 K and pH2O = 1.8 kPa under air flow (40 mL·min−1). Activated B-MFI refers to fresh or steamed B-MFI sample which was treated under ODHP condition (803 K, total gas flow = 40 mL·min−1, pC3H8 = 0.25 atm, pO2 = 0.125 atm with balancing N2) for at least 6 h to finish the induction period.
Synthesis of B-BEA zeolite
B-BEA zeolites with *BEA framework were prepared by a hydrothermal method. In a typical synthesis, 21.5 g of 25 wt% TEAOH was mixed with 11.0 g of water with stirring. Then, 0.10 g of NaOH and 0.16 g of NaBO2·4H2O were added, following by stirring for 30 min. Then 4.80 g of SiO2 was added into the solution and stirred for another 6 h. The mixed gel was transferred into a 100 mL Teflon autoclave and heated at 413 K for 6 days. After cooling down to room temperature, the obtained product was filtered, washed with water, dried at 373 K and finally calcined at 873 K for 4 h in flowing air. The as-prepared sample is referred to as fresh B-BEA. The steaming condition and the definition of activated B-BEA are same as B-MFI.
Characterization
X-ray diffraction (XRD) patterns were collected on a PANalytical X-Pert3 Powder equipped with a Cu Kα radiation (λ = 1.54056 Å). 2θ of X-ray diffraction pattern was recorded from 5° to 50° with a scan speed of 3°/min.
MAS 11B and 29Si solid-state NMR were performed on a Bruker AVANCE III 400 MHz spectrometer at resonance frequencies of 128.42 and 79.52 MHz for the 11B and 29Si nucleus, respectively, with a 2.5 mm MAS probe at a spinning rate of 20 kHz. The 11B MAS NMR spectra were collected using a single-pulse sequence with a π/12 pulse length of 3.3 μs and a recycle delay of 3 s. The 29Si MAS NMR spectra were collected using a single-pulse sequence with a π/12 pulse length of 0.4 μs and a recycle delay of 1 s. Before the NMR tests, the samples were dehydrated at 373 K under vacuum for 24 h. The parameters used for quantifying 11B NMR spectrum was obtained according to the reported method54. The estimated correction coefficient for the satellite transitions of tetra-coordinated boron Icl(ST, B[4]) is 1.04, and the estimated correction coefficient for the central transitions caused by quadrupolar coupling of tri-coordinated boron Icl(CT, B[3]) is 0.93. The corrected factor of measured 11B NMR area I(B[4]/B[3]) is 1.12.
The 1H spin-echo MAS NMR, 1H-11B symmetry-based rotational-echo double-resonance (REDOR) MAS NMR and two-dimensional (2D) 1H-1H DQ-SQ MAS NMR experiments were performed on a Bruker Avance III 600 spectrometer equipped with 14.1 T and 89 mm wide-bore magnet using 3.2 mm H-X-Y triple resonances MAS probe with the corresponding Larmor frequency of 600.13 MHz for 1H. Prior to the above experiments, the samples were dehydrated with a home-built vacuum line at 693 K for 12 h. The chemical shift of 1H NMR were referenced to adamantane at 1.74 ppm. The 1H spin-echo MAS NMR spectra were recorded with a π/2 pulse width of 4.0 μs, a recycle delay of 5 s, an echo time of 0.33 ms and a spinning rate of 12 kHz. The 1H-11B REDOR spectra were performed with a spinning rate of 12 kHz and a recycle delay of 5 s which accumulated 32 scans. A π/2 pulse length of 4.0 μs and π pulse length of 8.0 μs were used on the 1H channel with SR4 recoupling pulse. The recoupling time was 0.66 ms. The saturation pulse on the 11B channel was with duration of 125 μs (1.5 Tr). The 1H-1H DQ-SQ MAS NMR spectra were excited and reconverted with the POST-C7 pulse sequence with a spinning rate of 12 kHz and a recycle delay of 2 s. The increment in the indirect dimension (t1) was set to 83.34 μs, and 32 scans were acquired for each t1 increment.
Fourier transform infrared (FTIR) spectroscopy experiments were performed on a Bruker Invenio-S spectrometer in a custom transmission cell by using pyridine as the probe molecule. The samples were pressed into self-supporting wafers of about 10 mg and loaded into a sample holder for vertical alignment in the infrared beam. Spectra presented were 64 coadded scans per spectrum at a spectral resolution of 4 cm−1. Vacuum levels of < 0.01 mTorr in the transmission cell were achieved by connecting to a vacuum manifold equipped with a mechanical pump (Agilent) and a diffusion pump (Agilent). The transmission cell was heated by heating rings controlled by a temperature programmed PID controller. Prior to all experiments, the samples were evacuated at 823 K for 1 h to remove adsorbed molecules. The samples were then cooled down to 298 K under vacuum for further characterization.
Scanning electron microscopy (SEM) experiments were performed on a Zeiss Merlin Compact electron microscope with an acceleration voltage of 2 kV.
The element compositions of B-MFI, ZSM-5 and B-BEA zeolites were measured by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) over Teledyne Leeman Labs Prodigy 7 after digestion with 2 vol% HF.
The N2 adsorption-desorption isotherms were measured at 277 K over a Micromeritics Tristar II 3030 analyzer. Before the tests, all the materials were evacuated at 363 K for 1 h and 573 K for 6 h. Brunauer-Emmett-Teller (BET) surface area are calculated from adsorption data in range of p/p0 = 0.05–0.30. The micropore evaluation was used t-plot method based on Harkins-Jura model. The linearization of t-plot is in range from thickness = 3.5–5 Å, corresponding to p/p0 = 0.10–0.27.
Computational method
The unit cell of B-MFI is obtained from the peak positions in XRD patterns with the cell parameters of a = 20.07 Å, b = 19.88 Å, c = 13.39 Å, α = β = γ = 90°. DFT calculations using periodic boundary condition (PBC) models are carried out with the Quickstep module of the CP2K (version 7.1) package55. The Perdew-Burke-Ernzerhof (PBE)56 functional is used in combination with Godecker-Teter-Hutter (GTH) pseudopotentials57 and the molecularly optimized double-ζ polarization quality Gaussian basis sets (DZVP-MOLOPT-SR-GTH)58. An 800 Ry energy cutoff, a 70 Ry relative energy cut-off, and 4 grid levels are employed. The binding energy of hydroperoxyl radical (HOO•) on isolated boron sites with different degree of hydroxylation was calculated as
where EB-MFI-OOH, EB-MFI, EO2 and μH refer to the calculated energy of B-MFI-OOH intermediate, the isolated boron sites, O2 in gas phase and the chemical potential of H which referenced from water formation 1/2H2 + 1/4O2 = 1/2H2O (μH = 1/2EH2O – 1/4EO2). According to the definition of Ebind(HOO•), more positive values indicate less favorable binding between B-MFI and HOO•.
Catalytic testing
Catalytic properties of ODHP were evaluated in a quartz fixed-bed tubular reactor with an inner diameter of 6 mm under atmospheric pressure. In a typical experiment, 200 mg mixed sample and SiC with 20 to 40 mesh were used in tests of catalytic performance with a mass ratio of 1:3. The catalyst bed was supported by 1 cm quartz wool lug. During the steaming treatment, water vapor was introduced into the reactor by gas bubbler with the pH2O = 1.8 kPa. The reactor effluent was analyzed online by an Agilent 8890 gas chromatograph (GC) equipped with a TCD and an FID detector. O2, CO, CO2 and N2 were separated by 5 A column (Agilent J&W) and quantified by TCD. Hydrocarbons (C2H6, C3H8, C2H4, C3H6, CH4) were separated by HP-PLOT Al2O3 column (Agilent J&W) and quantified by FID. The C3H8 conversion was determined by Eq. (2).
F is the volumetric flow rate under standard temperature and pressure (STP). The specific rate of C3H6 formation was determined by Eq. (3).
L is the molar flow rate under STP and nboron is the molar amount of boron in the measured catalyst, which is determined by ICP-AES. The selectivity of product i was determined by Eq. (4).
where i represents the products C3H6, C2H4, CH4, CO and CO2 in the effluent gas, ni is the number of carbon atoms of component i, and Fi is the volumetric flow rate under STP. The carbon balance (Eq. (5)) in all experiments was higher than 98%,
The deactivation of catalysts was less than 5% in all cases.
Data availability
The authors declare that all the relevant data within this paper and its Supplementary Information file are available from the corresponding author upon a reasonable request. Source data are provided with this paper.
References
Sattler, J. J. H. B., Ruiz-Martinez, J., Santillan-Jimenez, E. & Weckhuysen, B. M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 114, 10613–10653 (2014).
Chen, S. et al. Propane dehydrogenation: catalyst development, new chemistry, and emerging technologies. Chem. Soc. Rev. 50, 3315–3354 (2021).
Lange, J. P., Schoonebeek, R. J., Mercera, P. D. L. & van Breukelen, F. W. Oxycracking of hydrocarbons: chemistry, technology and economic potential. Appl. Catal. A: Gen. 283, 243–253 (2005).
Cavani, F., Ballarini, N. & Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 127, 113–131 (2007).
Kondratenko, E. V. et al. Oxidative dehydrogenation of propane over V/MCM-41 catalysts: comparison of O2 and N2O as oxidants. J. Catal. 234, 131–142 (2005).
Murakami, Y., Otsuka, K., Wada, Y. & Morikawa, A. Partial Oxidation of Ethane over Boron Oxide Added Catalysts. Chem. Lett. 18, 535–538 (1989).
Buyevskaya, O. V. & Baerns, M. Catalytic selective oxidation of propane. Catal. Today 42, 315–323 (1998).
Grant, J. T. et al. Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts. Science 354, 1570–1573 (2016).
Gao, X. et al. Subsurface nickel boosts the low-temperature performance of a boron oxide overlayer in propane oxidative dehydrogenation. Nat. Commun. 14, 1478 (2023).
Venegas, J. M. & Hermans, I. The Influence of Reactor Parameters on the Boron Nitride-Catalyzed Oxidative Dehydrogenation of Propane. Org. Process Res. Dev. 22, 1644–1652 (2018).
Venegas, J. M. et al. Why Boron Nitride is such a Selective Catalyst for the Oxidative Dehydrogenation of Propane. Angew. Chem. Int Ed. Engl. 59, 16527–16535 (2020).
Tian, H. & Xu, B. Oxidative co-dehydrogenation of ethane and propane over h-BN as an effective means for C–H bond activation and mechanistic investigations. Chin. J. Catal. 43, 2173–2182 (2022).
Kraus, P. & Lindstedt, R. P. It’s a Gas: Oxidative Dehydrogenation of Propane over Boron Nitride Catalysts. J. Phys. Chem. C. 125, 5623–5634 (2021).
Liu, Z. et al. Auto-accelerated dehydrogenation of alkane assisted by in-situ formed olefins over boron nitride under aerobic conditions. Nat. Commun. 14, 73 (2023).
Zhang, Z. et al. Unraveling Radical and Oxygenate Routes in the Oxidative Dehydrogenation of Propane over Boron Nitride. J. Am. Chem. Soc. 145, 7910–7917 (2023).
Love, A. M. et al. Probing the Transformation of Boron Nitride Catalysts under Oxidative Dehydrogenation Conditions. J. Am. Chem. Soc. 141, 182–190 (2019).
Dorn, R. W. et al. Structure Determination of Boron-Based Oxidative Dehydrogenation Heterogeneous Catalysts With Ultrahigh Field 35.2 T 11B Solid-State NMR Spectroscopy. ACS Catal. 10, 13852–13866 (2020).
Yan H, et al. Identifying Boron Active Sites for the Oxidative Dehydrogenation of Propane. ACS Catal. 11, 9370–9376 (2021).
Altvater, N. R. et al. B-MWW Zeolite: The Case Against Single-Site Catalysis. Angew. Chem. Int Ed. Engl. 59, 6546–6550 (2020).
Qiu, B. et al. Oxidative dehydrogenation of propane using layered borosilicate zeolite as the active and selective catalyst. J. Catal. 385, 176–182 (2020).
Zhou, H. et al. Isolated boron in zeolite for oxidative dehydrogenation of propane. Science 372, 76–80 (2021).
Qiu, B. et al. Borosilicate zeolite enriched in defect boron sites boosting the low-temperature oxidative dehydrogenation of propane. J. Catal. 408, 133–141 (2022).
Qiu, B. et al. Boosting the propylene selectivity over embryonic borosilicate zeolite catalyst for oxidative dehydrogenation of propane. J. Catal. 417, 14–21 (2023).
Mitra, B., Wachs, I. E. & Deo, G. Promotion of the propane ODH reaction over supported V2O5/Al2O3 catalyst with secondary surface metal oxide additives. J. Catal. 240, 151–159 (2006).
Chen, K., Iglesia, E. & Bell, A. T. Kinetic Isotopic Effects in Oxidative Dehydrogenation of Propane on Vanadium Oxide Catalysts. J. Catal. 192, 197–203 (2000).
Phadke, N. M., Mansoor, E., Bondil, M., Head-Gordon, M. & Bell, A. T. Mechanism and Kinetics of Propane Dehydrogenation and Cracking over Ga/H-MFI Prepared via Vapor-Phase Exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 141, 1614–1627 (2019).
Tian, H., Liu, Y., Xu, B. Kinetic Investigations of Oxidative Dehydrogenation of Propane on Boron Oxide in Confined Spaces. Catal. Today 420, 114048 (2023).
Millini, R., Perego, G. & Bellussi, G. Synthesis and characterization of boron‐containing molecular sieves. Top. Catal. 9, 13 (1999).
Wiper, P. V., Amelse, J. & Mafra, L. Multinuclear solid-state NMR characterization of the Brønsted/Lewis acid properties in the BP HAMS-1B (H-[B]-ZSM-5) borosilicate molecular sieve using adsorbed TMPO and TBPO probe molecules. J. Catal. 316, 240–250 (2014).
Chu, C. T. W., Kuehl, G. H., Lago, R. M. & Chang, C. D. Isomorphous substitution in zeolite frameworks: II. Catalytic properties of [B]ZSM-5. J. Catal. 93, 451–458 (1985).
Suzuki, T. & Okuhara, T. Change in pore structure of MFI zeolite by treatment with NaOH aqueous solution. Microporous Mesoporous Mater. 43, 83–89 (2001).
Garcia Vargas, N., Stevenson, S. & Shantz, D. F. Simultaneous isomorphous incorporation of boron and germanium in MFI zeolites. Microporous Mesoporous Mater. 170, 131–140 (2013).
Howden, M. G. Zeolite ZSM-5 containing boron instead of aluminium atoms in the framework. Zeolites 5, 334–338 (1985).
Koller, H., Chen, C.-Y. & Zones, S. I. Selectivities in Post-Synthetic Modification of Borosilicate Zeolites. Top. Catal. 58, 451–479 (2015).
Scholle, K. F. M. G. J. & Veeman, W. S. The influence of hydration on the coordination state of boron in H-Boralite studied by 11B magic angle spinning n.m.r. Zeolites 5, 118–122 (1985).
Fild, C., Shantz, D. F., Lobo, R. F. & Koller, H. Cation-induced transformation of boron-coordination in zeolites. Phys. Chem. Chem. Phys. 2, 3091–3098 (2000).
Koller, H., Fild, C. & Lobo, R. F. Variable anchoring of boron in zeolite beta. Microporous Mesoporous Mater. 79, 215–224 (2005).
Kroeker, S. & Stebbins, J. F. Three-Coordinated Boron-11 Chemical Shifts in Borates. Inorg. Chem. 40, 6239–6246 (2001).
Medeiros-Costa, I. C. et al. Silanol defect engineering and healing in zeolites: opportunities to fine-tune their properties and performances. Chem. Soc. Rev. 50, 11156–11179 (2021).
Schroeder, C. et al. Hydrogen Bond Formation of Brønsted Acid Sites in Zeolites. Chem. Mater. 32, 1564–1574 (2020).
de Ruiter, R., Kentgens, A. P. M., Grootendorst, J., Jansen, J. C. & van Bekkum, H. Calcination and deboronation of [B]-MFI single crystals. Zeolites 13, 128–138 (1993).
Hwang, S.-J., Chen, C.-Y. & Zones, S. I. Boron Sites in Borosilicate Zeolites at Various Stages of Hydration Studied by Solid State NMR Spectroscopy. J. Phys. Chem. B 108, 18535–18546 (2004).
McDermott, W. P., Cendejas, M. C. & Hermans, I. Recent Advances in the Understanding of Boron-Containing Catalysts for the Selective Oxidation of Alkanes to Olefins. Top. Catal. 63, 1700–1707 (2020).
Tian, J. et al. Understanding the origin of selective oxidative dehydrogenation of propane on boron-based catalysts. Appl. Catal. A: Gen. 623, 118271 (2021).
van Bokhoven, J. A. et al. An Explanation for the Enhanced Activity for Light Alkane Conversion in Mildly Steam Dealuminated Mordenite: The Dominant Role of Adsorption. J. Catal. 202, 129–140 (2001).
Yi, X. et al. Origin and Structural Characteristics of Tri-coordinated Extra-framework Aluminum Species in Dealuminated Zeolites. J. Am. Chem. Soc. 140, 10764–10774 (2018).
Gao, B. et al. Dynamic Self-Dispersion of Aggregated Boron Clusters into Stable Oligomeric Boron Species on MFI Zeolite Nanosheets under Oxidative Dehydrogenation of Propane. ACS Catal. 12, 7368–7376 (2022).
Zhou, Y. et al. Peripheral-nitrogen effects on the Ru1 centre for highly efficient propane dehydrogenation. Nat. Catal. 5, 1145–1156 (2022).
Hough, A. et al. Boron removal and reinsertion studies in 10B–11B exchanged HAMS-1B (H-[B]-ZSM-5) borosilicate molecular sieves using solid-state NMR. J. Catal. 334, 14–22 (2016).
Zhou, Y. et al. Enhanced performance of boron nitride catalysts with induction period for the oxidative dehydrogenation of ethane to ethylene. J. Catal. 365, 14–23 (2018).
Jansen JC, de Ruiter R, Biron E, van Bekkum H. Isomorphous Substitution of Si in Zeolite Single Crystals. Part II. On The Boron Distribution and Coordination In [B]-ZSM-5. In: Studies in Surface Science and Catalysis (eds Jacobs PA, van Santen RA). Elsevier (1989).
Datka, J. & Piwowarska, Z. OH groups in boralites. J. Chem. Soc., Faraday Trans. 1: Phys. Chem. Condens. Phases 85, 837–841 (1989).
Coudurier, G. & Vedrine, J. C. Catalytic and acidic properties of boron pentasil zeolites. Pure Appl. Chem. 58, 1389–1396 (1986).
Massiot, D., Bessada, C., Coutures, J. P. & Taulelle, F. A quantitative study of 27Al MAS NMR in crystalline YAG. J. Magn. Reson. 90, 231–242 (1990).
Kühne, T. D. et al. CP2K: An electronic structure and molecular dynamics software package - Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 152, 194103 (2020).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703–1710 (1996).
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (22172001 and 22108006) and Beijing National Laboratory for Molecular Sciences. H.T. acknowledges the financial support from the Chinese Postdoctoral Science Foundation (No. 2020M680239 and 2022T150009).
Author information
Authors and Affiliations
Contributions
B.J.X. supervised the project. H.T. performed the experiment and characterization. W.Y.L. and H.X. carried out the DFT simulations and analysis. L.H.H., Y.Z.Z. and S.T.X. contributed to the characterization of borosilicate zeolites. All the authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, H., Li, W., He, L. et al. Rationalizing kinetic behaviors of isolated boron sites catalyzed oxidative dehydrogenation of propane. Nat Commun 14, 6520 (2023). https://doi.org/10.1038/s41467-023-42403-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42403-2
- Springer Nature Limited